Acute Exercise-Induced Epinephrine Elevation Promotes Post-Learning Memory Consolidation: A Narrative Review of Mechanisms and Implementation Strategies
Abstract
1. Introduction
2. Neurochemical Actions of Epinephrine: The Molecular Catalyst of Memory Consolidation
2.1. Activation of β-Adrenergic Receptors and Synaptic Plasticity
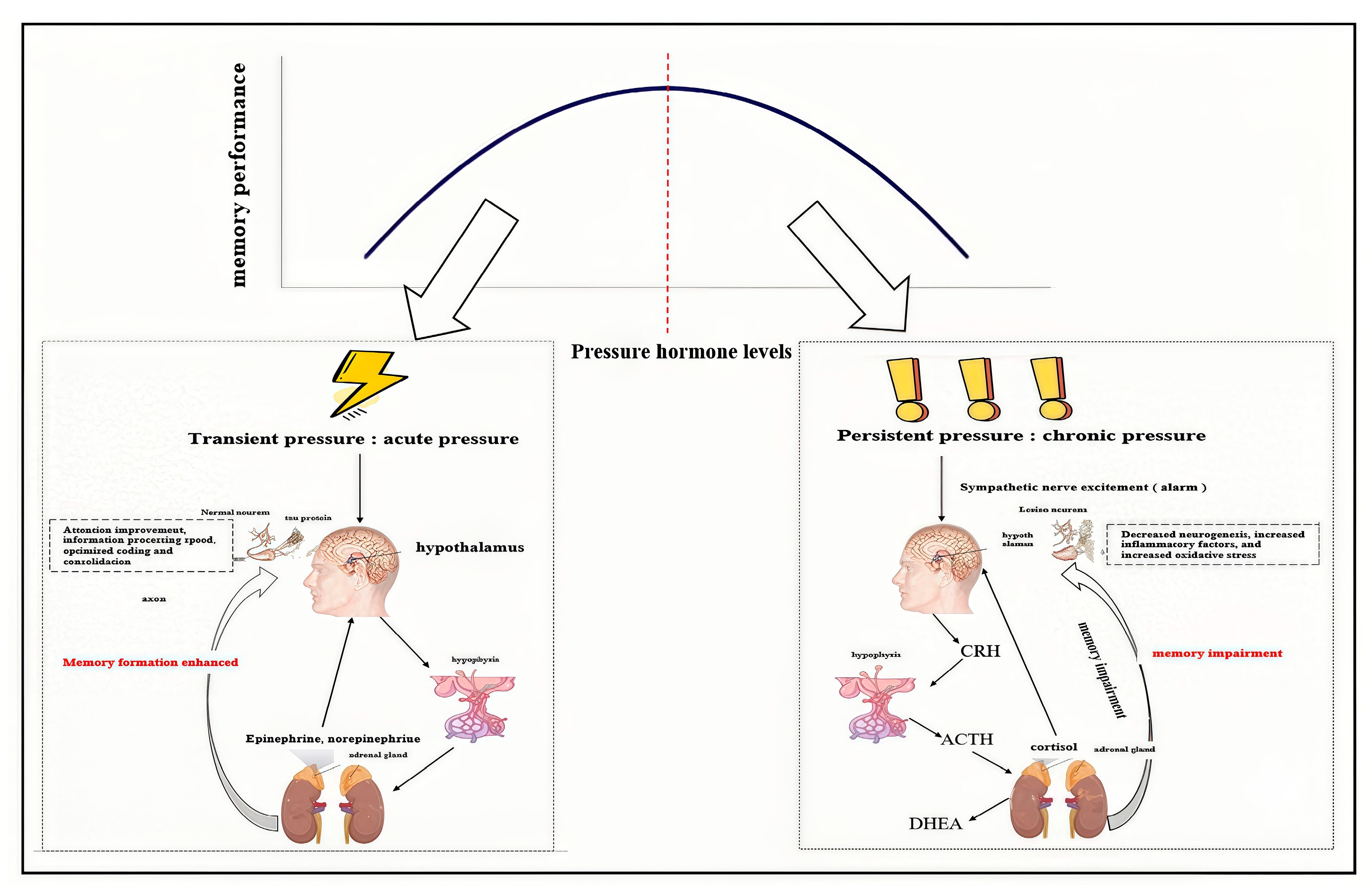
2.2. The Double-Edged Sword Effect of Stress on Memory Formation
3. Timing Determines Efficacy: The “Window Effect” of Post-Learning Epinephrine Surge
3.1. The Critical Time Window for Memory Consolidation
3.2. The “Increment Effect” of the Epinephrine Surge
3.3. Avoiding Interference During the Learning Process
4. The Dual Advantage of Exercise: Efficient Epinephrine Surge and Synergistic Neural Benefits
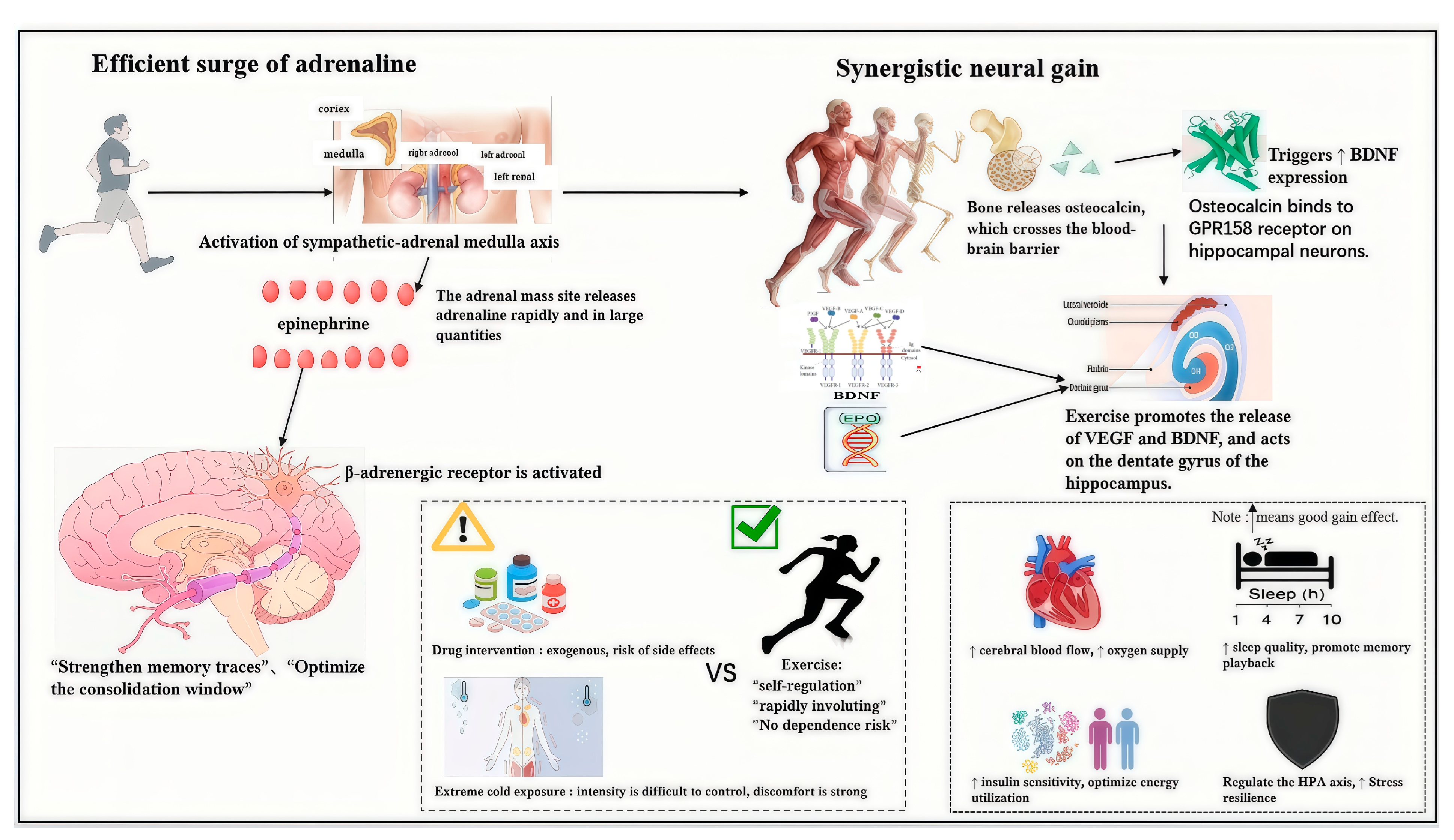
4.1. Rapidity and Safety of Exercise-Induced Epinephrine Release
4.2. Beyond Epinephrine: Multi-Pathway Synergistic Regulation of the Memory System by Exercise
4.3. Optimizing Exercise Intervention Strategies: Type, Dose, and Timing
5. From Theory to Practice: Application Prospects and Potential Challenges
5.1. Application Potential in the Fields of Education and Vocational Training
5.2. Challenges and Future Directions for Personalized Programs
5.3. Long-Term Adherence and Behavior Change
6. Conclusions and Outlook
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Dudai, Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004, 55, 51–86. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L. Emotional arousal regulation of memory consolidation. Curr. Opin. Behav. Sci. 2018, 19, 55–60. [Google Scholar] [CrossRef]
- Tully, K.; Bolshakov, V.Y. Emotional enhancement of memory: How norepinephrine enables synaptic plasticity. Mol. Brain 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Sapolsky, R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995, 5, 205–216. [Google Scholar] [CrossRef]
- Gao, V.; Suzuki, A.; Magistretti, P.J.; Lengacher, S.; Pollonini, G.; Steinman, M.Q.; Alberini, C.M. Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8526–8531. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Voss, M.W.; Nagamatsu, L.S.; Liu-Ambrose, T.; Kramer, A.F. Exercise, brain, and cognition across the life span. J. Appl. Physiol. 2011, 111, 1505–1513. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, W.; Li, C.; Tian, Y.; Wang, L.; Zhong, J.; Yan, X.; Wang, Y.; Wang, L.; Wang, H. The effects of high-intensity interval training on cognitive performance: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 32082. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef]
- McGaugh, J.L. Memory—A century of consolidation. Science 2000, 287, 248–251. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L.; Roozendaal, B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 2002, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Real, E.; Takamiya, K.; Kang, M.-G.; Ledoux, J.; Huganir, R.L.; Malinow, R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 2007, 131, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Manahan-Vaughan, D. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of β-adrenergic receptors. Cereb. Cortex 2015, 25, 1889–1896. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Joëls, M.; Pu, Z.; Wiegert, O.; Oitzl, M.S.; Krugers, H.J. Learning under stress: How does it work? Trends Cogn. Sci. 2006, 10, 152–158. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Stress and plasticity in the limbic system. Neurochem. Res. 2003, 28, 1735–1742. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Jacotte-Simancas, A.; Costa-Miserachs, D.; Torras-Garcia, M.; Coll-Andreu, M.; Portell-Cortés, I. Effect of voluntary physical exercise and post-training epinephrine on acquisition of a spatial task in the barnes maze. Behav. Brain Res. 2013, 247, 178–181. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, J.L. Consolidating memories. Annu. Rev. Psychol. 2015, 66, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.; Alkire, M.T. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol. Learn. Mem. 2003, 79, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 2000, 7, 73–84. [Google Scholar] [CrossRef]
- Cahill, L.; Gorski, L.; Le, K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn. Mem. 2003, 10, 270–274. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- van Dongen, E.V.; Kersten, I.H.; Wagner, I.C.; Morris, R.G.; Fernández, G. Physical exercise performed four hours after learning improves memory retention and increases hippocampal pattern similarity during retrieval. Curr. Biol. 2016, 26, 1722–1727. [Google Scholar] [CrossRef]
- Packard, M.G.; Cahill, L. Affective modulation of multiple memory systems. Curr. Opin. Neurobiol. 2001, 11, 752–756. [Google Scholar] [CrossRef]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Li, J.W.; O’Connor, H.; O’Dwyer, N.; Orr, R. The effect of acute and chronic exercise on cognitive function and academic performance in adolescents: A systematic review. J. Sci. Med. Sport 2017, 20, 841–848. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Krueger, K.; Fromme, A.; Korsukewitz, C.; Floel, A. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Suzuki, W.A. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2016, 2, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Tallis, J.; Miller, A.; Clarke, N.D.; Guimarães-Ferreira, L.; Duncan, M.J. The effect of exercise intensity on cognitive performance during short duration treadmill running. J. Hum. Kinet. 2016, 51, 27. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.-S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat. Rev. Endocrinol. 2018, 14, 174–182. [Google Scholar] [CrossRef]
- Van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef]
- Fabel, K.; Fabel, K.; Tam, B.; Kaufer, D.; Baiker, A.; Simmons, N.; Kuo, C.J.; Palmer, T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003, 18, 2803–2812. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of physical activity on neurocognitive function: A review at multiple levels of analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Brothers, R.M.; Castelli, D.M.; Glowacki, E.M.; Chen, Y.T.; Salinas, M.M.; Kim, J.; Jung, Y.; Calvert, H.G. Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci. Lett. 2016, 630, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements—A systematic review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Osman, J.; Cabral, D.F.; Morris, T.P.; McInerney, K.; Cahalin, L.P.; Rundek, T.; Oliveira, A.; Pascual-Leone, A. Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurol. Clin. Pract. 2018, 8, 257–265. [Google Scholar] [CrossRef]
- Roig, M.; Skriver, K.; Lundbye-Jensen, J.; Kiens, B.; Nielsen, J.B. A single bout of exercise improves motor memory. PLoS ONE 2012, 7, e44594. [Google Scholar] [CrossRef]
- Schmidt-Kassow, M.; Deusser, M.; Thiel, C.; Otterbein, S.; Montag, C.; Reuter, M.; Banzer, W.; Kaiser, J. Physical exercise during encoding improves vocabulary learning in young female adults: A neuroendocrinological study. PLoS ONE 2013, 8, e64172. [Google Scholar] [CrossRef]
- Segal, S.K.; Cotman, C.W.; Cahill, L.F. Exercise-induced noradrenergic activation enhances memory consolidation in both normal aging and patients with amnestic mild cognitive impairment. J. Alzheimer’s Dis. 2012, 32, 1011–1018. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Blough, J.; Crawford, L.; Ryu, S.; Zou, L.; Li, H. The temporal effects of acute exercise on episodic memory function: Systematic review with meta-analysis. Brain Sci. 2019, 9, 87. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. Aerobic-Exercise and resistance-training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev. Cogn. Neurosci. 2019, 37, 100572. [Google Scholar] [CrossRef]
- Vazou, S.; Pesce, C.; Lakes, K.; Smiley-Oyen, A. More than one road leads to Rome: A narrative review and meta-analysis of physical activity intervention effects on cognition in youth. Int. J. Sport Exerc. Psychol. 2019, 17, 153–178. [Google Scholar] [CrossRef]
- Singh, B.; Bennett, H.; Miatke, A.; Dumuid, D.; Curtis, R.; Ferguson, T.; Brinsley, J.; Szeto, K.; Petersen, J.M.; Gough, C. Effectiveness of exercise for improving cognition, memory and executive function: A systematic umbrella review and meta-meta-analysis. Br. J. Sports Med. 2025, 59, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Mang, C.S.; Brown, K.E.; Neva, J.L.; Snow, N.J.; Campbell, K.L.; Boyd, L.A. Promoting motor cortical plasticity with acute aerobic exercise: A role for cerebellar circuits. Neural Plast. 2016, 2016, 6797928. [Google Scholar] [CrossRef] [PubMed]
- Logana, N.E. A review of the effects of physical activity on cognition and brain health across children and adolescence. In Building Future Health and Well-Being of Thriving Toddlers and Young Children: Nestlé Nutrition Institute Workshop, Basel, Switzerland, 14–17 September 2020; Karger: Basel, Switzerland, 2020; pp. 116–126. [Google Scholar]
- Snigdha, S.; De Rivera, C.; Milgram, N.W.; Cotman, C.W. Exercise enhances memory consolidation in the aging brain. Front. Aging Neurosci. 2014, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.; Zhang, Z.; Zhu, F.; Tang, Y.; Li, Z. Effects of Exergaming on executive function and motor ability in children: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0309462. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E. Physical activity, cognition, and brain outcomes: A review of the 2018 physical activity guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242. [Google Scholar] [CrossRef]
- Saanijoki, T.; Nummenmaa, L.; Eskelinen, J.-J.; Savolainen, A.M.; Vahlberg, T.; Kalliokoski, K.K.; Hannukainen, J.C. Affective responses to repeated sessions of high-intensity interval training. Med. Sci. Sports Exerc. 2015, 47, 2604–2611. [Google Scholar] [CrossRef]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Basso, J.C.; Shang, A.; Elman, M.; Karmouta, R.; Suzuki, W.A. Acute exercise improves prefrontal cortex but not hippocampal function in healthy adults. J. Int. Neuropsychol. Soc. 2015, 21, 791–801. [Google Scholar] [CrossRef]
- Howlett, N.; Trivedi, D.; Troop, N.A.; Chater, A.M. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl. Behav. Med. 2019, 9, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Gronwald, T.; Törpel, A.; Herold, F.; Budde, H. Perspective of dose and response for individualized physical exercise and training prescription. J. Funct. Morphol. Kinesiol. 2020, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.A. The evolution of physical activity promotion. Am. J. Nurs. 2015, 115, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Wurz, A.; Bradshaw, A.; Saunders, S.; West, M.A.; Brunet, J. Physical activity and quality of life in cancer survivors: A meta-synthesis of qualitative research. Cancers 2017, 9, 53. [Google Scholar] [CrossRef]
- Škola, F.; Tinková, S.; Liarokapis, F. Progressive training for motor imagery brain-computer interfaces using gamification and virtual reality embodiment. Front. Hum. Neurosci. 2019, 13, 329. [Google Scholar] [CrossRef]
- Lee, S.; Kim, W.; Park, T.; Peng, W. The psychological effects of playing exergames: A systematic review. Cyberpsychol. Behav. Soc. Netw. 2017, 20, 513–532. [Google Scholar] [CrossRef]
- Daly-Smith, A.; Quarmby, T.; Archbold, V.S.; Corrigan, N.; Wilson, D.; Resaland, G.K.; Bartholomew, J.B.; Singh, A.; Tjomsland, H.E.; Sherar, L.B. Using a multi-stakeholder experience-based design process to co-develop the Creating Active Schools Framework. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 13. [Google Scholar] [CrossRef]
- Direito, A.; Dale, L.P.; Shields, E.; Dobson, R.; Whittaker, R.; Maddison, R. Do physical activity and dietary smartphone applications incorporate evidence-based behaviour change techniques? BMC Public Health 2014, 14, 646. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Willumsen, J.; Bull, F.; Chou, R.; Ekelund, U.; Firth, J.; Jago, R.; Ortega, F.B.; Katzmarzyk, P.T. 2020 WHO guidelines on physical activity and sedentary behaviour for children and adolescents aged 5–17 years: Summary of the evidence. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 141. [Google Scholar] [CrossRef]
- Carden, L.; Wood, W. Habit formation and change. Curr. Opin. Behav. Sci. 2018, 20, 117–122. [Google Scholar] [CrossRef]
| Application Field | Specific Application Scenarios | Implementation Suggestions | Expected Benefits |
|---|---|---|---|
| Basic Education | After classroom teaching | Schedule 10–15 min of moderate-intensity activity, such as brisk walking, aerobics, jumping rope, after main lessons | Reinforces knowledge and memory, alleviates sedentary fatigue, improves subsequent attention, and significantly enhances memory retention rates and test scores |
| Higher Education and Professional Training | After language learning, skill training, and lectures | Conduct directed physical activity, such as 20–30 min of jogging or ball sports, after lectures or training sessions | Enhances memory of complex knowledge structures; promotes consolidation of procedural motor skills, such as instrument operation |
| Remote and Blended Learning | After online courses | Develop home-based exercise guidance programs, such as follow-along videos and app reminders | Compensates for the lack of physical activity in virtual learning environments and extends cognitive benefits to online learning scenarios |
| Universal Value | Curriculum and health policy design | Integrate “exercise micro-breaks” into timetables and teaching plans | Low cost, high benefit. Requires no expensive equipment/medication, combines dual advantages of enhancing cognition and promoting physical health, and reduces medical expenditure |
| Challenge Category | Specific Manifestations and Root Causes | Individualized and Precise Coping Strategies | Behavioral Change and Technical Support Strategy |
|---|---|---|---|
| Individual Response Differences | Effectiveness is influenced by age, gender, baseline health status, and genetic factors such as brain-derived neurotrophic factor gene polymorphism; “one-size-fits-all” plans have limited effect. | Develop precise exercise “prescriptions”: customize exercise parameters (type, intensity, duration) based on physiological characteristics, genotype, and preferences. Use digital health technology such as wearables + machine learning for dynamic adjustment. | Consider psychological characteristics (motivation, self-efficacy) and temporal preferences (morning/evening type) to improve plan acceptance. |
| Insufficient Long-Term Adherence | >50% of individuals quit an exercise plan within 6 months. Barriers include time constraints, lack of motivation, environmental obstacles, and insufficient self-regulatory capacity. | Offer flexible plans, allow intensity adjustment based on state, avoid “all-or-nothing” mindset, and facilitate restarting after interruptions. | Multi-level Behavioral Interventions: (1) Individual level: Goal setting, self-monitoring, and immediate feedback. (2) Social level: Peer support, group exercise, and social sharing. (3) Environmental level: Optimize venue design with features like standing desks and prominent stairs. |
| Technology Integration and Sustainability | Integrating interventions seamlessly and enjoyably into daily life is a major challenge. | / | Technological Innovation and Gamification: (1) Mobile apps: Personalized reminders and recommendations. (2) Wearable devices: Gamification elements, such as achievement badges and progress bars. (3) Virtual reality (VR): Create immersive, enjoyable exercise experiences. (4) Habit fusion: Combine exercise with daily routines, such as active commuting or lunch walks. Do not treat exercise as an extra burden. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhang, Y.; Lin, X.; Li, G.; Wang, S. Acute Exercise-Induced Epinephrine Elevation Promotes Post-Learning Memory Consolidation: A Narrative Review of Mechanisms and Implementation Strategies. Life 2026, 16, 13. https://doi.org/10.3390/life16010013
Zhang Y, Lin X, Li G, Wang S. Acute Exercise-Induced Epinephrine Elevation Promotes Post-Learning Memory Consolidation: A Narrative Review of Mechanisms and Implementation Strategies. Life. 2026; 16(1):13. https://doi.org/10.3390/life16010013
Chicago/Turabian StyleZhang, Yiwan, Xuewan Lin, Gen Li, and Songtao Wang. 2026. "Acute Exercise-Induced Epinephrine Elevation Promotes Post-Learning Memory Consolidation: A Narrative Review of Mechanisms and Implementation Strategies" Life 16, no. 1: 13. https://doi.org/10.3390/life16010013
APA StyleZhang, Y., Lin, X., Li, G., & Wang, S. (2026). Acute Exercise-Induced Epinephrine Elevation Promotes Post-Learning Memory Consolidation: A Narrative Review of Mechanisms and Implementation Strategies. Life, 16(1), 13. https://doi.org/10.3390/life16010013





