Biosynthesis and Function of Extracellular Glycans in Cyanobacteria
Abstract
:1. Introduction
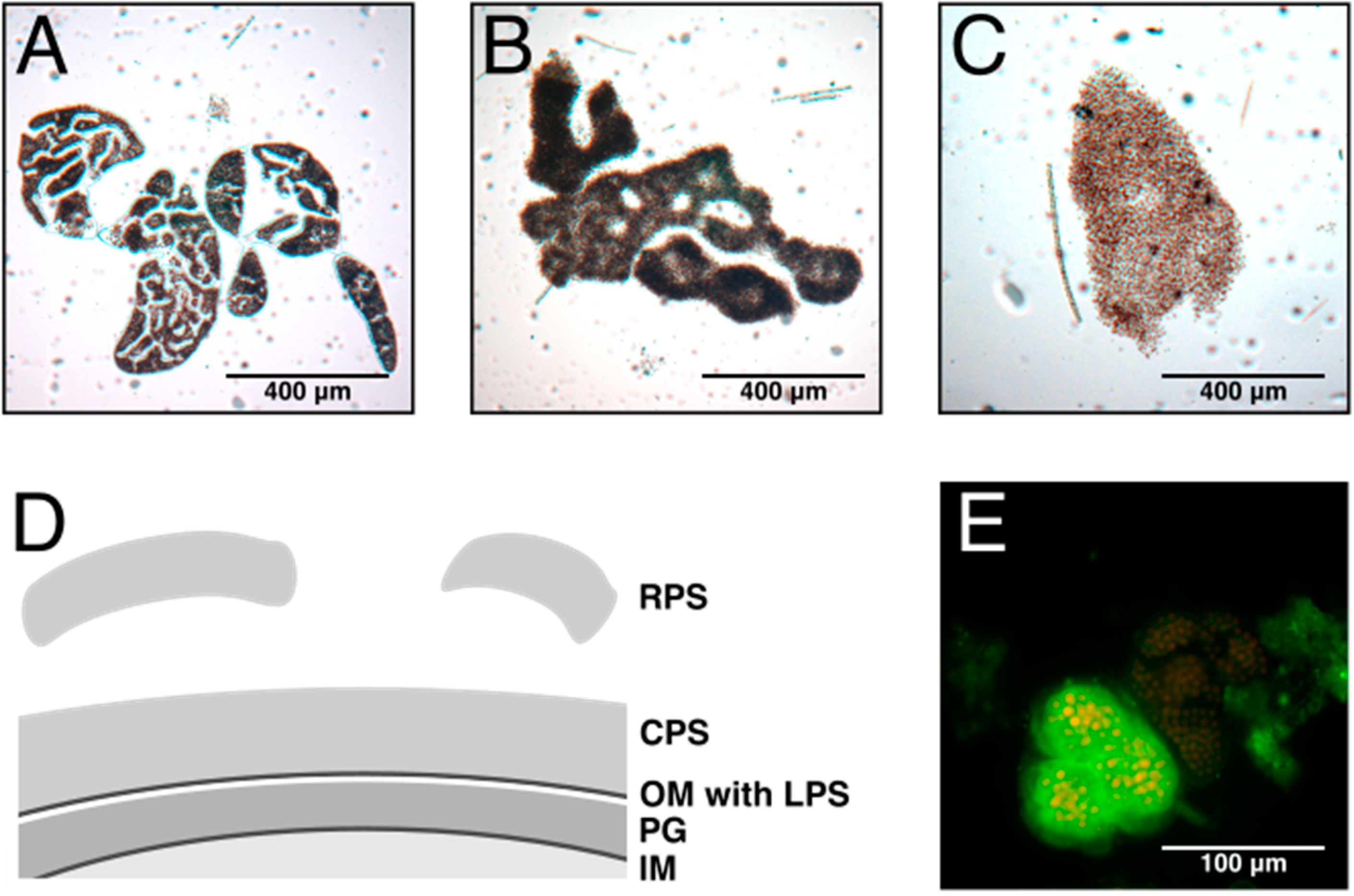
2. Composition and Structure of Cyanobacterial EPS
3. Unique Features of Cyanobacterial Lipopolysaccharides
4. Biosynthesis of Extracellular Glycans


5. Overview of Lectins in Cyanobacteria
| Lectin | Organism | Reference |
|---|---|---|
| microvirin (MVN) | Microcystis aeruginosa PCC 7806 | [44,46] |
| cyanovirin-N (CV-N) | Nostoc ellipsosporum | [47,48] |
| scytovirin (SVN) | Scytonema varium | [49] |
| Oscillatoria agardhii agglutinin (OAA) | Oscillatoria agardhii | [50,51] |
| Microcystis viridis lectin (MVL) | Microcystis viridis | [45] |
| MAL | Microcystis aeruginosa M228 | [52] |
6. The Role of Exopolysaccharides in Cyanobacteria
6.1. Colony Formation
6.2. Symbiosis
6.3 LPS Are Receptors for Cyanophages
6.4 EPS and Environmental Stress
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nut. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Schussler, A.; Meyer, T.; Gehrig, H.; Kluge, M. Variations of lectin binding sites in extracellular glycoconjugates during the life cycle of Nostoc punctiforme, a potentially endosymbiotic cyanobacterium. Eur. J. Phycol. 1997, 32, 233–239. [Google Scholar] [CrossRef]
- De Philippis, R.; Sili, C.; Paperi, R.; Vincenzini, M. Exopolysaccharide-producing cyanobacteria and their possible exploitation: A review. J. Appl. Phycol. 2001, 13, 293–299. [Google Scholar]
- De Philippis, R.; Margheri, M.C.; Materassi, R.; Vincenzini, M. Potential of unicellular cyanobacteria from saline environments as exopolysaccharide producers. Appl Environ. Microb. 1998, 64, 1130–1132. [Google Scholar]
- De Philippis, R.; Sili, C.; Faraloni, C.; Vincenzini, M. Occurrence and significance of exopolysaccharide-producing cyanobacteria in the benthic mucilaginous aggregates of the Tyrrhenian Sea (Tuscan Archipelago). Ann. Microbiol. 2002, 52, 1–11. [Google Scholar]
- Di Pippo, F.; Ellwood, N.T.W.; Gismondi, A.; Bruno, L.; Rossi, F.; Magni, P.; De Philippis, R. Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J. Appl. Phycol. 2013, 25, 1697–1708. [Google Scholar]
- Moreno, J.; Vargas, M.A.; Madiedo, J.M.; Munoz, J.; Rivas, J.; Guerrero, M.G. Chemical and rheological properties of an extracellular polysaccharide produced by the cyanobacterium Anabaena sp ATCC 33047. Biotechnol. Bioeng. 2000, 67, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Micheletti, E.; Bruno, L.; Adhikary, S.P.; Albertano, P.; De Philippis, R. Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on stone monuments. Biofouling 2012, 28, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Weckesser, J.; Drews, G.; Mayer, H. Lipopolysaccharides of photosynthetic prokaryotes. Annu Rev. Microbiol. 1979, 33, 215–239. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechol. 2011, 92, 697–708. [Google Scholar] [CrossRef]
- Nobles, D.R.; Romanovicz, D.K.; Brown, R.M., Jr. Cellulose in cyanobacteria. Origin of vascular plant cellulose synthase? Plant. Physiol. 2001, 127, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Werz, D.B.; Ranzinger, R.; Herget, S.; Adibekian, A.; von der Lieth, C.W.; Seeberger, P.H. Exploring the structural diversity of mammalian carbohydrates (“glycospace”) by statistical databank analysis. ACS Chem. Biol. 2007, 2, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Garcia, V.; Rios-Leal, E.; Calderon-Dominguez, G.; Canizares-Villanueva, R.O.; Olvera-Ramirez, R. Detection, isolation, and characterization of exopolysaccharide produced by a strain of Phormidium 94a isolated from an arid zone of mexico. Biotechnol. Bioeng. 2004, 85, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Snyder, D.S.; McIntosh, T.J. The lipopolysaccharide barrier: Correlation of antibiotic susceptibility with antibiotic permeability and fluorescent probe binding kinetics. Biochemistry (USA) 2000, 39, 11777–11787. [Google Scholar] [CrossRef]
- Sutherland, I.W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Keleti, G.; Sykora, J.L.; Lippy, E.C.; Shapiro, M.A. Composition and biological properties of lipopolysaccharides isolated from Schizothrix calcicola (Ag.) Gomont (Cyanobacteria). Appl Environ. Microb. 1979, 38, 471–477. [Google Scholar]
- Snyder, D.S.; Brahamsha, B.; Azadi, P.; Palenik, B. Structure of compositionally simple lipopolysaccharide from marine Synechococcus. J. Bacteriol. 2009, 191, 5499–5509. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Kaniuk, N.; Frirdich, E. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J. Endotoxin Res. 2003, 9, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Lam, J.S. Synthesis of bacterial polysaccharides via the Wzx/Wzy-dependent pathway. Can. J. Microbiol. 2014, 60, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.K.; Whitfield, C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr. Res. 2012, 356, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Whitney, J.C.; Howell, P.L. Synthase-dependent exopolysaccharide secretion in Gram-negative bacteria. Trends Microbiol. 2013, 21, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, L.; Kos, V.; Whitfield, C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol. Mol. Biol. Rev. 2010, 74, 341–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.N.; Wang, Q.; Reeves, P.R.; Wang, L. Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 2008, 32, 627–653. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.M.; Whitfield, C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr. Res. 2013, 378, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Keenleyside, W.J.; Whitfield, C. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar borreze. J. Biol. Chem. 1996, 271, 28581–28592. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Rice, J.D.; Goller, C.; Pannuri, A.; Taylor, J.; Meisner, J.; Beveridge, T.J.; Preston, J.F.; Romeo, T. Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-β-1,6-N-acetyl-d-glucosamine. J. Bacteriol. 2008, 190, 3670–3680. [Google Scholar] [CrossRef] [PubMed]
- Romling, U. Molecular biology of cellulose production in bacteria. Res. Microbiol. 2002, 153, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.L.; Allen, R.; Luo, Y.Q.; Curtiss, R. Export of extracellular polysaccharides modulates adherence of the cyanobacterium Synechocystis. PloS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Jittawuttipoka, T.; Planchon, M.; Spalla, O.; Benzerara, K.; Guyot, F.; Cassier-Chauvat, C.; Chauvat, F. Multidisciplinary evidences that Synechocystis PCC6803 exopolysaccharides operate in cell sedimentation and protection against salt and metal stresses. PloS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Simkovsky, R.; Daniels, E.F.; Tang, K.; Huynh, S.C.; Golden, S.S.; Brahamsha, B. Impairment of O-antigen production confers resistance to grazing in a model amoeba-cyanobacterium predator-prey system. Proc. Natl. Acad. Sci. USA 2012, 109, 16678–16683. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Saotome, T.; Ochiai, Y.; Katayama, M.; Narikawa, R.; Ikeuchi, M. Cellulose accumulation and a cellulose synthase gene are responsible for cell aggregation in the cyanobacterium Thermosynechococcus vulcanus RKN. Plant Cell Physiol. 2011, 52, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Lam, J.S. Wzx flippase-mediated membrane translocation of sugar polymer precursors in bacteria. Environ. Microbiol. 2013, 15, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.T.; Taylor, V.L.; Qi, M.; Lam, J.S. Membrane topology mapping of the O-antigen flippase (Wzx), polymerase (Wzy), and ligase (Waal) from Pseudomonas aeruginosa PAO1 reveals novel domain architectures. mBio 2010, 1. [Google Scholar] [CrossRef]
- Fiore, M.F.; Alvarenga, D.O.; Varani, A.M.; Hoff-Risseti, C.; Crespim, E.; Ramos, R.T.; Silva, A.; Schaker, P.D.; Heck, K.; Rigonato, J.; et al. Draft genome sequence of the brazilian toxic bloom-forming cyanobacterium Microcystis aeruginosa strain SPC777. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Gugger, H.; Jurich, M.; Swalen, J.D.; Sievers, A.J. Reply to “comment on ‘observation of an index-of-refraction-induced change in the drude parameters of ag films’”. Phys. Rev. B 1986, 34, 1322–1324. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakajima, N.; Okamoto, S.; Suzuki, I.; Tanabe, Y.; Tamaoki, M.; Nakamura, Y.; Kasai, F.; Watanabe, A.; Kawashima, K.; et al. Complete genomic structure of the bloom-forming toxic cyanobacterium Microcystis aeruginosa NIES-843. DNA Res. 2007, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, W.; Ren, M.; Song, L.; Li, T.; Zhao, J. Whole-genome sequence of Microcystis aeruginosa TAIHU98, a nontoxic bloom-forming strain isolated from Taihu Lake, China. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Sharon, N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. How proteins bind carbohydrates: Lessons from legume lectins. J. Agric. Food Chem. 2002, 50, 6586–6591. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Schols, D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs 2012, 10, 1476–1497. [Google Scholar] [CrossRef] [PubMed]
- Kehr, J.C.; Zilliges, Y.; Springer, A.; Disney, M.D.; Ratner, D.D.; Bouchier, C.; Seeberger, P.H.; de Marsac, N.T.; Dittmann, E. A mannan binding lectin is involved in cell-cell attachment in a toxic strain of Microcystis aeruginosa. Mol. Microbiol. 2006, 59, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ogawa, T.; Muramoto, K.; Jimbo, M.; Kamiya, H. Effects of culture conditions on the expression level of lectin in Microcystis aeruginosa (freshwater cyanobacterium). Fish. Sci. 2000, 66, 665–669. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ogawa, T.; Muramoto, K.; Kamio, Y.; Jimbo, M.; Kamiya, H. Isolation and characterization of a mannan-binding lectin from the freshwater cyanobacterium (blue-green algae) Microcystis viridis. Biochem. Biophys. Res. Commun. 1999, 265, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Ferir, G.; Vermeire, K.; Kehr, J.C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a novel α(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-n but a much higher safety profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [PubMed]
- Bewley, C.A.; Gustafson, K.R.; Boyd, M.R.; Covell, D.G.; Bax, A.; Clore, G.M.; Gronenborn, A.M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 1998, 5, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.Y.; O’Keefe, B.R.; Botos, I.; Wlodawer, A.; McMahon, J.B. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr. Purif. 2006, 46, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Murakami, M.; Miyazawa, K.; Hori, K. Purification and characterization of a novel lectin from a freshwater cyanobacterium, Oscillatoria agardhii. Comp. Biochem. Phys. B 2000, 125, 169–177. [Google Scholar] [CrossRef]
- Sato, Y.; Okuyama, S.; Hori, K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J. Biol. Chem. 2007, 282, 11021–11029. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, M.; Yamaguchi, M.; Muramoto, K.; Kamiya, H. Cloning of the Microcystis aeruginosa M228 lectin (MAL) gene. Biochem. Biophys. Res. Commun. 2000, 273, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.J.; Sigee, D.C.; White, K.N. Characterization of surface sugars on algal cells with fluorescein isothiocyanate-conjugated lectins. Protoplasma 2005, 225, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zippel, B.; Neu, T.R. Characterization of glycoconjugates of extracellular polymeric substances in Tufa-associated biofilms by using fluorescence lectin-binding analysis. Appl. Environ. Microb. 2011, 77, 505–516. [Google Scholar] [CrossRef]
- Baulina, O.I.; Titel, K.; Gorelova, O.A.; Malai, O.V.; Ehwald, R. Permeability of cyanobacterial mucous surface structures for macromolecules. Microbiology 2008, 77, 198–205. [Google Scholar] [CrossRef]
- Gan, N.Q.; Xiao, Y.; Zhu, L.; Wu, Z.X.; Liu, J.; Hu, C.L.; Song, L.R. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol. 2012, 14, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, S.; Salencon, M.J.; Thebault, J.M. Functional analysis of Microcystis vertical migration: A dynamic model as a prospecting tool: I—Processes analysis. Ecol. Model. 2005, 188, 386–403. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Y.; Xie, P.; Tao, M.; Yang, X. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw. Biol. 2011, 56, 1065–1080. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.X.; Shi, X.L.; Zhang, M.; Xing, P.; Cao, H.S. Changes in the morphology and polysaccharide content of Microcystis aeruginosa (cyanobacteria) during flagellate grazing. J. Phycol. 2008, 44, 716–720. [Google Scholar] [CrossRef]
- Zhang, M.; Kong, F.; Tan, X.; Yang, Z.; Cao, H.; Xing, P. Biochemical, morphological, and genetic variations in Microcystis aeruginosa due to colony disaggregation. World J. Microbiol. Biotechnol. 2007, 23, 663–670. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Mikkat, S.; Bouchier, C.; de Marsac, N.T.; Borner, T.; Dittmann, E. An extracellular glycoprotein is implicated in cell-cell contacts in the toxic cyanobacterium Microcystis aeruginosa PCC 7806. J. Bacteriol. 2008, 190, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Zhao, J.; Li, J.H.; Li, S.S.; Zhang, L.H.; Wu, M. Effects of calcium levels on colonial aggregation and buoyancy of Microcystis aeruginosa. Curr. Microbiol. 2011, 62, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Via-Ordorika, L.; Fastner, J.; Kurmayer, R.; Hisbergues, M.; Dittmann, E.; Komarek, J.; Erhard, M.; Chorus, I. Distribution of microcystin-producing and non-microcystin-producing Microcystis sp. in European freshwater bodies: Detection of microcystins and microcystin genes in individual colonies. Syst. Appl. Microbiol. 2004, 27, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, W.; Gao, L.; Lu, L. Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J. Appl. Phycol. 2013, 25, 1023–1030. [Google Scholar] [CrossRef]
- Becker, A.; Fraysse, N.; Sharypova, L. Recent advances in studies on structure and symbiosis-related function of rhizobial K-antigens and lipopolysaccharides. Mol. Plant-Microbe Interact. 2005, 18, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; Oliva, M.D.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, I.; Vanderleyden, J. O-antigen structural variation: Mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol. Rev. 2002, 26, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr. Opin. Plant Biol. 1999, 2, 320–326. [Google Scholar] [CrossRef]
- Meeks, J.C.; Campbell, E.L.; Summers, M.L.; Wong, F.C. Cellular differentiation in the cyanobacterium Nostoc punctiforme. Arch. Microbiol. 2002, 178, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.B.; Gadd, G.M.; Rowell, P.; Stewart, W.D. A phytohaemahglutinin from the azolla-Anabaena symbiosis. Biochem. Biophys. Res. Commun. 1981, 99, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.M.; Sacristan, M.; Legaz, M.E.; Vicente, C. Isolation and characterization of a cyanobacterium-binding protein and its cell wall receptor in the lichen Peltigera canina. Plant Signal. Behav. 2009, 4, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Vivas, M.; Sacristan, M.; Legaz, M.E.; Vicente, C. The cell recognition model in chlorolichens involving a fungal lectin binding to an algal ligand can be extended to cyanolichens. Plant Biol. 2010, 12, 615–621. [Google Scholar] [PubMed]
- Chaturongakul, S.; Ounjai, P. Phage-host interplay: Examples from tailed phages and Gram-negative bacterial pathogens. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.; Mann, N.H. Marine cyanophages and light. Environ. Microbiol. 2006, 8, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.H. Phages of the marine cyanobacterial picophytoplankton. FEMS Microbiol. Rev. 2003, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, T.; Deng, F.; Hu, Z. Freshwater cyanophages. Virol. Sin. 2013, 28, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Samimi, B.; Drews, G. Adsorption of cyanophage AS-1 to unicellular cyanobacteria and isolation of receptor material from Anacystis nidulans. J. Virol. 1978, 25, 164–174. [Google Scholar] [PubMed]
- Xu, X.D.; Khudyakov, I.; Wolk, C.P. Lipopolysaccharide dependence of cyanophage sensitivity and aerobic nitrogen fixation in Anabaena sp. strain PCC 7120. J. Bacteriol. 1997, 179, 2884–2891. [Google Scholar] [PubMed]
- Ozturk, S.; Aslim, B. Modification of exopolysaccharide composition and production by three cyanobacterial isolates under salt stress. Environ. Sci. Pollut. Res. 2010, 17, 595–602. [Google Scholar] [CrossRef]
- Garcia-Meza, J.V.; Barrangue, C.; Admiraal, W. Biofilm formation by algae as a mechanism for surviving on mine tailings. Environ. Toxicol. Chem. 2005, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol 1997, 179, 1940–1945. [Google Scholar] [PubMed]
- Sommaruga, R.; Chen, Y.; Liu, Z. Multiple strategies of bloom-forming Microcystis to minimize damage by solar ultraviolet radiation in surface waters. Microbial. Ecol. 2009, 57, 667–674. [Google Scholar] [CrossRef]
- Böhm, G.A.; Pfleiderer, W.; Böger, P.; Scherer, S. Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J. Biol. Chem. 1995, 270, 8536–8539. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Wingard, C.E.; Castenholz, R.W. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 1993, 59, 170–176. [Google Scholar] [PubMed]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Wang, G.H.; Hong, S.; Liu, A.; Li, C.; Liu, Y.D. UV-B-induced oxidative damage and protective role of exopolysaccharides in desert cyanobacterium Microcoleus vaginatus. J. Integr. Plant. Biol. 2009, 51, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Potts, M. Mechanisms of desiccation tolerance in cyanobacteria. Eur. J. Phycol. 1999, 34, 319–328. [Google Scholar] [CrossRef]
- Hill, D.R.; Keenan, T.W.; Helm, R.F.; Potts, M.; Crowe, L.M.; Crowe, J.H. Extracellular polysaccharide of Nostoc commune (cyanobacteria) inhibits fusion of membrane vesicles during desiccation. J. Appl. Phycol. 1997, 9, 237–248. [Google Scholar] [CrossRef]
- Tamaru, Y.; Takani, Y.; Yoshida, T.; Sakamoto, T. Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl. Environ. Microbiol. 2005, 71, 7327–7333. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Vincenzini, M. Nostoc (cyanophyceae) goes nude: Extracellular polysaccharides serve as a sink for reducing power under unbalanced C/N metabolism. J. Phycol. 2004, 40, 74–81. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kehr, J.-C.; Dittmann, E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life 2015, 5, 164-180. https://doi.org/10.3390/life5010164
Kehr J-C, Dittmann E. Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life. 2015; 5(1):164-180. https://doi.org/10.3390/life5010164
Chicago/Turabian StyleKehr, Jan-Christoph, and Elke Dittmann. 2015. "Biosynthesis and Function of Extracellular Glycans in Cyanobacteria" Life 5, no. 1: 164-180. https://doi.org/10.3390/life5010164
APA StyleKehr, J.-C., & Dittmann, E. (2015). Biosynthesis and Function of Extracellular Glycans in Cyanobacteria. Life, 5(1), 164-180. https://doi.org/10.3390/life5010164





