Landmarks in the Evolution of (t)-RNAs from the Origin of Life up to Their Present Role in Human Cognition
Abstract
:1. Introduction
2. RNA Aminoacylation and the Origin of tRNA
3. tRNA Modification “Then and Today”
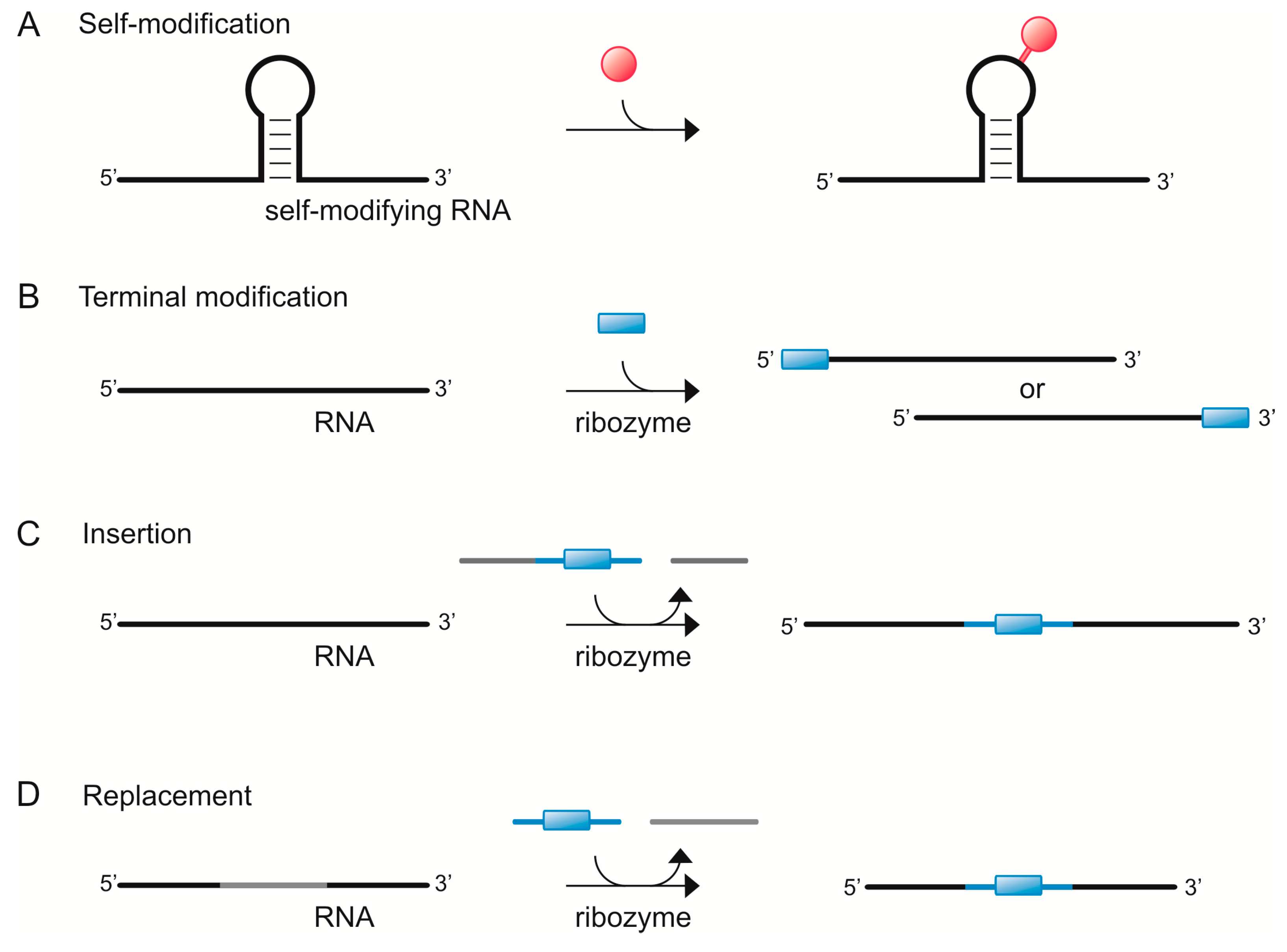
4. tRNA at an Evolutionary High Point
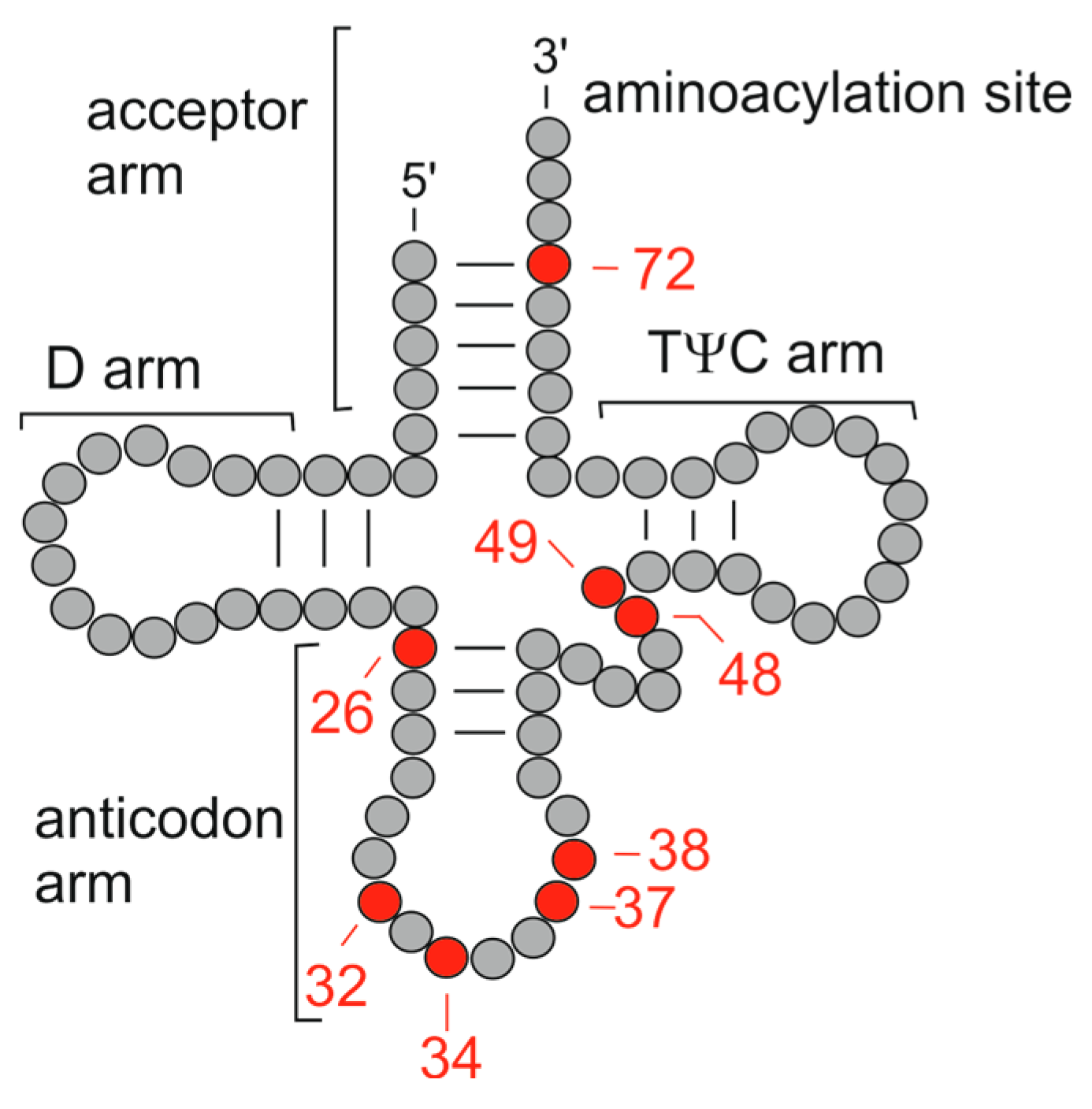
5. Conclusions
Conflicts of Interest
References
- Higgs, P.G.; Lehman, N. The RNA World: Molecular cooperation at the origins of life. Nat. Rev. Genet. 2015, 16, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Pressman, A.; Blanco, C.; Chen, I.A. The RNA World as a model system to study the origin of life. Curr. Biol. 2015, 25, R953–R963. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Crick, F.H. The origin of the genetic code. J. Mol. Biol. 1968, 38, 367–379. [Google Scholar] [CrossRef]
- Orgel, L.E. Evolution of the genetic apparatus. J. Mol. Biol. 1968, 38, 381–393. [Google Scholar] [CrossRef]
- Woese, C.R. The fundamental nature of the genetic code: Prebiotic interactions between polynucleotides and polyamino acids or their derivatives. Proc. Natl. Acad. Sci. USA 1968, 59, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35, 849–857. [Google Scholar] [CrossRef]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- White III, H.B. Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 1976, 7, 101–104. [Google Scholar] [CrossRef]
- Sprengel, G.; Follmann, H. Evidence for the reductive pathway of deoxyribonucleotide synthesis in an archaebacterium. FEBS Lett. 1981, 132, 207–209. [Google Scholar] [CrossRef]
- Jenne, A.; Famulok, M. A novel ribozyme with ester transferase activity. Chem. Biol. 1998, 5, 23–34. [Google Scholar] [CrossRef]
- Lohse, P.A.; Szostak, J.W. Ribozyme-catalysed amino-acid transfer reactions. Nature 1996, 381, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Bessho, Y.; Wei, K.; Szostak, J.W.; Suga, H. Ribozyme-catalyzed tRNA aminoacylation. Nat. Struct. Biol. 2000, 7, 28–33. [Google Scholar] [PubMed]
- Lee, N.; Suga, H. A minihelix-loop RNA acts as a trans-aminoacylation catalyst. RNA 2001, 7, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Bessho, Y.; Hodgson, D.R.; Suga, H. A tRNA aminoacylation system for non-natural amino acids based on a programmable ribozyme. Nat. Biotechnol. 2002, 20, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Illangasekare, M.; Yarus, M. A tiny RNA that catalyzes both aminoacyl-RNA and peptidyl-RNA synthesis. RNA 1999, 5, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Chumachenko, N.V.; Novikov, Y.; Yarus, M. Rapid and simple ribozymic aminoacylation using three conserved nucleotides. J. Am. Chem. Soc. 2009, 131, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.M.; Chumachenko, N.V.; Yarus, M. Multiple translational products from a five-nucleotide ribozyme. Proc. Natl. Acad. Sci. USA 2010, 107, 4585–4589. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.M.; Illangasekare, M.; Yarus, M. Catalyzed and spontaneous reactions on ribozyme ribose. J. Am. Chem. Soc. 2011, 133, 6044–6050. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Yarus, M. RNA-catalyzed amino acid activation. Biochemistry 2001, 40, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- El Yacoubi, B.; Bailly, M.; Crecy-Lagard, V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012, 46, 69–95. [Google Scholar] [CrossRef] [PubMed]
- Phizicky, E.M.; Hopper, A.K. tRNA biology charges to the front. Genes Dev. 2010, 24, 1832–1860. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Batlle, E.; de Ribas Pouplana, L. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014, 20, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Towns, W.L.; Begley, T.J. Transfer RNA methytransferases and their corresponding modifications in budding yeast and humans: Activities, predications, and potential roles in human health. DNA Cell Biol. 2012, 31, 434–454. [Google Scholar] [CrossRef] [PubMed]
- Illangasekare, M.; Sanchez, G.; Nickles, T.; Yarus, M. Aminoacyl-RNA synthesis catalyzed by an RNA. Science 1995, 267, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Appel, B.; Balke, D.; Wichert, C.; Müller, S. RNA aminoacylation mediated by sequential action of two ribozymes and a nonactivated amino acid. ChemBioChem 2014, 15, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F. Evolution of protein synthesis from an RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003681. [Google Scholar] [CrossRef] [PubMed]
- De Ribas Pouplana, L.; Turner, R.J.; Steer, B.A.; Schimmel, P. Genetic code origins: tRNAs older than their synthetases? Proc. Natl. Acad. Sci. USA 1998, 95, 11295–11300. [Google Scholar] [CrossRef]
- Di Giulio, M. A comparison among the models proposed to explain the origin of the tRNA molecule: A synthesis. J. Mol. Evol. 2009, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Morgens, D.W. The protein invasion: A broad review on the origin of the translational system. J. Mol. Evol. 2013, 77, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.E. On the origin of the ribosome: Co-evolution of sub-domains of tRNA and rRNA. In The RNA World; Gesteland, R.F., Atkins, J.E., Eds.; Cold Spring Harbour Laboratory Press: New York, NY, USA, 1993; pp. 137–156. [Google Scholar]
- Maizels, N.; Weiner, A.M. The genomic tag hypothesis: Modern viruses as molecular fossils of ancient strategies for genomic replication. In The RNA World; Gesteland, R.F., Atkins, J.E., Eds.; Cold Spring Harbour Laboratory Press: NewYork, NY, USA, 1993; pp. 577–602. [Google Scholar]
- Maizels, N.; Weiner, A.M. Phylogeny from function: Evience from the molecular fossilrecord that tRNA originated in replication, not translation. Proc. Natl. Acad. Sci. USA 1994, 91, 6729–6734. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, P.; Giege, R.; Moras, D.; Yokoyama, S. An operational RNA code for amino acids and possible relationship to genetic code. Proc. Natl. Acad. Sci. USA 1993, 90, 8763–8768. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, P.; Henderson, B. Possible role of aminoacyl-RNA complexes in non-coded peptide synthesis and origin of coded synthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 11283–11286. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. The origin of the tRNA molecule: Independent data favor a specific model of its evolution. Biochimie 2012, 94, 1464–1466. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Peng, D.; Yin, X.; Zhou, X.; Cheng, H.; Zhou, R. Genome-wide analysis reveals origin of transfer RNA genes from tRNA halves. Mol. Biol. Evol. 2013, 30, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.P.; Martinis, S.A.; Schimmel, P. RNA tetraloops as minimalist substrates for aminoacylation. Biochemistry 1992, 31, 4931–4936. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.M.; Maizels, N. tRNA-like structures tag the 3′-ends of genomic RNA molecules for replication: Implications for the origin of protein synthesis. Proc. Natl. Acad. Sci. USA 1987, 84, 7383–7387. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.M.; Maizels, N. The genomic tag hypothesis: Modern viruses as molecular fossils of ancient strategies for genomic replication, and clues regarding the origin of protein synthesis. Biol. Bull. 1999, 196, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kourouklis, D.; Suga, H. An in vitro evolved precursor tRNA with aminoacylation activity. EMBO J. 2001, 20, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Murakami, H.; Suga, H.; Ferré-D′Amaré, A. Structural basis of specific tRNA aminoacylation by a small in vitro selected ribozyme. Nature 2008, 454, 358–361. [Google Scholar] [CrossRef] [PubMed]
- The RNA Modification Database: Modifications. Available online: http://www.webcitation.org/6caSH8n5P (accessed on 16 December 2015).
- Wilson, C.; Szostak, J.W. In vitro evolution of a self-alkylating ribozyme. Nature 1995, 374, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Plant, J.J.; Rangel, A.E.; Meek, K.N.; Anamisis, A.J.; Hollien, J.; Heemstra, J.M. Fluorescent RNA labeling using self-alkylating ribozymes. ACS Chem. Biol. 2014, 9, 1680–1684. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.E.; Müller, U.F. A ribozyme that triphosphorylates RNA 5′-hydroxyl groups. Nucleic Acids Res. 2014, 42, 4767–4778. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.K.; Unrau, P.J.; Lawrence, M.S.; Glasner, M.E.; Bartel, D.P. RNA-catalyzed RNA polymerization: Accurate and general RNA-templated primer extension. Science 2001, 292, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Ekland, E.H.; Bartel, D.P. RNA-catalysed RNA polymerization using nucleoside triphosphates. Nature 1996, 382, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P.; Szostak, J.W. Isolation of new ribozymes from a large pool of random sequences. Science 1993, 261, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Ellington, A.D. In vitro selection of an allosteric ribozyme that transduces analytes to amplicons. Nat. Biotechnol. 1999, 17, 62–66. [Google Scholar] [PubMed]
- Rogers, J.; Joyce, G.F. The effect of cytidine on the structure and function of an RNA ligase ribozyme. RNA 2001, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- McGinness, K.E.; Joyce, G.F. RNA-catalyzed RNA ligation on an external RNA template. Chem. Biol. 2002, 9, 297–307. [Google Scholar] [CrossRef]
- Ikawa, Y.; Tsuda, K.; Matsumura, S.; Inoue, T. De novo synthesis and development of an RNA enzyme. Proc. Natl. Acad. Sci. USA 2004, 101, 13750–13755. [Google Scholar] [CrossRef] [PubMed]
- Baskerville, S.; Bartel, D.P. A ribozyme that ligates RNA to protein. Proc. Natl. Acad. Sci. USA 2002, 99, 9154–9159. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.S.; Watkins, R.A.; Unrau, P.J. Two independently selected capping ribozymes share similar substrate requirements. RNA 2006, 12, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Yarus, M. 5′-RNA self-capping from guanosine diphosphate. Biochemistry 1997, 36, 6557–6563. [Google Scholar] [CrossRef] [PubMed]
- Unrau, P.J.; Bartel, D.P. RNA-catalysed nucleotide synthesis. Nature 1998, 395, 260–263. [Google Scholar] [PubMed]
- Lau, M.W.; Cadieux, K.E.; Unrau, P.J. Isolation of fast purine nucleotide synthase ribozymes. J. Am. Chem. Soc. 2004, 126, 15686–15693. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.W.; Unrau, P.J. A promiscuous ribozyme promotes nucleotide synthesis in addition to ribose chemistry. Chem. Biol. 2009, 16, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Fusz, S.; Eisenführ, A.; Srivatsan, S.G.; Heckel, A.; Famulok, M. A ribozyme for the aldol reaction. Chem. Biol. 2005, 12, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, T.W.; Janssen, R.C.; Eaton, B.E. Selection of RNA amide synthases. Chem. Biol. 1997, 4, 675–683. [Google Scholar] [CrossRef]
- Seelig, B.; Jäschke, A. A small catalytic RNA motif with Diels-Alderase activity. Chem. Biol. 1999, 6, 167–176. [Google Scholar] [CrossRef]
- Prudent, J.R.; Uno, T.; Schultz, P.G. Expanding the scope of RNA catalysis. Science 1994, 264, 1924–1927. [Google Scholar] [CrossRef] [PubMed]
- Sengle, G.; Eisenführ, A.; Arora, P.S.; Nowick, J.S.; Famulok, M. Novel RNA catalysts for the Michael reaction. Chem. Biol. 2001, 8, 459–473. [Google Scholar] [CrossRef]
- Mutschler, H.; Holliger, P. Non-canonical 3′-5′ extension of RNA with prebiotically plausible ribonucleoside 2′,3′-cyclic phosphates. J. Am. Chem. Soc. 2014, 136, 5193–5196. [Google Scholar] [CrossRef] [PubMed]
- Dotson, P.P., II; Frommeyer, K.N.; Testa, S.M. Ribozyme mediated trans insertion-splicing of modified oligonucleotides into RNA. Arch. Biochem. Biophys. 2008, 478, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Welz, R.; Bossmann, K.; Klug, C.; Schmidt, C.; Fritz, H.J.; Müller, S. Site-directed alteration of RNA sequence mediated by an engineered twin ribozyme. Angew. Chem. Int. Ed. 2003, 42, 2424–2427. [Google Scholar] [CrossRef] [PubMed]
- Drude, I.; Vauléon, S.; Müller, S. Twin ribozyme mediated removal of nucleotides from an internal RNA site. Biochem. Biophys. Res. Commun. 2007, 363, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vauléon, S.; Ivanov, S.A.; Gwiazda, S.; Müller, S. Site-specific fluorescent and affinity labelling of RNA by using a small engineered twin ribozyme. ChemBioChem 2005, 6, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Balke, D.; Zieten, I.; Strahl, A.; Müller, O.; Müller, S. Design and characterization of a twin ribozyme for potential repair of a deletion mutation within the oncogenic CTNNB1-ΔS45 mRNA. ChemMedChem 2014, 9, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.P.; Phizicky, E.M. Two-subunit enzymes involved in eukaryotic post-transcriptional tRNA modification. RNA Biol. 2014, 11, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Hori, H. Methylated nucleosides in tRNA and tRNA methyltransferases. Front. Genet. 2014, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Motorin, Y.; Lyko, F.; Helm, M. 5-methylcytosine in RNA: Detection, enzymatic formation and biological functions. Nucleic Acids Res. 2010, 38, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Auxilien, S.; Guerineau, V.; Szweykowska-Kulinska, Z.; Golinelli-Pimpaneau, B. The human tRNA m5C methyltransferase Misu is multisite-specific. RNA Biol. 2012, 9, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, G.; Ney, M.; Gaspar, I.; Aigueperse, C.; Schaefer, M.; Kellner, S.; Helm, M.; Motorin, Y. Eukaryotic rRNA Modification by Yeast 5-Methylcytosine-Methyltransferases and Human Proliferation-Associated Antigen p120. PLoS ONE 2015, 10, e0133321. [Google Scholar] [CrossRef] [PubMed]
- Edelheit, S.; Schwartz, S.; Mumbach, M.R.; Wurtzel, O.; Sorek, R. Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genet. 2013, 9, e1003602. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Aleksic, J.; Blanco, S.; Dietmann, S.; Frye, M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 2013, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; Patel, H.R.; Nousch, M.; Sibbritt, T.; Humphreys, D.T.; Parker, B.J.; Suter, C.M.; Preiss, T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012, 40, 5023–5033. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Redman, K.L. Trm4 and Nsun2 RNA:m5C methyltransferases form metabolite-dependent, covalent adducts with previously methylated RNA. Biochemistry 2014, 53, 7132–7144. [Google Scholar] [CrossRef] [PubMed]
- Raina, M.; Ibba, M. tRNAs as regulators of biological processes. Front. Genet. 2014, 5, 171. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Ropers, H.H. Genetics of recessive cognitive disorders. Trends Genet. 2014, 30, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Vallianatos, C.N.; Iwase, S. Disrupted intricacy of histone H3K4 methylation in neurodevelopmental disorders. Epigenomics 2015, 7, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, T.; Singh, A.K.; Chen, T. Genetic alterations of DNA methylation machinery in human diseases. Epigenomics 2015, 7, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Francke, U. Mechanisms of disease: Neurogenetics of MeCP2 deficiency. Nat. Clin. Pract. Neurol. 2006, 2, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Iwase, S.; Lan, F.; Bayliss, P.; de la Torre-Ubieta, L.; Huarte, M.; Qi, H.H.; Whetstine, J.R.; Bonni, A.; Roberts, T.M.; Shi, Y. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell 2007, 128, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.R.; Amende, M.; Gurok, U.; Moser, B.; Gimmel, V.; Tzschach, A.; Janecke, A.R.; Tariverdian, G.; Chelly, J.; Fryns, J.P.; et al. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am. J. Hum. Genet. 2005, 76, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Brzezicha, B.; Schmidt, M.; Makalowska, I.; Jarmolowski, A.; Pienkowska, J.; Szweykowska-Kulinska, Z. Identification of human tRNA:m5C methyltransferase catalysing intron-dependent m5C formation in the first position of the anticodon of the pre-tRNALeu (CAA). Nucleic Acids Res. 2006, 34, 6034–6043. [Google Scholar] [CrossRef] [PubMed]
- Goll, M.G.; Kirpekar, F.; Maggert, K.A.; Yoder, J.A.; Hsieh, C.L.; Zhang, X.; Golic, K.G.; Jacobsen, S.E.; Bestor, T.H. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 2006, 311, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Xie, S.; Li, E. Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res. 1998, 26, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Kurowski, A.; Nichols, J.; Watt, F.M.; Benitah, S.A.; Frye, M. The RNA-methyltransferase Misu (NSun2) poises epidermal stem cells to differentiate. PLoS Genet. 2011, 7, e1002403. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Chidester, S.; Zavala, C.V.; Manos, E.J.; James, S.R.; Karpf, A.R.; Jones, D.A.; Cairns, B.R. Dnmt2 functions in the cytoplasm to promote liver, brain, and retina development in zebrafish. Genes Dev. 2007, 21, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Tuorto, F.; Liebers, R.; Musch, T.; Schaefer, M.; Hofmann, S.; Kellner, S.; Frye, M.; Helm, M.; Stoecklin, G.; Lyko, F. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat. Struct. Mol. Biol. 2012, 19, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.; Watt, F.M. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr. Biol. 2006, 16, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Benavente, S.B.; Nascimento, E.; Dragoni, I.; Kurowski, A.; Gillich, A.; Humphreys, P.; Frye, M. The nucleolar RNA methyltransferase Misu (NSun2) is required for mitotic spindle stability. J. Cell Biol. 2009, 186, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Tuorto, F.; Menon, S.; Blanco, S.; Cox, C.; Flores, J.V.; Watt, S.; Kudo, N.R.; Lyko, F.; Frye, M. The mouse cytosine-5 RNA methyltransferase NSun2 is a component of the chromatoid body and required for testis differentiation. Mol. Cell Biol. 2013, 33, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Sakita-Suto, S.; Kanda, A.; Suzuki, F.; Sato, S.; Takata, T.; Tatsuka, M. Aurora-B regulates RNA methyltransferase NSUN2. Mol. Biol. Cell 2007, 18, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Moheb, L.; Mertel, S.; Gonsior, M.; Nouri-Vahid, L.; Kahrizi, K.; Cirak, S.; Wieczorek, D.; Motazacker, M.M.; Esmaeeli-Nieh, S.; Cremer, K.; et al. Mutations in NSUN2 cause autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2012, 90, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Rafiq, M.A.; Noor, A.; Hussain, S.; Flores, J.V.; Rupp, V.; Vincent, A.K.; Malli, R.; Ali, G.; Khan, F.S.; et al. Mutation in NSUN2, which encodes an RNA methyltransferase, causes autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2012, 90, 856–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, F.J.; Lee, J.H.; Lee, J.E.; Blanco, S.; Nickerson, E.; Gabriel, S.; Frye, M.; Al-Gazali, L.; Gleeson, J.G. Whole exome sequencing identifies a splicing mutation in NSUN2 as a cause of a Dubowitz-like syndrome. J. Med. Genet. 2012, 49, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Fahiminiya, S.; Almuriekhi, M.; Nawaz, Z.; Staffa, A.; Lepage, P.; Ali, R.; Hashim, L.; Schwartzentruber, J.; Abu Khadija, K.; Zaineddin, S.; et al. Whole exome sequencing unravels disease-causing genes in consanguineous families in Qatar. Clin. Genet. 2014, 86, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Blanco, S.; Dietmann, S.; Flores, J.V.; Hussain, S.; Kutter, C.; Humphreys, P.; Lukk, M.; Lombard, P.; Treps, L.; Popis, M.; et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014, 33, 2020–2039. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsen, K.; Ketema, M.; Truong, H.; Sonnenberg, A. KASH-domain proteins in nuclear migration, anchorage and other processes. J. Cell Sci. 2006, 119, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.; Warda, A.S.; Kretschmer, J.; Gunnigmann, M.A.; Hobartner, C.; Bohnsack, M.T. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. RNA 2015, 21, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Delgado-Olguin, P. Expression of NOL1/NOP2/sun domain (Nsun) RNA methyltransferase family genes in early mouse embryogenesis. Gene Expr. Patterns 2013, 13, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Straby, K.B. The human tRNA(m22G26)dimethyltransferase: Functional expression and characterization of a cloned hTRM1 gene. Nucleic Acids Res. 2000, 28, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Najmabadi, H.; Hu, H.; Garshasbi, M.; Zemojtel, T.; Abedini, S.S.; Chen, W.; Hosseini, M.; Behjati, F.; Haas, S.; Jamali, P.; et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 2011, 478, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Davarniya, B.; Hu, H.; Kahrizi, K.; Musante, L.; Fattahi, Z.; Hosseini, M.; Maqsoud, F.; Farajollahi, R.; Wienker, T.F.; Ropers, H.H.; et al. The Role of a Novel TRMT1 Gene Mutation and Rare GRM1 Gene Defect in Intellectual Disability in Two Azeri Families. PLoS ONE 2015, 10, e0129631. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.R.; Lenzner, S.; Moser, B.; Freude, K.; Tzschach, A.; Wei, C.; Fryns, J.P.; Chelly, J.; Turner, G.; Moraine, C.; et al. X-linked mental retardation: A comprehensive molecular screen of 47 candidate genes from a 7.4 Mb interval in Xp11. Eur. J. Med. Genet. 2007, 15, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Freude, K.; Hoffmann, K.; Jensen, L.R.; Delatycki, M.B.; des Portes, V.; Moser, B.; Hamel, B.; van Bokhoven, H.; Moraine, C.; Fryns, J.P.; et al. Mutations in the FTSJ1 gene coding for a novel S-adenosylmethionine-binding protein cause nonsyndromic X-linked mental retardation. Am. J. Hum. Genet. 2004, 75, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ramser, J.; Winnepenninckx, B.; Lenski, C.; Errijgers, V.; Platzer, M.; Schwartz, C.E.; Meindl, A.; Kooy, R.F. A splice site mutation in the methyltransferase gene FTSJ1 in Xp11.23 is associated with non-syndromic mental retardation in a large Belgian family (MRX9). J. Med. Genet. 2004, 41, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Gregoire, M.J.; Brochet, K.; Raffo, E.; Leheup, B.; Jonveaux, P. Pure de-novo 5 Mb duplication at Xp11.22-p11.23 in a male: Phenotypic and molecular characterization. J. Hum. Genet. 2006, 51, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Bugl, H.; Fauman, E.B.; Staker, B.L.; Zheng, F.; Kushner, S.R.; Saper, M.A.; Bardwell, J.C.; Jakob, U. RNA methylation under heat shock control. Mol. Cell 2000, 6, 349–360. [Google Scholar] [CrossRef]
- Hager, J.; Staker, B.L.; Bugl, H.; Jakob, U. Active site in RrmJ, a heat shock-induced methyltransferase. J. Biol. Chem. 2002, 277, 41978–41986. [Google Scholar] [CrossRef] [PubMed]
- Fromont-Racine, M.; Senger, B.; Saveanu, C.; Fasiolo, F. Ribosome assembly in eukaryotes. Gene 2003, 313, 17–42. [Google Scholar] [CrossRef]
- Pintard, L.; Bujnicki, J.M.; Lapeyre, B.; Bonnerot, C. MRM2 encodes a novel yeast mitochondrial 21S rRNA methyltransferase. EMBO J. 2002, 21, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Pintard, L.; Kressler, D.; Lapeyre, B. Spb1p is a yeast nucleolar protein associated with Nop1p and Nop58p that is able to bind S-adenosyl-l-methionine in vitro. Mol. Cell Biol. 2000, 20, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Pintard, L.; Lecointe, F.; Bujnicki, J.M.; Bonnerot, C.; Grosjean, H.; Lapeyre, B. Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. EMBO J. 2002, 21, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.P.; Shaw, M.; Weiner, C.L.; Hobson, L.; Stark, Z.; Rose, K.; Kalscheuer, V.M.; Gecz, J.; Phizicky, E.M. Defects in tRNA anticodon loop 2′-O-methylation are implicated in nonsyndromic X-linked intellectual disability due to mutations in FTSJ1. Hum. Mutat. 2015. [Google Scholar] [CrossRef] [PubMed]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balke, D.; Kuss, A.; Müller, S. Landmarks in the Evolution of (t)-RNAs from the Origin of Life up to Their Present Role in Human Cognition. Life 2016, 6, 1. https://doi.org/10.3390/life6010001
Balke D, Kuss A, Müller S. Landmarks in the Evolution of (t)-RNAs from the Origin of Life up to Their Present Role in Human Cognition. Life. 2016; 6(1):1. https://doi.org/10.3390/life6010001
Chicago/Turabian StyleBalke, Darko, Andreas Kuss, and Sabine Müller. 2016. "Landmarks in the Evolution of (t)-RNAs from the Origin of Life up to Their Present Role in Human Cognition" Life 6, no. 1: 1. https://doi.org/10.3390/life6010001
APA StyleBalke, D., Kuss, A., & Müller, S. (2016). Landmarks in the Evolution of (t)-RNAs from the Origin of Life up to Their Present Role in Human Cognition. Life, 6(1), 1. https://doi.org/10.3390/life6010001





