A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond
Abstract
:1. Introduction
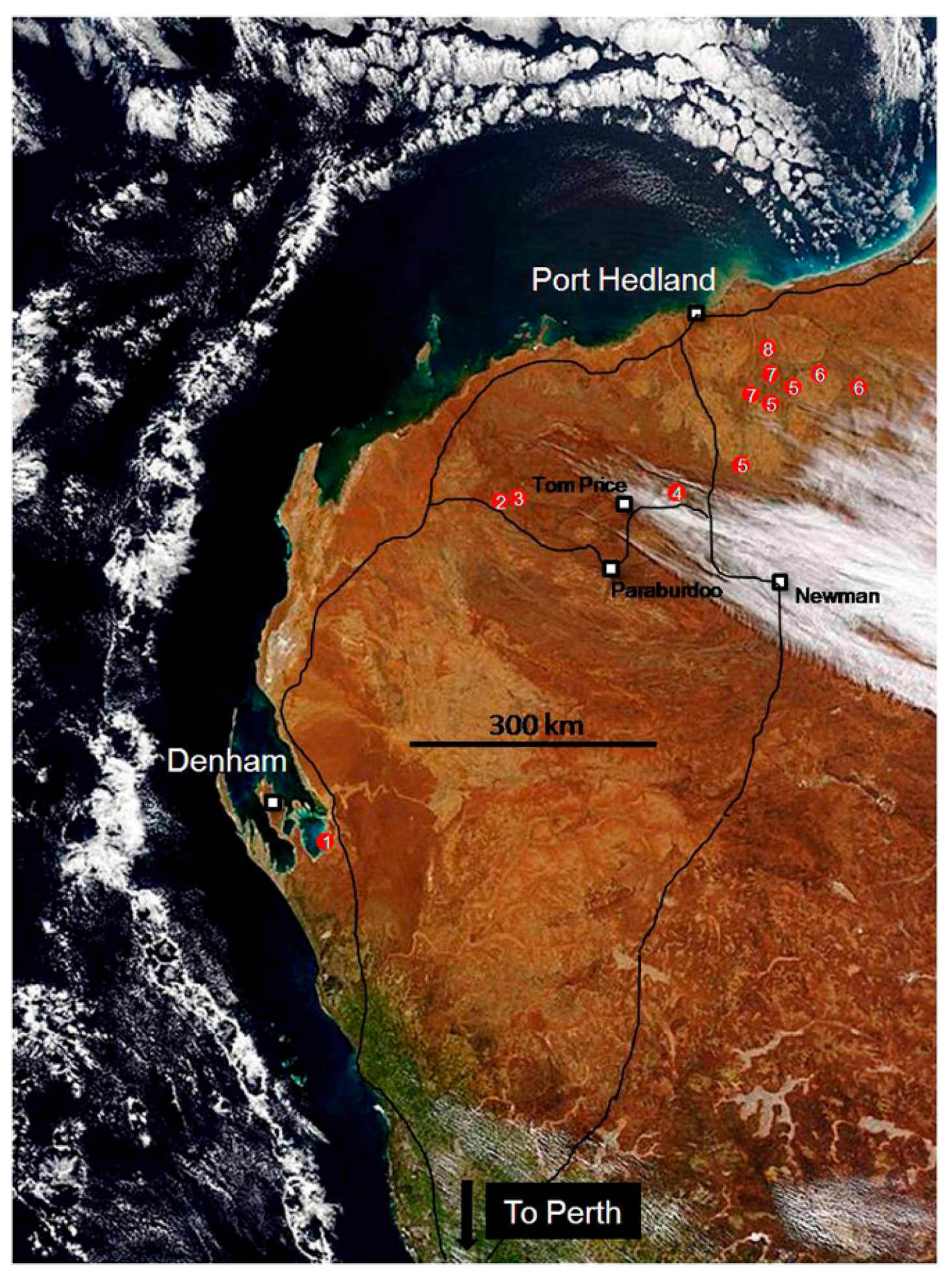
2. A Field Trip in Deep Time to the Archaean
3. Living Marine Stromatolites and Inland Microbial Communities
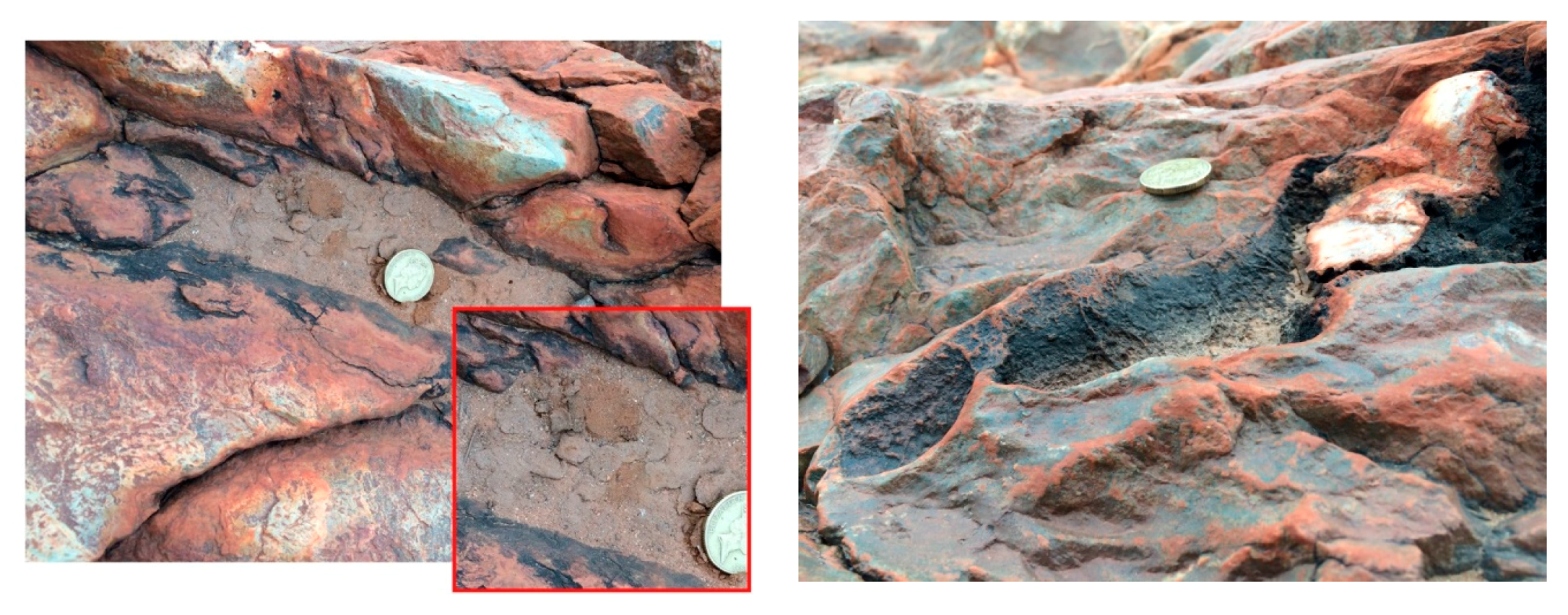
4. Fossil Stromatolites
5. A novel Model for a Terrestrial Origin of Life
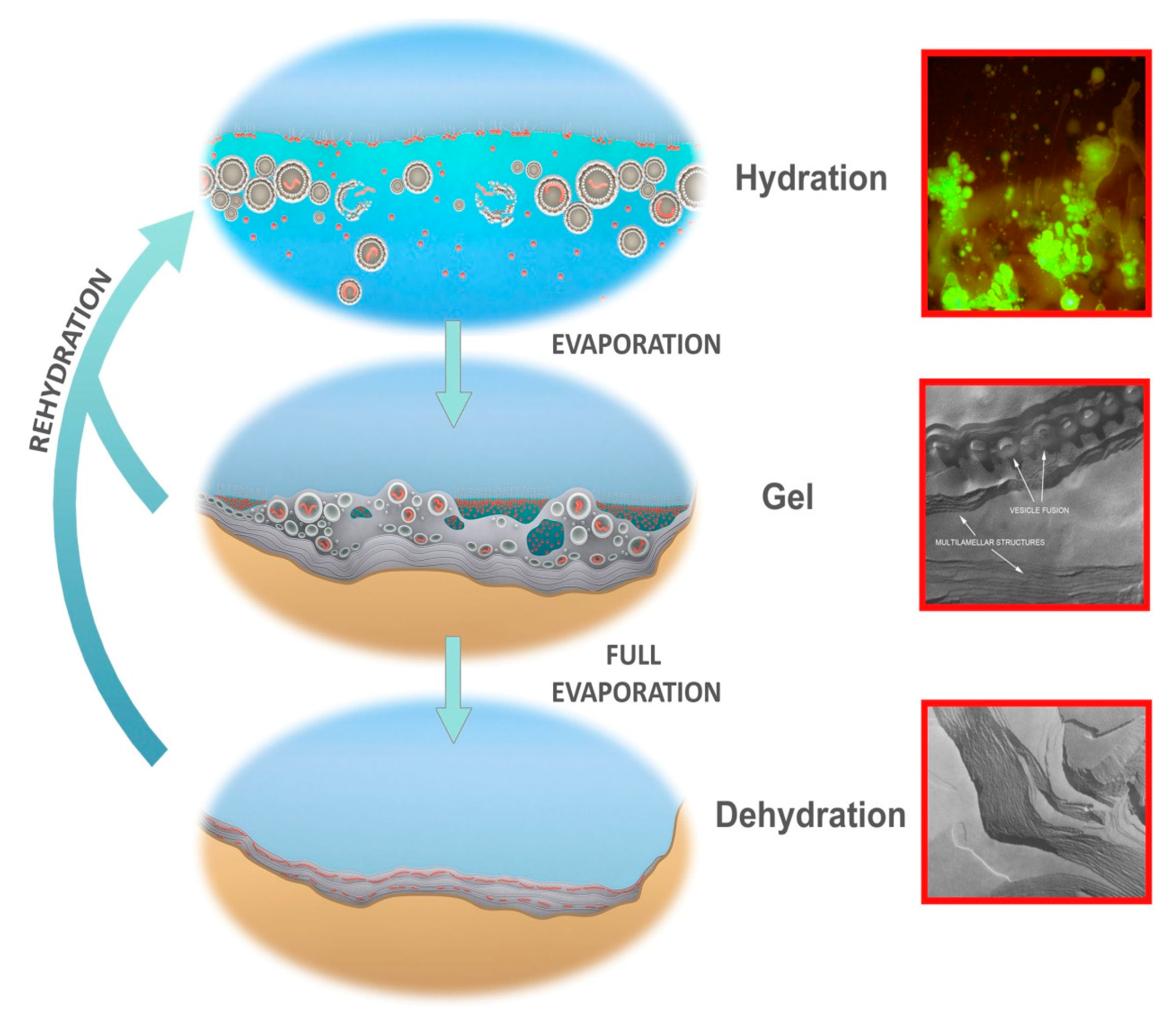
6. Linking Chemical Reactions to the Physical Properties of Hydrothermal Pools
7. A Gel Phase as Candidate Progenote
8. The Adaptive Radiation of the Progenote
9. Summary of Novel Features and Testable Predictions of the Model
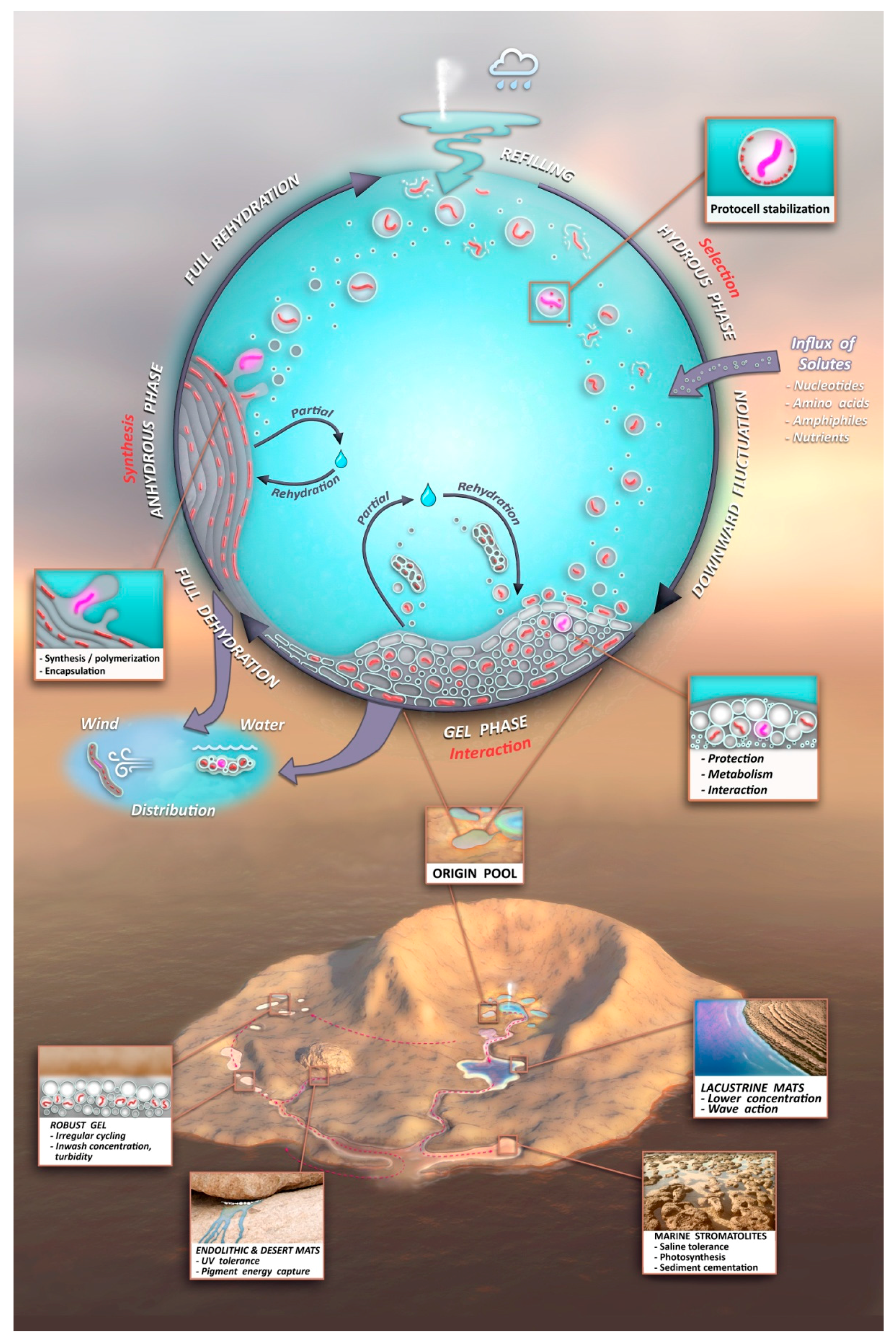
- A kinetic trap cycling polymers through coupled phases of hydration and dehydration will produce large numbers of random molecular systems synthesized between dehydrated lipid lamellae (Figure 9, upper left), which become encapsulated within large numbers of lipid compartments (protocells).
- Combinatorial selection of encapsulated functional polymers will be observed within this kinetic trap. The initial selection criterion will be the ability of encapsulated polymers to enhance the stability of their membranous compartments, which will be tested in the hydrous phase (Figure 9, upper right).
- During dehydration, stable protocells will crowd together with concentrated solutes forming a third, gel phase (Figure 9, center) which provides a protective environment. Interaction of protocells within the gel will enable early forms of metabolism, competition, and sharing of functions and products across the gel.
- These three phases will provide continuous resources and subject molecular systems to a variety of selective pressures, driving the system to surmount hurdles to achieve stepwise molecular evolution of new functions.
- Pure self assembly and random synthesis will enable the initial stages of the model, but eventually, active functions expressed through sets of robust, heritable proto-genomic instructions will enable Darwinian evolution to take hold.
- Degradation rates of synthesized polymers will set upper bounds on the permitted dwell time within each phase suggesting the necessity of reliable, repetitive fluid refilling typical of hydrothermal fields. Expressing a pattern emerging later as the life cycle, degraded polymers and other inert byproducts must be periodically reused or expelled and functional systems continually re-synthesized through proto-genomic blueprints drawing from solute sources (Figure 9, upper right).
- A candidate protocell gel progenote capable of growth and evolutionary adaptation will emerge and become robust to distribution to a variety of watery venues in a plausible Archaean volcanic landscape. Extensive laboratory growth, testing, and analysis of such progenotes will provide insight to possible pathways to the first microbial communities and viable free-living cells.
10. Conclusions
Acknowledgments
Resources
Conflicts of Interest
References
- Verrecchia, E.; Yair, A.; Kidron, G.; Verrecchia, K. Physical properties of the psammophile cryptogamic crust and their consequences to the water regime of sandy soils, north-western Negev Desert, Israel. J. Arid Environ. 1995, 29, 427–437. [Google Scholar] [CrossRef]
- Hoshino, Y.; George, S. Cyanobacterial Inhabitation on Archean Rock Surfaces in the Pilbara Craton, Western Australia. Astrobiology 2015, 15, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, E. Endolithic microbial life in hot and cold deserts. Orig. Life 1980, 10, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Corliss, J.; Baross, J.; Hoffman, S. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol. Acta 1981, 4, 59–69. [Google Scholar]
- Baross, J.; Hoffman, S. Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig. Life Evol. Biosphere 1985, 15, 327–345. [Google Scholar] [CrossRef]
- Russell, M.; Hall, A. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. Lond. 1997, 154, 377–402. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Martin, W.F. The origin of membrane bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Singaram, S.; Rajamani, S.; Kompanichenko, V.; Guggenheim, S. Self-assembly processes in the prebiotic environment. Phil. Trans. Roy. Soc. B Biol. Sci. 2006, 361, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.; Bychkov, A.; Dibrova, D.; Galperin, M.; Koonin, E. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 1985, 317, 792–794. [Google Scholar] [CrossRef]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.; Smith, K.; Cleaves, H.; Ruzicka, J.; Stern, J.; Glavin, D.; House, C.; Dworkin, J. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. PNAS 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed]
- Cody, G.W. Geochemical Connections to Primitive Metabolism. Elements 2005, 3, 139–143. [Google Scholar] [CrossRef]
- Cleaves, H.; Michalkova Scott, A.; Hill, F.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef] [PubMed]
- Stribling, R.; Miller, S. Energy yields for hydrogen cyanide and formaldehyde syntheses: The HCN and amino acid concentrations in the primitive ocean. Orig. Life Evol. Biosphere 1987, 17, 261–273. [Google Scholar] [CrossRef]
- Hofmann, H.; Grey, K.; Hickman, A.; Thorpe, R. Origin of 3.45 Ga coniform stromatolites in Warrawoona Group, Western Australia. Geol. Soc. Am. Bull. 1999, 111, 1256–1262. [Google Scholar] [CrossRef]
- Allwood, A.; Walter, M.; Kamber, B.; Marshall, C.; Burch, I. Stromatolite reef from the Early Archaean era of Australia. Nature 2006, 441, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Van Kranendonk, M. Stromatolite morphology as an indicator of biogenicity for Earth’s oldest fossils from the 3.5 to 3.4 Ga Pilbara Craton, Western Australia. In Advances in Stromatolite Geobiology; Lecture Notes in Earth Sciences; Reitner, J., Queric, N.-V., Arp, G., Eds.; Springer: Berlin, Germany, 2011; pp. 517–534. [Google Scholar]
- Sugitani, K.; Lepot, K.; Mimura, K.; Van Kranendonk, M.; Oehler, D.; Walter, M. Biogenicity of morphologically diverse carbonaceous microstructures from the ca. 3400 Ma Strelley Pool Formation, in the Pilbara Craton, Western Australia. Astrobiology 2010, 10, 899–920. [Google Scholar] [CrossRef] [PubMed]
- Van Kranendonk, M.; Webb, G.; Kamber, B. Geological and trace element evidence for a marine sedimentary environment of deposition and biogenicity of 3.45 Ga stromatolitic carbonates in the Pilbara Craton, and support for a reducing Archean ocean. Geobiology 2003, 1, 91–108. [Google Scholar] [CrossRef]
- Sugitani, K.; Mimura, K.; Takeuchi, M.; Yamaguchi, T.; Suzuki, K.; Senda, R.; Asahara, Y.; Van Kranendonk, M. A Paleoarchean coastal hydrothermal field inhabited by diverse microbial communities: The Strelley Pool Formation, Pilbara Craton, Western Australia. Geobiology 2015. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Buick, R.; Dunlop, J. Stromatolites, 3400–3500 Myr old from the North Pole area, Western Australia. Nature 1980, 284, 443–445. [Google Scholar] [CrossRef]
- Van Kranendonk, M.; Philippot, P.; Lepot, K.; Bodorkos, S.; Pirajno, F. Geological setting of Earth’s oldest fossils in the c. 3.5 Ga Dresser Formation, Pilbara Craton, Western Australia. Precambrian Res. 2008, 167, 93–124. [Google Scholar] [CrossRef]
- Van Kranendonk, M.; Djokic, T.; Poole, G.; Nakamura, E. The elements of life at a 3.5 Ga subaerial hotspring: Evidence from the Dresser Formation, Pilbara Craton, Australia. In Proceedings of the Astrobiology Science Conference 2015, Chicago, IL, USA, 15–19 June 2015.
- Darwin, C. Darwin Correspondence Project, “Letter No. 7471”, 1871. Available online: http://www.darwinproject.ac.uk/DCP-LETT-7471 (accessed on 19 March 2016).
- Damer, B.; Deamer, D. Coupled phases and combinatorial selection in fluctuating hydrothermal pools: A scenario to guide experimental approaches to the origin of cellular life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- King, G. Recycling, reproduction, and life’s origins. BioSystems 1982, 15, 89–97. [Google Scholar] [CrossRef]
- Vaidya, N.; Walker, S.; Lehman, N. Recycling of informational units leads to selection of replicators in a prebiotic soup. Chem. Biol. 2013, 20, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.; Georgiou, C. Hydrothermal Conditions and the Origin of Cellular Life. Astrobiology 2015, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Toppozini, L.; Dies, H.; Deamer, D.; Rheinstädter, M. Adenosine Monophosphate Forms Ordered Arrays in Multilamellar Lipid Matrices: Insights into Assembly of Nucleic Acid for Primitive Life. PLoS ONE 2013, 8, e62810. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.; Gerland, B.; Sutherland, J. Synthesis of activated pyrimidine nucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D. Lipid-assisted synthesis of RNA-like polymers from mononucleotides. Orig. Life Evol. Biosphere 2008, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, V.; Shenasa, H.; Vercoutere, W.; Deamer, D. Generation of oligonucleotides under hydrothermal conditions by non-enzymatic polymerization. J. Mol. Evol. 2014, 78, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.; Maurel, M.C.; Deamer, D. Salt-promoted synthesis of RNA-like molecules in simulatehydrothermal conditions. J. Mol. Evol. 2014, 80, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, G.; Yu, S.; Mamajanov, I.; Grover, A.; Krishnamurthy, R.; Fernandez, M.; Hud, N. Ester-mediated amide bond formation driven by wet-dry cycles: A possible path to polypeptides on the prebiotic Earth. Angew. Chem. Int. Ed. Engl. 2015, 54, 9871–9875. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Percivalle, C.; Ritson, D.; Duffy, C.; Sutherland, J. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Black, R.; Blosser, M.; Keller, S. Amino Acids and Peptides Stabilize Fatty Acid Membranes against Salt-Induced Flocculation. Biophys. J. 2015, 108, 542a. [Google Scholar] [CrossRef]
- Gilbert, W. The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Joyce, G. The antiquity of RNA based evolution. Nat. Insight 2002, 418, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Olasagasti, F.; Kim, H.; Pourmand, N.; Deamer, D. Non-enzymatic transfer of sequence information under plausible prebiotic conditions. Biochimie 2011, 93, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.; Szostak, J. Isolation of new ribozymes from a large pool of random sequences. Science 1993, 261, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Wochner, A.; Attwater, J.; Coulson, A.; Holliger, P. Ribozyme-Catalyzed Transcription of an Active Ribozyme. Science 2011, 332, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, T.; Joyce, G. Self-sustained replication of an RNA enzyme. Science 2009, 323, 1229. [Google Scholar] [CrossRef] [PubMed]
- Trevors, J.; Pollack, G. Hypothesis: The origin of life in a hydrogel environment. Prog. Biophys. Mol. Biol. 2005, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, H. Beginnings of Cellular Life: Metabolism Recapitulates Biogenesis; Yale University Press: New Haven, CT, USA, 1992. [Google Scholar]
- Odling-Smee, J.; Laland, K.; Feldman, M. Niche Construction: The Neglected Process in Evolution. In Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Mereschkowsky, K. Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Ent-stehung der Organismen. Biol. Centralbl. 1910, 30, 353–367. [Google Scholar]
- Margulis, L. Symbiogenesis. A new principle of evolution rediscovery of Boris Mikhaylovich Kozo-Polyansky (1890–1957). Paleontol. J. 2011, 44, 1525–1539. [Google Scholar] [CrossRef]
- Pereira, L.; Rodrigues, T.; Carrapico, F. A symbiogenic way in the origin of life. In Genesis-in the Beginning: Precursors of Life, Chemical Models and Early Biological Evolution, Cellular Origin; Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 22, pp. 723–742. [Google Scholar]
- Walter, M. Stromatolites: The main geological source of information on the evolution of the early benthos. In Early Life on Earth; Bengtson, S., Ed.; Columbia University Press: New York, NY, USA, 1994; Volume 84, pp. 270–286. [Google Scholar]
- Burns, B.; Goh, F.; Allen, M.; Neilan, B. Microbial diversity of extant stromatolites in the hypersaline marine environment of Shark Bay, Australia. Environ. Microbiol. 2004, 6, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, W.F. Uprooting the tree of life. Sci. Am. 2000, 282, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Roberts, R.; Szostak, J. The emergence of competition between model protocells. Science 2004, 305, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.; Szostak, J. Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 2013, 5, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.; Fox, G. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [PubMed]
- Woese, C. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar] [PubMed]





© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damer, B. A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond. Life 2016, 6, 21. https://doi.org/10.3390/life6020021
Damer B. A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond. Life. 2016; 6(2):21. https://doi.org/10.3390/life6020021
Chicago/Turabian StyleDamer, Bruce. 2016. "A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond" Life 6, no. 2: 21. https://doi.org/10.3390/life6020021
APA StyleDamer, B. (2016). A Field Trip to the Archaean in Search of Darwin’s Warm Little Pond. Life, 6(2), 21. https://doi.org/10.3390/life6020021





