Inflammatory Profile and Risk of Hypertension in Infants Following Coarctation of the Aorta Repair: The Role of IL-6/TNF-α Ratio
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Coarctation of the Aorta—Characteristics
3.3. Inflammatory Biomarkers
3.4. Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torok, R.D.; Campbell, M.J.; Fleming, G.A.; Hill, K.D. Coarctation of the aorta: Management from infancy to adulthood. World J. Cardiol. 2015, 7, 765–775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vergales, J.E.; Gangemi, J.J.; Rhueban, K.S.; Lim, D.S. Coarctation of the aorta—The current state of surgical and transcatheter therapies. Curr. Cardiol. Rev. 2013, 9, 211–219. [Google Scholar] [CrossRef]
- Margarint, I.-M.; Youssef, T.; Rotaru, I.; Popescu, A.; Untaru, O.; Filip, C.; Stiru, O.; Constantin, A.-A.; Iliescu, V.A.; Vladareanu, R. Association of Plasma Renin Activity with Risk of Late Hypertension in Pediatric Patients with Early Aortic Coarctation Repair: A Retrospective Study. Life 2025, 15, 656. [Google Scholar] [CrossRef] [PubMed]
- Moutafi, A.C.; Alissafi, T.; Chamakou, A.; Chryssanthopoulos, S.; Thanopoulos, V.; Dellos, C.; Xanthou, G.; Tousoulis, D.; Stefanadis, C.; Gatzoulis, M.A.; et al. Neurohormonal activity and vascular properties late after aortic coarctation repair. Int. J. Cardiol. 2012, 159, 211–216. [Google Scholar] [CrossRef]
- Brili, S.; Tousoulis, D.; Antoniades, C.; Aggeli, C.; Roubelakis, A.; Papathanasiu, S.; Stefanadis, C. Evidence of vascular dysfunction in young patients with successfully repaired coarctation of aorta. Atherosclerosis 2005, 182, 97–103. [Google Scholar] [CrossRef]
- Brili, S.; Tousoulis, D.; Antoniades, C.; Vasiliadou, C.; Karali, M.; Papageorgiou, N.; Ioakeimidis, N.; Marinou, K.; Stefanadi, E.; Stefanadis, C. Effects of ramipril on endothelial function and the expression of proinflammatory cytokines and adhesion molecules in young normotensive subjects with successfully repaired coarctation of aorta: A randomized cross-over study. J. Am. Coll. Cardiol. 2008, 51, 742–749. [Google Scholar] [CrossRef]
- Damas, P.; Ledoux, D.; Nys, M.; Vrindts, Y.; De Groote, D.; Franchimont, P.; Lamy, M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann. Surg. 1992, 215, 356–362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walters, H.M.; Pan, N.; Lehman, T.J.; Adams, A.; Kalliolias, G.D.; Zhu, Y.S.; Santiago, F.; Nguyen, J.; Sitaras, L.; Cunningham-Rundles, S.; et al. The impact of disease activity and tumour necrosis factor-α inhibitor therapy on cytokine levels in juvenile idiopathic arthritis. Clin. Exp. Immunol. 2016, 184, 308–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouchart, F.; Dubar, A.; Tabley, A.; Litzler, P.Y.; Haas-Hubscher, C.; Redonnet, M.; Bessou, J.P.; Soyer, R. Coarctation of the aorta in adults: Surgical results and long-term follow-up. Ann. Thorac. Surg. 2000, 70, 1483–1488; discussion 1488–1489. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Burkhart, H.M.; Connolly, H.M.; Dearani, J.A.; Cetta, F.; Li, Z.; Oliver, W.C.; Warnes, C.A.; Schaff, H.V. Coarctation of the aorta: Lifelong surveillance is mandatory following surgical repair. J. Am. Coll. Cardiol. 2013, 62, 1020–1025. [Google Scholar] [CrossRef]
- Toro-Salazar, O.H.; Steinberger, J.; Thomas, W.; Rocchini, A.P.; Carpenter, B.; Moller, J.H. Long-term follow-up of patients after coarctation of the aorta repair. Am. J. Cardiol. 2002, 89, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.M.; Erasmus, M.E.; Ebels, T. Hypertension in children after repair of coarctation of the aorta: Still a cause for concern. Ann. Thorac. Surg. 2011, 91, 1901–1906. [Google Scholar]
- Trojnarska, O.; Szczepaniak-Chicheł, L.; Mizia-Stec, K.; Gabriel, M.; Bartczak, A.; Grajek, S.; Gąsior, Z.; Kramer, L.; Tykarski, A. Vascular remodeling in adults after coarctation repair: Impact of descending aorta stenosis and age at surgery. Clin. Res. Cardiol. 2011, 100, 447–455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niebauer, J.; Cooke, J.P. Cardiovascular effects of exercise: Role of endothelial shear stress. J. Am. Coll. Cardiol. 1996, 28, 1652–1660. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Anghel, A.; Taranu, G.; Seclaman, E.; Rata, A.; Tamas, L.; Moldovan, H.; Ursoniu, S.; Samoila, C.; Ionac, M.; Popa-Wagner, A. Safety of Vascular Endothelial and Hepatocyte Growth Factor Gene Therapy in Patients with Critical Limb Ischemia. Curr. Neurovasc. Res. 2011, 8, 183–189. [Google Scholar] [CrossRef] [PubMed]
- de Divitiis, M.; Pilla, C.; Kattenhorn, M.; Zadinello, M.; Donald, A.; Leeson, P.; Wallace, S.; Redington, A.; Deanfield, J.E. Vascular dysfunction after repair of coarctation of the aorta: Impact of early surgery. Circulation 2001, 104, I165–I170. [Google Scholar] [CrossRef]
- Ou, P.; Celermajer, D.S.; Jolivet, O.; Buyens, F.; Herment, A.; Sidi, D.; Bonnet, D.; Mousseaux, E. Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. Am. Heart J. 2008, 155, 187–193. [Google Scholar] [CrossRef]
- Shang, Q.; Sarikouch, S.; Patel, S.; Schuster, A.; Steinmetz, M.; Ou, P.; Danford, D.A.; Beerbaum, P.; Kutty, S. Assessment of ventriculo-vascular properties in repaired coarctation using cardiac magnetic resonance-derived aortic, left atrial and left ventricular strain. Eur. Radiol. 2017, 27, 167–177. [Google Scholar] [CrossRef]
- Róg, B.; Okólska, M.; Dziedzic-Oleksy, H.; Sałapa, K.; Rubiś, P.; Kopeć, G.; Podolec, P.; Tomkiewicz-Pająk, L. Arterial stiffness in adult patients after coarctation of aorta repair and with bicuspid aortic valve. Acta Cardiol. 2019, 74, 517–524. [Google Scholar] [CrossRef]
- Çetiner, N.; Erolu, E.; Baran Him, N.; Şaylan Çevik, B.; Akalın, F. Vascular Wall Changes and Arterial Functions in Children with Surgically Repaired Aortic Coarctation. Turk. Arch. Pediatr. 2022, 57, 193–199. [Google Scholar] [CrossRef]
- Donazzan, L.; Crepaz, R.; Stuefer, J.; Stellin, G. Abnormalities of aortic arch shape, central aortic flow dynamics, and distensibility predispose to hypertension after successful repair of aortic coarctation. World J. Pediatr. Congenit. Heart Surg. 2014, 5, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Radke, R.M.; Diller, G.-P.; Duck, M.; Orwat, S.; Hartmann, D.; Thum, T.; Baumgartner, H. Endothelial function in contemporary patients with repaired coarctation of aorta. Heart 2014, 100, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.; De Wolf, D.; Vandekerckhove, K. Hypertension after coarctation repair—A systematic review. Transl. Pediatr. 2022, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Timofte, D.; Tanasescu, M.-D.; Balcangiu-Stroescu, A.-E.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, A.; Constantin, P.C.; Cosconel, C.-I.; et al. Dyselectrolytemia-management and implications in hemodialysis (Review). Exp. Ther. Med. 2021, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Recinos, A., 3rd; Eledrisi, M.S. Vascular inflammation and the renin-angiotensin system. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Angelone, D.F.; Wessels, M.R.; Coughlin, M.; Suter, E.; Valentini, P.; A Kalish, L.; Levy, O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr. Res. 2006, 60, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Tal, Y.; Shoenfeld, Y. Interleukin 6 and cardiovascular disease. Autoimmun. Rev. 2004, 3, 84–90. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, M.; Menéndez-Suso, J.J.; Cámara-Hijón, C.; Río-García, M.; Laplaza-González, M.; Amores-Hernández, I.; Romero-Gómez, M.P.; Álvarez-Rojas, E.; Salas-Mera, D.; López-Granados, E.; et al. Cytokine Profile in Children with Severe Multisystem Inflammatory Syndrome Related to the Coronavirus Disease 2019. J. Pediatr. Intensive Care 2021, 11, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tulin, R.; Geana, R.C.; Robu, M.; Iliescu, V.A.; Stiru, O.; Nayyerani, R.; Chibulcutean, A.S.; Bacalbasa, N.; Balescu, I.; Tulin, A.; et al. Predictors of Late Mortality in Patients With Surgically Resected Cardiac Myxomas: A Single-Center Experience. Cureus 2022, 14, e20866. [Google Scholar] [CrossRef]
- Peer, S.M.; Sharma, A.; Hote, M.P. Arterial stiffness following repair of coarctation of the aorta: Comparison of endovascular and surgical treatment. J. Am. Coll. Cardiol. 2009, 54, 1120–1126. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Margarint, I.-M.; Youssef, T.; Robu, M.; Rotaru, I.; Popescu, A.; Untaru, O.; Filip, C.; Stiru, O.; Iliescu, V.A.; Vladareanu, R. The Management of Aortic Coarctation Associated with Hypoplastic Arches and Particular Arch Anatomies: A Literature Review. J. Pers. Med. 2024, 14, 732. [Google Scholar] [CrossRef]
- Filip, C.; Vasile, C.M.; Nicolae, G.; Margarint, I.; Popa, L.; Bizubac, M.; Ganea, G.; Rusu, M.; Murzi, B.; Balgradean, M.; et al. Gemella sanguinis Infective Endocarditis-Challenging Management of an 8-Year-Old with Duchenne Dystrophy and Undiagnosed Congenital Heart Disease: A Case Report. Antibiotics 2023, 12, 706. [Google Scholar] [CrossRef]
- Costa, D.; Scalise, E.; Ielapi, N.; Bracale, U.M.; Andreucci, M.; Serra, R. Metalloproteinases as Biomarkers and Sociomarkers in Human Health and Disease. Biomolecules 2024, 14, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aflyatumova, G.N.; Nigmatullina, R.R.; Sadykova, D.I.; Chibireva, M.D.; Fugetto, F.; Serra, R. Endothelin-1, nitric oxide, serotonin and high blood pressure in male adolescents. Vasc. Health Risk Manag. 2018, 18, 213–223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
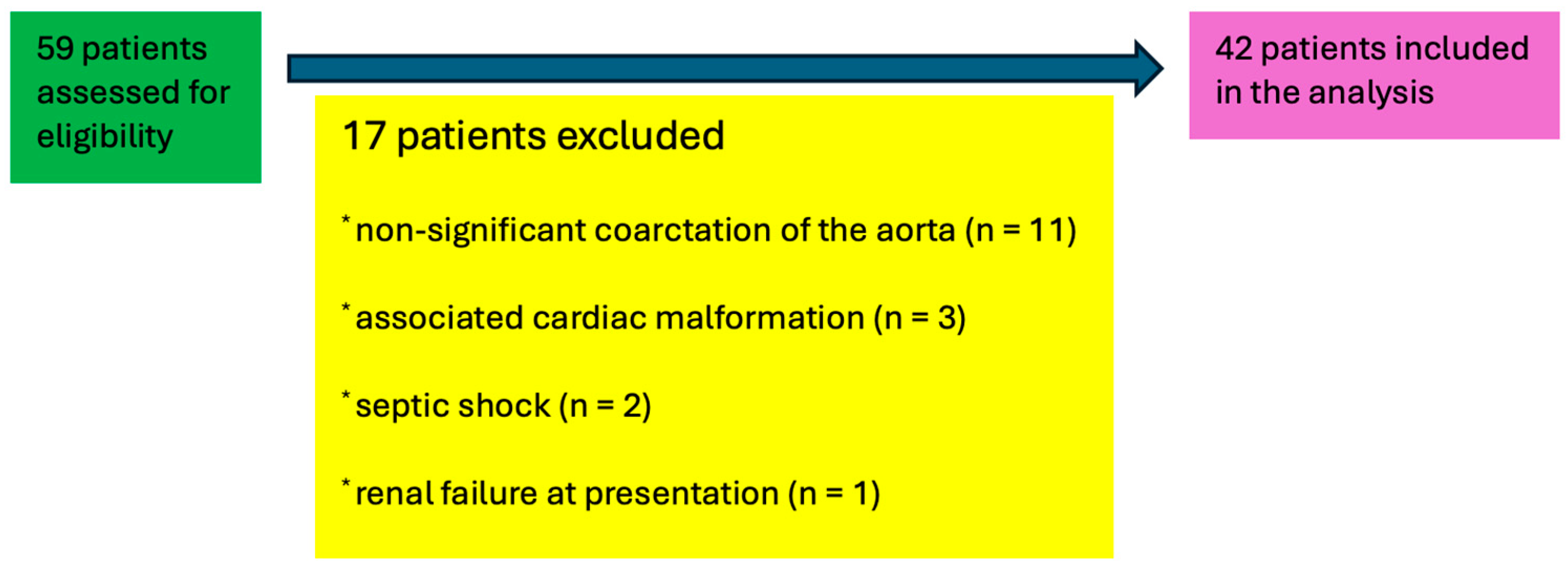
| Parameter (Unit) | N = 42 (100%) |
|---|---|
| Preoperative | |
| Age (mean, SD months) | 5.5 ± 3.67 |
| Weight (mean, SD grams) | 5024.1 ± 2214.73 |
| Height (mean, SD cm) | 63.7 ± 9.72 |
| Male sex (n, %) | 36 (87.8) |
| Premature (n, %) | 6 (14.6) |
| Moderate left ventricular disfunction (n, %) | 5 (12.2) |
| Severe left ventricular disfunction (n, %) | 4 (9.8) |
| Diameter of the aorta at the isthmus (mean, SD mm) | 0.95 ± 0.17 |
| Z score (mean, SD) | −3.85 ± 1.16 |
| Bicuspid aortic valve (n, %) | 6 (14.6) |
| Gothic aortic arch (n, %) | 12 (29.3) |
| Crenel gothic arch (n, %) | 3 (7.3) |
| Bovine aortic arch (n, %) | 5 (12.2) |
| Ascending aortic diameter (mean, SD mm) | 8.41 ± 1.76 |
| Proximal aortic arch diameter (mean, SD mm) | 6.98 ± 2 |
| Distal aortic arch diameter (mean, SD mm) | 4.8 ± 1.34 |
| Peak systolic gradient at the aortic isthmus (mean, SD mmHg) | 38.31 ± 23.2 |
| Peak velocity at the isthmus (mean, SD m/s) | 2.97 ± 0.87 |
| Inotropic support (n, %) | 3 (7.3) |
| Inotropic and vasopressor support (n %) | 6 (14.6) |
| Biomarkers (plasma concentration) | |
| Renin (mean, SD pg/mL) | 56,32 ± 61.24 |
| Renin over 28 pg/mL (n, %) | 32 (78) |
| vWF (mean, SD UI/mL) | 77.9 ± 63.13 |
| vWF over 150 UI/mL (n, %) | 5 (12.2) |
| IL-6 (mean, SD, pg/mL) | 54.52 ± 64.77 |
| IL-6 over 7 pg/mL (n, %) | 35 (85.4) |
| TNF-alpha (mean, SD, pg/mL) | 30.5 ± 32.08 |
| TNF-alpha over 8.1 pg/mL (n, %) | 39 (95.1) |
| IL-6/TNF-alpha over 2 | 16 (39) |
| Intraoperative | |
| Duration of surgery (mean, SD min) | 96.2 ± 20,71 |
| Aortic clamp time (mean, SD min) | 28.95 ± 7.97 |
| Surgery under one month (n, %) | 4 (9.75) |
| Extracorporeal circulation (n, %) | 1 (2.43) |
| Postoperative | |
| Peak systolic gradient at the aortic isthmus (mean, SD m/s) | 12 ± 6.27 |
| Peak velocity (mean, SD m/s) | 1.7 ± 0.4 |
| Inotropic and/or vasopressor support (n, %) | 4 (9.8) |
| Complications | 5 (12.2) |
| Chylothorax (n, %) | 1 (2.43) |
| Superficial wound infection (n,%) | 1 (2.43) |
| Cardiogenic shock (n, %) | 3 (7.3) |
| Hypertension (n, %) | 16 (39) |
| Parameter (Unit) | HTA (0) N = 26 | HTA (1) N = 16 | p |
|---|---|---|---|
| Preoperative | |||
| Age (mean, SD months) | 40.4 ± 41.2 | 37.7 ± 54.8 | 0.86 |
| Weight (mean, SD grams) | 5400 ± 700 | 5100 ± 800 | 0.22 |
| Height (mean, SD cm) | 61.2 ± 3.5 | 59.8 ± 4.0 | 0.25 |
| Male sex (n, %) | 10 (38.46) | 16 (100) | 0.37 |
| Premature (n, %) | 3 (11.5) | 3 (18.75) | 0.83 |
| Moderate left ventricular disfunction (n, %) | 4 (24%) | 4 (17%) | 0.88 |
| Severe left ventricular disfunction (n, %) | 0 (0%) | 5 (21%) | 0.13 |
| Diameter of the aorta at the isthmus (mean, SD mm) | 2.8 ± 0.9 | 2.7 ± 1.0 | 0.77 |
| Z score (mean, SD) | −1.3 ± 0.5 | −1.6 ± 0.7 | 0.19 |
| Bicuspid aortic valve (n, %) | 6 (35%) | 10 (42%) | 0.63 |
| Gothic aortic arch (n, %) | 5 (29%) | 2 (8%) | 0.18 |
| Crenel aortic arch (n, %) | 3 (18%) | 5 (21%) | 0.72 |
| Bovine aortic arch (n, %) | 2 (12%) | 6 (25%) | 0.31 |
| Ascending aortic diameter (mean, SD mm) | 8.7 ± 2.3 | 7.6 ± 1.3 | 0.08 |
| Proximal aortic arch diameter (mean, SD mm) | 5.7 ± 1.2 | 6.0 ± 1.1 | 0.47 |
| Distal aortic arch diameter (mean, SD mm) | 4.7 ± 1.2 | 5.0 ± 1.1 | 0.41 |
| Peak systolic gradient at the aortic isthmus (mean, SD mmHg) | 46.6 ± 19.3 | 57.0 ± 18.7 | 0.09 |
| Peak velocity at the isthmus (mean, SD m/s) | |||
| Inotropic and vasopressor support (n, %) | 2 (12%) | 6 (25%) | 0.31 |
| Inotropic support (n, %) | 3 (18%) | 7 (29%) | 0.38 |
| Biomarkers (plasma concentration) | |||
| Renin (mean, SD pg/mL) | 22 ± 7 | 35 ± 10 | 0.01 |
| vWF (mean, SD UI/mL) | 140 ± 30 | 170 ± 40 | 0.03 |
| IL-6 (mean, SD, pg/mL) | 4.5 ± 1.8 | 6.9 ± 2.3 | 0.02 |
| TNF-alpha (mean, SD, pg/mL) | 4.8 ± 1.7 | 7.2 ± 2.1 | 0.01 |
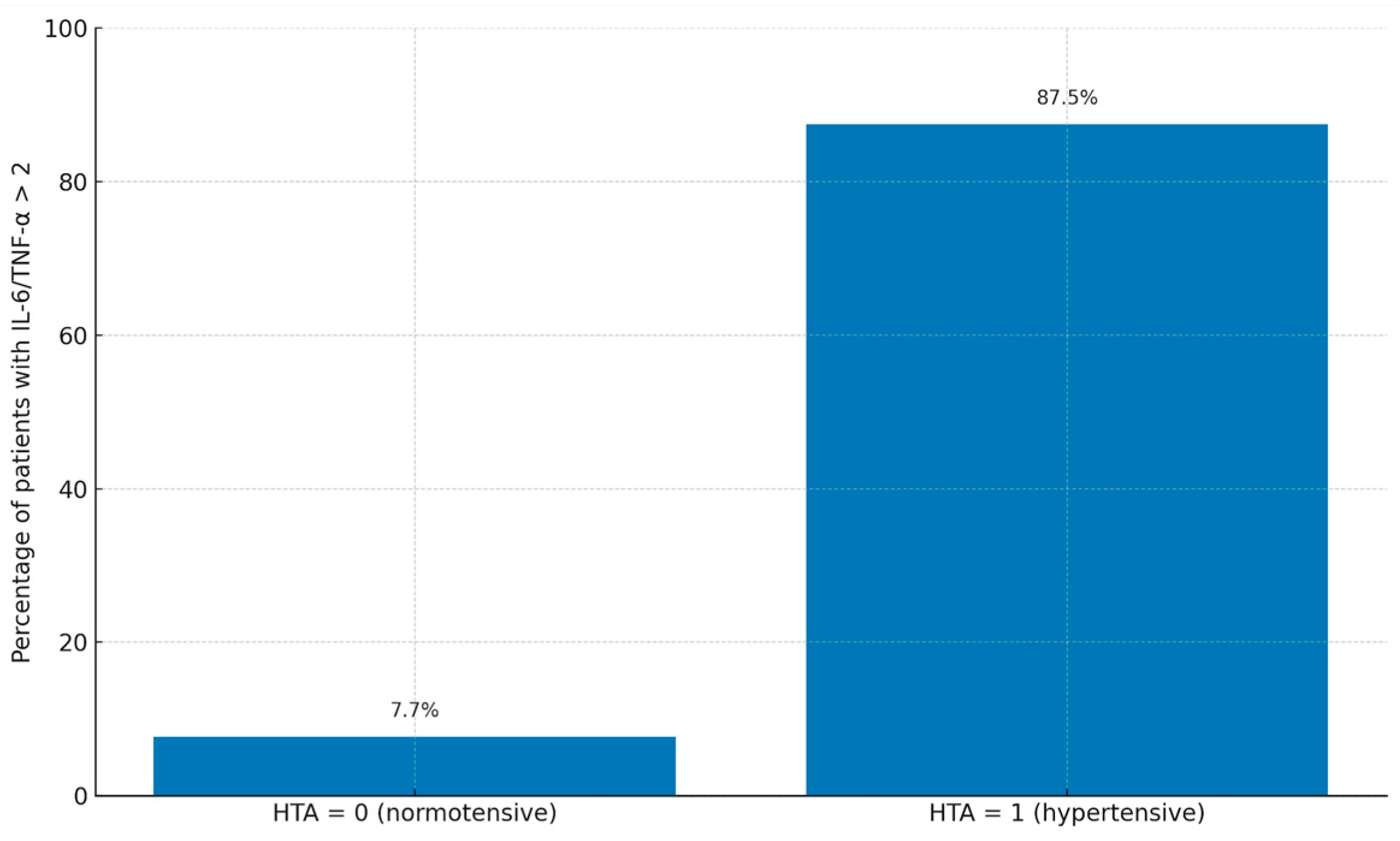
| IL-6/TNF-alpha over 2 | 2 (7.7%) | 14 (87.5%) | 0.03 |
| Intraoperative | |||
| Duration of surgery (mean, SD min) | 130 ± 20 | 140 ± 25 | 0.17 |
| Aortic clamp time (mean, SD min) | 25 ± 6 | 27 ± 5 | 0.36 |
| Surgery under one month (n, %) | 6 (35%) | 7 (29%) | 0.68 |
| Extracorporeal circulation (n, %) | 0 (0%) | 1 (6.25%) | 0.82 |
| Postoperative | |||
| Peak systolic gradient at the aortic isthmus (mean, SD m/s) | 12 ± 6.27 | 13 ± 3.28 | 0.39 |
| Peak velocity (mean, SD m/s) | 1.7 ± 0.4 | 1.9 ± 0.2 | 0.46 |
| Inotropic and/or vasopressor support (n, %) | 2 (12%) | 5 (21%) | 0.41 |
| Chylothorax (n, %) | 0 (0%) | 2 (8%) | 0.18 |
| Superficial wound infection (n,%) | 1 (6%) | 3 (12%) | 0.52 |
| Cardiogenic shock (n, %) | 0 (0%) | 2 (8%) | 0.18 |
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| IL-6/TNF-alpha ratio | 7.4 | 2.4–22.9 | <0.001 | 5.2 | 1.6–17.1 | 0.02 |
| Renin plasma concentration | 3.30 | 1.1–10.5 | 0.05 | 2.4 | 1.1–5.9 | 0.03 |
| Gothic arch | 4.80 | 1.2–20.9 | 0.03 | |||
| Aortic clamp time | 3.60 | 2.1–13.2 | 0.045 | 3 | 1.2–9.2 | 0.046 |
| Age | 1.04 | 1.5–26.4 | 0.040 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Margarint, I.-M.; Iliescu, V.A.; Youssef, T.; Rotaru, I.; Popescu, A.; Untaru, O.; Vladareanu, R. Inflammatory Profile and Risk of Hypertension in Infants Following Coarctation of the Aorta Repair: The Role of IL-6/TNF-α Ratio. Life 2025, 15, 1481. https://doi.org/10.3390/life15091481
Margarint I-M, Iliescu VA, Youssef T, Rotaru I, Popescu A, Untaru O, Vladareanu R. Inflammatory Profile and Risk of Hypertension in Infants Following Coarctation of the Aorta Repair: The Role of IL-6/TNF-α Ratio. Life. 2025; 15(9):1481. https://doi.org/10.3390/life15091481
Chicago/Turabian StyleMargarint, Irina-Maria, Vlad Anton Iliescu, Tammam Youssef, Iulian Rotaru, Alexandru Popescu, Olguta Untaru, and Radu Vladareanu. 2025. "Inflammatory Profile and Risk of Hypertension in Infants Following Coarctation of the Aorta Repair: The Role of IL-6/TNF-α Ratio" Life 15, no. 9: 1481. https://doi.org/10.3390/life15091481
APA StyleMargarint, I.-M., Iliescu, V. A., Youssef, T., Rotaru, I., Popescu, A., Untaru, O., & Vladareanu, R. (2025). Inflammatory Profile and Risk of Hypertension in Infants Following Coarctation of the Aorta Repair: The Role of IL-6/TNF-α Ratio. Life, 15(9), 1481. https://doi.org/10.3390/life15091481






