Minimally-Invasive Imaging of Sublingual Vessels—A New Method to Study Microvascular Changes in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
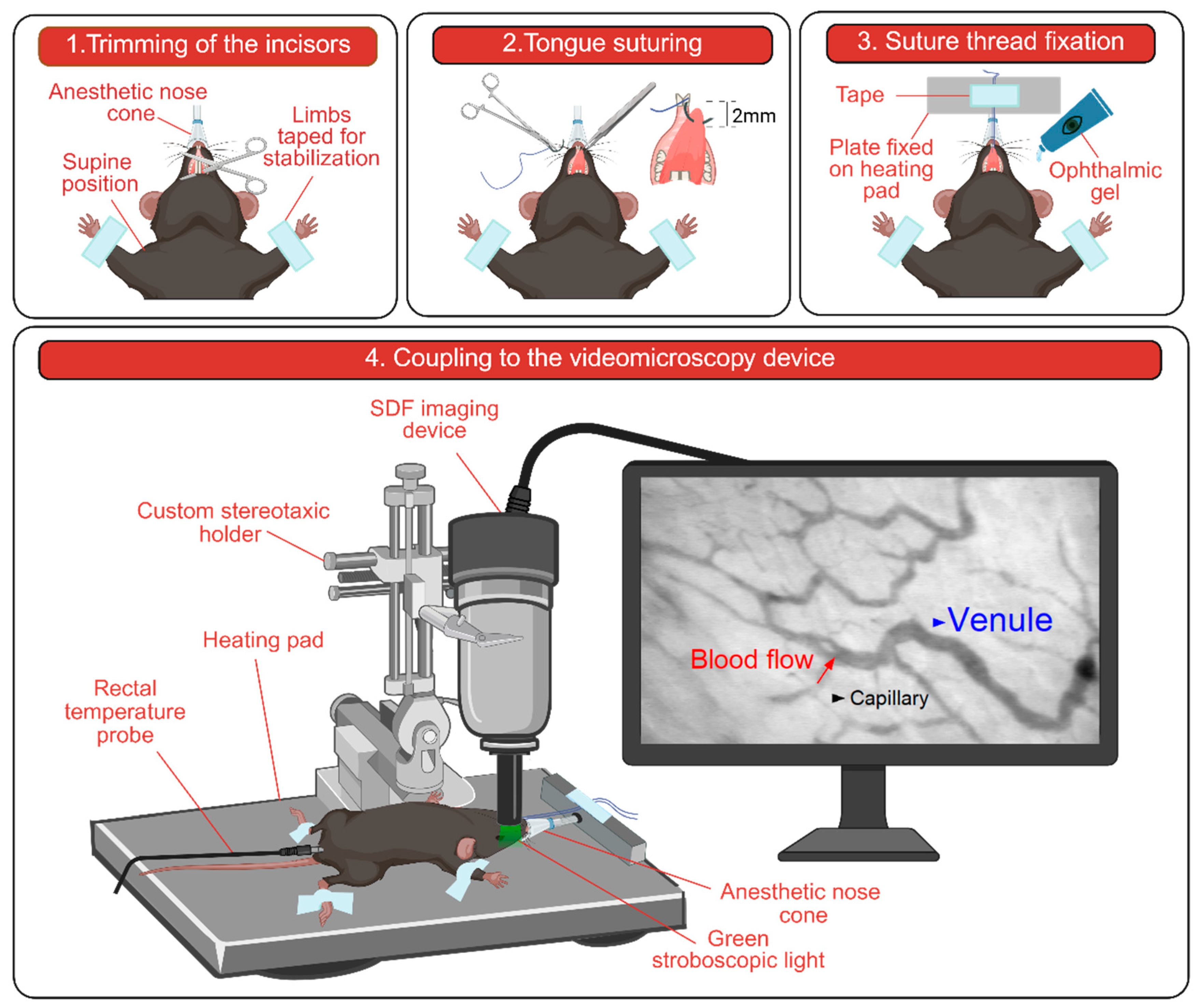
2.2. Mouse Preparation for In Vivo Imaging
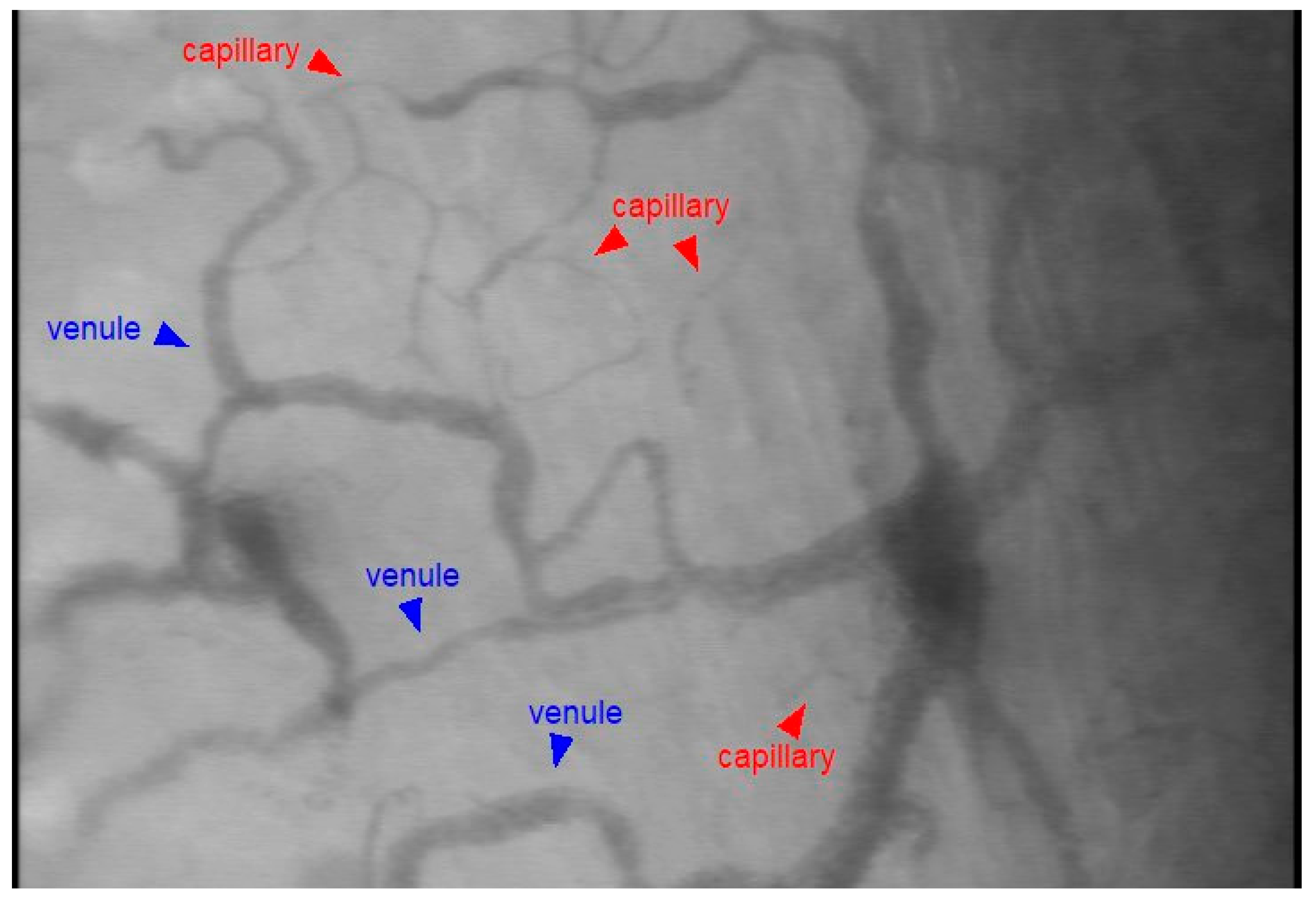
2.3. Setup and Image Acquisition
2.4. Image Analysis
2.5. Statistics
3. Results
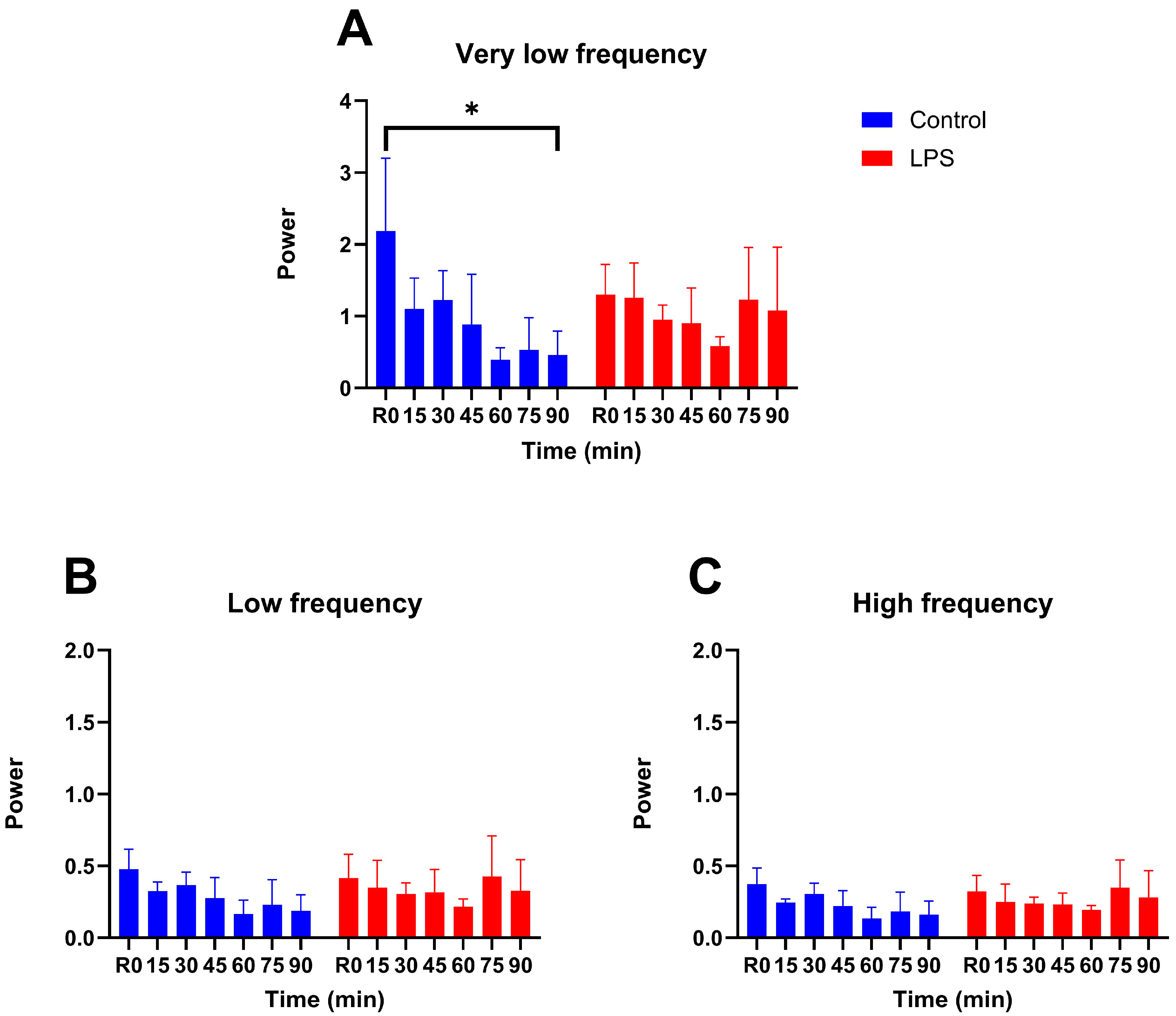
3.1. Microcirculatory Perfusion
3.2. Vasomotion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- De Backer, D.; Cortes, D.O.; Donadello, K.; Vincent, J.L. Pathophysiology of micraocirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014, 5, 73–79. [Google Scholar] [CrossRef]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef]
- Damiani, E.; Carsetti, A.; Casarotta, E.; Domizi, R.; Scorcella, C.; Donati, A.; Adrario, E. Microcirculation-guided resuscitation in sepsis: The next frontier? Front. Media 2023, 10, 1212321. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Ricottilli, F.; Ospina-Tascón, G.A. Septic shock: A microcirculation disease. Curr. Opin. Anaesthesiol. 2021, 34, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lelubre, C.; Vincent, J.L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.G.; Bateman, R.M.; Sharpe, M.D.; Sibbald, W.J.; Gill, R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H156–H164. [Google Scholar] [CrossRef]
- Nilsson, H. Vasomotion: Mechanisms and Physiological Importance. Mol. Interv. 2003, 3, 79–89. [Google Scholar] [CrossRef]
- Aalkjær, C.; Boedtkjer, D.; Matchkov, V. Vasomotion–what is currently thought? Acta Physiol. 2011, 202, 253–269. [Google Scholar] [CrossRef]
- Intaglietta, M. Vasomotion and flowmotion: Physiological mechanisms and clinical evidence. Vasc. Med. Rev. 2017, 1, 101–112. [Google Scholar] [CrossRef]
- Kowalewska, P.M.; Kowalewski, J.E.; Milkovich, S.L.; Sové, R.J.; Wang, L.; Whitehead, S.N.; Ellis, C.G. Spectroscopy detects skeletal muscle microvascular dysfunction during onset of sepsis in a rat fecal peritonitis model. Sci. Rep. 2022, 12, 6339. [Google Scholar] [CrossRef]
- Mendelson, A.A.; Rajaram, A.; Bainbridge, D.; Lawrence, K.S.; Bentall, T.; Sharpe, M.; Diop, M.; Ellis, C.G. Dynamic tracking of microvascular hemoglobin content for continuous perfusion monitoring in the intensive care unit: Pilot feasibility study. J. Clin. Monit. Comput. 2021, 35, 1453–1465. [Google Scholar] [CrossRef]
- Eskandari, R.; Milkovich, S.; Kamar, F.; Goldman, D.; Welsh, D.G.; Ellis, C.G.; Diop, M. Non-invasive point-of-care optical technique for continuous in vivo assessment of microcirculatory function: Application to a preclinical model of early sepsis. FASEB J. 2024, 38, e70204. [Google Scholar] [CrossRef]
- Fredriksson, I.; Larsson, M.; Strömberg, T.; Iredahl, F. Vasomotion analysis of speed resolved perfusion, oxygen saturation, red blood cell tissue fraction, and vessel diameter: Novel microvascular perspectives. Ski. Res. Technol. 2022, 28, 142–152. [Google Scholar] [CrossRef]
- Yajnik, V.; Maarouf, R. Sepsis and the microcirculation: The impact on outcomes. Curr. Opin. Anaesthesiol. 2022, 35, 230–235. [Google Scholar] [CrossRef]
- Tang, A.; Shi, Y.; Dong, Q.; Wang, S.; Ge, Y.; Wang, C.; Gong, Z.; Zhang, W.; Chen, W. Prognostic Value of Sublingual Microcirculation in Sepsis: A Systematic Review and Meta-analysis. J. Intensive Care Med. 2024, 39, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.S.K.; Enrico, C.; Laviolle, B.; Vazquez, A.R.; Ince, C.; Dubin, A. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit. Care Med. 2012, 40, 1443–1448. [Google Scholar] [CrossRef]
- De Backer, D.; Creteur, J.; Dubois, M.-J.; Sakr, Y.; Koch, M.; Verdant, C.; Vincent, J.-L. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit. Care Med. 2006, 34, 403–408. [Google Scholar] [CrossRef]
- Massey, M.J.; Hou, P.C.; Filbin, M.; Wang, H.; Ngo, L.; Huang, D.T.; Aird, W.C.; Novack, V.; Trzeciak, S.; Yealy, D.M.; et al. Microcirculatory perfusion disturbances in septic shock: Results from the ProCESS trial. Crit. Care 2018, 22, 308. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Donadello, K.; Sakr, Y.; Ospina-Tascon, G.; Salgado, D.; Scolletta, S.; Vincent, J.-L.M. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit. Care Med. 2013, 41, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Ellis, C.G. Bench-to-bedside review: Microvascular dysfunction in sepsis-Hemodynamics, oxygen transport, and nitric oxide. Crit. Care 2003, 7, 359–373. [Google Scholar] [CrossRef]
- Lala, R.; Homes, R.; Pratt, S.; Goodwin, W.; Midwinter, M. Comparison of sublingual microcirculatory parameters measured by sidestream darkfield videomicroscopy in anesthetized pigs and adult humans. Anim. Model. Exp. Med. 2023, 6, 499–503. [Google Scholar] [CrossRef]
- Bar, S.; Diaper, J.; Fontao, F.; Belin, X.; Abrard, S.; Albu, G.; Dupont, H.; Habre, W.; Schiffer, E. Early And Concomitant Administration of Norepinephrine and Ilomedin Improves Microcirculatory Perfusion Without Impairing Macrocirculation in an Intestinal Ischemia-Reperfusion Injury Swine Model: A Randomized Experimental Trial. Shock 2024, 63, 606–613. [Google Scholar] [CrossRef]
- Wang, C.; Bischof, E.; Xu, J.; Guo, Q.; Zheng, G.; Ge, W.; Hu, J.; Margarint, E.L.G.; Bradley, J.L.; Peberdy, M.A.; et al. Effects of Methylprednisolone on Myocardial Function and Microcirculation in Post-resuscitation: A Rat Model. Front. Cardiovasc. Med. 2022, 9, 894004. [Google Scholar] [CrossRef]
- Ge, W.; Zheng, G.; Ji, X.; He, F.; Hu, J.; Bradley, J.L.; Moore, C.E.; Peberdy, M.A.; Ornato, J.P.; Mangino, M.J.; et al. Effects of Polyethylene Glycol-20k on Coronary Perfusion Pressure and Postresuscitation Myocardial and Cerebral Function in a Rat Model of Cardiac Arrest. J. Am. Hear. Assoc. 2020, 9, e014232. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Edul, V.S.K.; Martins, E.; Canales, H.S.; Canullán, C.; Murias, G.; Pozo, M.O.; Estenssoro, E.; Ince, C.; Dubin, A. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. J. Appl. Physiol. 2016, 120, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Takala, J.; Jakob, S.M. Shedding light on microcirculation? Intensive Care Med. 2009, 35, 394–396. [Google Scholar] [CrossRef][Green Version]
- Hessler, M.; Arnemann, P.-H.; Zamit, F.; Seidel, L.; Kampmeier, T.-G.; Kathöfer, U.; Alnawaiseh, M.; Tchaichian, S.; Rehberg, S.; Ertmer, C. Monitoring of Conjunctival Microcirculation Reflects Sublingual Microcirculation in Ovine Septic and Hemorrhagic Shock. Shock 2019, 51, 479–486. [Google Scholar] [CrossRef]
- Astapenko, D.; Dostalova, V.; Kraus, J.; Radochova, V.; Dostal, P.; Ticha, A.; Hyspler, R.; Lehmann, C.; Cerny, V. Effect of acute hypernatremia induced by hypertonic saline administration on endothelial glycocalyx in rabbits. Clin. Hemorheol. Microcirc. 2019, 72, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Wu, J.; Qu, H.-P.; Tang, Y.-Q.; Chen, D.-C. Time of dissociation between microcirculation, macrocirculation, and lactate levels in a rabbit model of early endotoxemic shock. Chin. Med. J. 2020, 133, 2153–2160. [Google Scholar] [CrossRef]
- Dostalova, V.; Dostalova, V.J.; Kraus, J.; Cerny, V.; Ticha, A.R.; Hyspler, R.; Radochova, V.D.; Paral, J.; Dostal, P. The Effect of Fluid Loading and Hypertonic Saline Solution on Cortical Cerebral Microcirculation and Glycocalyx Integrity. J. Neurosurg. Anesthesiol. 2019, 31, 434–443. [Google Scholar] [CrossRef]
- Milstein, D.M.; Helmers, R.; Hackmann, S.; Belterman, C.N.; van Hulst, R.A.; de Lange, J. Sublingual microvascular perfusion is altered during normobaric and hyperbaric hyperoxia. Microvasc. Res. 2016, 105, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, P.T.; Khalilzada, M.; Bezemer, R.; Merza, J.; Ince, C. Sidestream Dark Field (SDF) imaging: A novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt. Express 2007, 15, 15101–15114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, L.; Zhao, H.; Hu, H.; Lai, S.; Zhao, X.; Zhang, H.; Ke, J.; Hu, Q. Imaging and observation of microcirculation in bowel mucosa using sidestream dark field imaging. J. Microsc. 2024, 297, 203–214. [Google Scholar] [CrossRef]
- Xu, T.; Gao, X.; Yuan, H.; Li, S.; Zhou, Z.; Gong, G.; Jia, G.; Zhao, G. Real-time semi-quantitative assessment of anastomotic blood perfusion in mini-invasive rectal resections by Sidestream Dark Field (SDF) imaging technology: A prospective in vivo pilot study. Langenbecks Arch. Surg. 2023, 408, 186. [Google Scholar] [CrossRef]
- Zweifach, B.W. Direct observation of the mesenteric circulation in experimental animals. Rec. 1954, 120, 277–291. [Google Scholar] [CrossRef]
- Gavins, F.N.E.; Chatterjee, B.E. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: Focus on the mesentery and cremaster preparations. J. Pharmacol. Toxicol. Methods 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Coste, A.; Oktay, M.H.; Condeelis, J.S.; Entenberg, D. Intravital Imaging Techniques for Biomedical and Clinical Research. Cytometry. Part A J. Int. Soc. Anal. Cytol. 2020, 97, 448–457. [Google Scholar] [CrossRef]
- Pittet, M.J.; Weissleder, R. Intravital imaging. Cell 2011, 147, 983–991. [Google Scholar] [CrossRef]
- Choi, M.; Kwok, S.J.J.; Yun, S.H. In vivo fluorescence microscopy: Lessons from observing cell behavior in their native environment. Am. Physiol. Soc. 2015, 30, 40–49. [Google Scholar] [CrossRef]
- Mota-Silva, I.; Castanho, M.A.R.B.; Silva-Herdade, A.S. Towards Non-Invasive Intravital Microscopy: Advantages of Using the Ear Lobe Instead of the Cremaster Muscle. Life 2023, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Kurata, T.; Li, Z.; Oda, S.; Kawahira, H.; Haneishi, H. Impact of vessel diameter and bandwidth of illumination in sidestream dark-field oximetry. Biomed. Opt. Express 2015, 6, 1616–1631. [Google Scholar] [CrossRef]
- Jansen, S.M.; De Bruin, D.M.; Faber, D.; Dobbe, J.; Heeg, E.; Milstein, D.M.J.; Strackee, S.D.; van Leeuwen, T. Applicability of quantitative optical imaging techniques for intraoperative perfusion diagnostics: A comparison of laser speckle contrast imaging, sidestream dark-field microscopy, and optical coherence tomography. J. Biomed. Opt. 2017, 22, 086004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef]
- Gengenbacher, N.; Singhal, M.; Augustin, H.G. Preclinical mouse solid tumour models: Status quo, challenges and perspectives. Nat. Rev. Cancer 2017, 17, 751–765. [Google Scholar] [CrossRef]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef]
- Treu, C.M.; Lupi, O.; Bottino, D.A.; Bouskela, E. Sidestream dark field imaging: The evolution of real-time visualization of cutaneous microcirculation and its potential application in dermatology. Arch. Dermatol. Res. 2011, 303, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Boyd, J.G.; Wood, N.; Frank, J.; Girard, T.D.; Ross-White, A.; Chopra, A.; Foster, D.; Griesdale, D.E.G. The Use of Near-Infrared Spectroscopy and/or Transcranial Doppler as Non-Invasive Markers of Cerebral Perfusion in Adult Sepsis Patients with Delirium: A Systematic Review. J. Intensive Care Med. 2022, 37, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- McDowell, K.P.; Berthiaume, A.-A.; Tieu, T.; Hartmann, D.A.; Shih, A.Y. VasoMetrics: Unbiased spatiotemporal analysis of microvascular diameter in multi-photon imaging applications. Quant. Imaging Med. Surg. 2020, 11, 969–982. [Google Scholar] [CrossRef]
- Boerma, E.C.; Mathura, K.R.; van der Voort, P.H.; Spronk, P.E.; Ince, C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: A prospective validation study. Crit. Care 2005, 9, R601–R606. [Google Scholar] [CrossRef]
- Spanos, A.; Jhanji, S.; Vivian-Smith, A.; Harris, T.; Pearse, R.M. Early microvascular changes in sepsis and severe sepsis. Shock 2010, 33, 387–391. [Google Scholar] [CrossRef]
- Inoue, S.; Suzuki-Utsunomiya, K.; Suzuki-Utsunomiya, K.; Sato, T.; Chiba, T.; Hozumi, K. Impaired innate and adaptive immunity of accelerated-aged Klotho mice in sepsis. Crit. Care 2012, 16, P1. [Google Scholar] [CrossRef]
- Taccone, F.S.; Su, F.; Pierrakos, C.; He, X.; James, S.; Dewitte, O.; Vincent, J.-L.; De Backer, D. Cerebral microcirculation is impaired during sepsis: An experimental study. Crit. Care 2010, 14, R140. [Google Scholar] [CrossRef]
- Verdant, C.L.; De Backer, D.; Bruhn, A.; Clausi, C.M.; Su, F.; Wang, Z.; Rodriguez, H.; Pries, A.R.; Vincent, J.-L.M. Evaluation of sublingual and gut mucosal microcirculation in sepsis: A quantitative analysis. Crit. Care Med. 2009, 37, 2875–2881. [Google Scholar] [CrossRef]
- Secor, D.; Li, F.; Ellis, C.G.; Sharpe, M.D.; Gross, P.L.; Wilson, J.X.; Tyml, K. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Care Med. 2010, 36, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Patil, R.; Mani, S.; Malarvizhi, R.; Vasanthi, H.R. Refinement of LPS induced Sepsis in SD Rats to Mimic Human Sepsis. Biomed. Pharmacol. J. 2020, 13, 335–346. [Google Scholar] [CrossRef]
- Cornell, T.T.; Rodenhouse, P.; Cai, Q.; Sun, L.; Shanley, T.P. Mitogen-activated protein kinase phosphatase 2 regulates the inflammatory response in sepsis. Infect. Immun. 2010, 78, 2868–2876. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokari, R.; Matsunaga, H.; Takebayashi, K.; Watanabe, C.; Komoto, S.; Okada, Y.; Kurihara, C.; Kawaguchi, A.; Nagao, S.; et al. P-selectin-dependent monocyte recruitment through platelet interaction in intestinal microvessels of LPS-treated mice. Microcirculation 2008, 15, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Malik, A.B.; Liu, S.F. NF-B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Cell. Mol. Physiol. 2006, 290, 622–645. [Google Scholar] [CrossRef]
- Hernandez, G.; Bruhn, A.; Ince, C. Microcirculation in Sepsis: New Perspectives. Curr. Vasc. Pharmacol. 2013, 11, 161–169. [Google Scholar] [CrossRef]
- CInce; Sinaasappel, M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit. Care Med. 1999, 27, 1369–1377. [Google Scholar] [CrossRef]
- Trzeciak, S.; Cinel, I.; Dellinger, R.P.; Shapiro, N.I.; Arnold, R.C.; Parrillo, J.E.; Hollenberg, S.M. Resuscitating the microcirculation in sepsis: The central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad. Emerg. Med. 2008, 15, 399–413. [Google Scholar] [CrossRef]
- Lidington, D.; Ouellette, Y.; Li, F.; Tyml, K. Conducted vasoconstriction is reduced in a mouse model of sepsis. J. Vasc. Res. 2003, 40, 149–158. [Google Scholar] [CrossRef]
- Ince, C. The microcirculation is the motor of sepsis. Crit. Care 2005, 9, S13–S19. [Google Scholar] [CrossRef]
- Tyml, K.; Wang, X.; Lidington, D.; Ouellette, Y. Lipopolysaccharide reduces intercellular coupling in vitro and arteriolar conducted response in vivo. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, 1397–1406. [Google Scholar] [CrossRef]
- Condon, M.R.; Kim, J.E.; Deitch, E.A.; Machiedo, G.W.; Spolarics, Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, 2177–2184. [Google Scholar] [CrossRef]
- Singel, D.J.; Stamler, J.S. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu. Rev. Physiol. 2005, 67, 99–145. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Balarini, M.; Caixeta, D.; Bouskela, X.E. Microcirculatory dysfunction in sepsis: Pathophysiology, clinical monitoring, and potential therapies. Am. J. Physiol. Circ. Physiol. 2016, 311, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, J.; Mazur, A.; Postnov, D.D.; Straarup, M.S.; Soendergaard, A.M.; Staehr, C.; Brøndum, E.; Aalkjaer, C.; Matchkov, V.V. Intravital investigation of rat mesenteric small artery tone and blood flow. J. Physiol. 2017, 595, 5037–5053. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Granger, D.N. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free. Radic. Biol. Med. 2012, 52, 556–592. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Li, B.; Liu, X.; Li, A.; Li, H.; Shi, X.; Han, J. Tumor-Derived Exosomes Promote Tumor Growth Through Modulating Microvascular Hemodynamics in a Human Ovarian Cancer Xenograft Model. Microcirculation 2024, 31, e12876. [Google Scholar] [CrossRef]
- Goldman, D.; Popel, A.S. A computational study of the effect of vasomotion on oxygen transport from capillary networks. J. Theor. Biol. 2001, 209, 189–199. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Roger, C.; Zieleskiewicz, L.; Demattei, C.; Lakhal, K.; Piton, G.; Louart, B.; Constantin, J.-M.; Chabanne, R.; Faure, J.-S.; Mahjoub, Y.; et al. Time course of fluid responsiveness in sepsis: The fluid challenge revisiting (FCREV) study. Crit. Care 2019, 23, 179. [Google Scholar] [CrossRef]
- Farkas, E.; Luiten, P.G.M. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog. Neurobiol. 2001, 64, 575–611. [Google Scholar] [CrossRef]
- Hundley, W.G.; Renaldo, G.J.; Levasseur, J.E.; Kontos, H.A. Vasomotion in cerebral microcirculation of awake rabbits. Am. J. Physiol. Circ. Physiol. 1988, 254, H67–H71. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.; Leaders, F.E. Effect of drugs and confinement on vasomotion in the conscious rat. Life Sci. 1967, 6, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Colantuoni, A.; Bertuglia, S.; Intaglietta, M. Effects of anesthesia on the spontaneous activity of the microvasculature. Int. J. Microcirc. Clin. Exp. 1984, 3, 13–28. [Google Scholar]
- Garcia, L.L.; Vicentini, Y.F.; Nagashima, J.K.; de Souza, A.F.; Pereira, M.A.A.; da Silva, L.C.L.C.; Fantoni, D.T. Sublingual microcirculation in isoflurane-anesthetized horses receiving dexmedetomidine and lidocaine constant rate infusion. Am. J. Veter-Res. 2025, 86, 1–8. [Google Scholar] [CrossRef]
- Kang, C.; Cho, A.-R.; Kim, H.; Kwon, J.-Y.; Lee, H.J.; Kim, E. Sedation with propofol and isoflurane differs in terms of microcirculatory parameters: A randomized animal study using dorsal skinfold chamber mouse model. Microvasc. Res. 2024, 153, 104655. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gramanzini, M.; Liuzzi, R.; Greco, A.; Brunetti, A.; Vesce, G. Effects of some anesthetic agents on skin microcirculation evaluated by laser Doppler perfusion imaging in mice. BMC Veter-Res. 2013, 9, 255. [Google Scholar] [CrossRef]
- Sullender, C.T.; Richards, L.M.; He, F.; Luan, L.; Dunn, A.K. Dynamics of isoflurane-induced vasodilation and blood flow of cerebral vasculature revealed by multi-exposure speckle imaging. J. Neurosci. Methods 2021, 366, 109434. [Google Scholar] [CrossRef]
- Park, K.W.; Dai, H.B.; Lowenstein, E.; Darvish, A.; Sellke, F.W. Heterogeneous Vasomotor Responses of Rabbit Coronary Microvessels to Isoflurane. Anesthesiology 1994, 81, 1190–1197. [Google Scholar] [CrossRef]
- Schumacher, J.; Pörksen, M.; Klotz, K.-F. Effects of isoflurane, enflurane, and halothane on skeletal muscle microcirculation in the endotoxemic rat. J. Crit. Care 2001, 16, 1–7. [Google Scholar] [CrossRef]
- Lee, J.G.; Hudetz, A.G.; Smith, J.J.; Hillard, C.J.; Bosnjak, Z.J.; Kampine, J.P. The Effects of Halothane and Isoflurane on Cerebrocortical Microcirculation and Autoregulation as Assessed by Laser-Doppler Flowmetry. Anesth. Analg. 1994, 79, 58–65. [Google Scholar] [CrossRef]
- Matta, B.F.; Heath, K.J.; Tipping, K.; Summors, A.C. Direct Cerebral Vasodilatory Effects of Sevoflurane and Isoflurane. Anesthesiology 1999, 91, 677. [Google Scholar] [CrossRef]
- Hutchings, S.D.; Wendon, J.; Watts, S.; Kirkman, E. The Cytocam video microscope. A new method for visualising the microcirculation using incident dark field (IDF) technology. Intensive Care Med. Exp. 2015, 3, A601. [Google Scholar] [CrossRef]
- Han, J.; Choi, P.; Choi, M. µTongue: A Microfluidics-Based Functional Imaging Platform for the Tongue In Vivo. J. Vis. Exp. 2021, 2021, e62361. [Google Scholar] [CrossRef]
- Choi, M.; Lee, W.M.; Yun, S.H. Intravital microscopic interrogation of peripheral taste sensation. Sci. Rep. 2015, 5, 8661. [Google Scholar] [CrossRef]
- Hammoudeh, S.M.; Ng, Y.; Wei, B.-R.; Madsen, T.D.; Yadav, M.P.; Simpson, R.M.; Weigert, R.; Randazzo, P.A. Tongue orthotopic xenografts to study fusion-negative rhabdomyosarcoma invasion and metastasis in live animals. Cell Rep. Methods 2024, 4, 100802. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyminski Parente Ribeiro, E.; Dastan, M.; Bellut-Staeck, U.; Zhou, J.; Lehmann, C. Minimally-Invasive Imaging of Sublingual Vessels—A New Method to Study Microvascular Changes in Mice. Life 2025, 15, 1478. https://doi.org/10.3390/life15091478
Dyminski Parente Ribeiro E, Dastan M, Bellut-Staeck U, Zhou J, Lehmann C. Minimally-Invasive Imaging of Sublingual Vessels—A New Method to Study Microvascular Changes in Mice. Life. 2025; 15(9):1478. https://doi.org/10.3390/life15091478
Chicago/Turabian StyleDyminski Parente Ribeiro, Ellen, Maryam Dastan, Ursula Bellut-Staeck, Juan Zhou, and Christian Lehmann. 2025. "Minimally-Invasive Imaging of Sublingual Vessels—A New Method to Study Microvascular Changes in Mice" Life 15, no. 9: 1478. https://doi.org/10.3390/life15091478
APA StyleDyminski Parente Ribeiro, E., Dastan, M., Bellut-Staeck, U., Zhou, J., & Lehmann, C. (2025). Minimally-Invasive Imaging of Sublingual Vessels—A New Method to Study Microvascular Changes in Mice. Life, 15(9), 1478. https://doi.org/10.3390/life15091478







