The Clinical Value of the Ferning Test in Monitoring Dry Eye Syndrome in Patients with Sarcoidosis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DED | Dry Eye Disease |
| FT | Ferning Test |
| S-DED | Sarcoidosis and Dry Eye Disease |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| 95%CI | Confidence Interval |
| NaCl | Sodium Chloride |
References
- O’Keefe, G.A.D.; Rao, N.A. Progress in the diagnosis of ocular sarcoidosis. Indian J. Ophthalmol. 2022, 70, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Sève, P.; Pacheco, Y.; Durupt, F.; Jamilloux, Y.; Gerfaud-Valentin, M.; Isaac, S.; Boussel, L.; Calender, A.; Androdias, G.; Valeyre, D.; et al. Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis. Cells 2021, 10, 766. [Google Scholar] [CrossRef]
- Leinonen, S. Ocular sarcoidosis, to screen or not to screen? Front. Med. 2024, 11, 1348435. [Google Scholar] [CrossRef]
- Pasadhika, S.; Rosenbaum, J.T. Ocular Sarcoidosis. Clin. Chest Med. 2015, 36, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Yokoi, N.; Nagata, K.; Deguchi, H.; Sekiyama, Y.; Sotozono, C. Investigation of the relationship between ocular sarcoidosis and dry eye. Sci. Rep. 2022, 12, 3469. [Google Scholar] [CrossRef]
- Baughman, R.P.; Field, S.; Costabel, U.; Crystal, R.G.; Culver, D.A.; Drent, M.; Judson, M.A.; Wolff, G. Sarcoidosis in America. Analysis Based on Health Care Use. Ann. Am. Thorac. Soc. 2016, 13, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.-M.; Tchah, H.-W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Alanazi, M.A.; El-Hiti, G.A.; Almaymuni, R.; Baashen, M.A.; Fagehi, R.; Masmali, A.M. Tear Ferning Test as a valuable tool to determine the quality of tear film in ani-mals and humans. EC Ophthalmol. 2021, 12, 41–55. [Google Scholar]
- Murube, J. Tear Crystallization Test: Two Centuries of History. Ocul. Surf. 2004, 2, 7–9. [Google Scholar] [CrossRef]
- López Solís, R.; Traipe Castro, L.; Salinas Toro, D.; Srur, M.; Toledo Araya, H. Microdesiccates produced from normal human tears display four distinctive morphological components. Biol. Res. 2013, 46, 299–305. [Google Scholar] [CrossRef]
- Masmali, A.M.; Murphy, P.J.; Purslow, C. Development of a new grading scale for tear ferning. Contact Lens Anterior Eye 2014, 37, 178–184. [Google Scholar] [CrossRef]
- Stapleton, F.; Argüeso, P.; Asbell, P.; Azar, D.; Bosworth, C.; Chen, W.; Ciolino, J.; Craig, J.P.; Gallar, J.; Galor, A.; et al. TFOS DEWS III Digest Report. Am. J. Ophthalmol. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; An, Y.; Hwang, W.J. Gender differences in dry eye disease symptoms associated with psychological health indicators among adults using mobile mental health apps. PLoS ONE 2023, 18, e0278921. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, M.; Frings, A.; Geerling, G.; Finis, D. Gender-Specific Differences in Signs and Symptoms of Dry Eye Disease. Curr. Eye Res. 2020, 46, 294–301. [Google Scholar] [CrossRef]
- Donica, V.C.; Donica, A.L.; Pavel, I.A.; Danielescu, C.; Alexa, A.I.; Bogdănici, C.M. Variabilities in Retinal Hemodynamics Across the Menstrual Cycle in Healthy Women Identified Using Optical Coherence Tomography Angiography. Life 2024, 15, 22. [Google Scholar] [CrossRef]
- Ungprasert, P.; Tooley, A.A.; Crowson, C.S.; Matteson, E.L.; Smith, W.M. Clinical Characteristics of Ocular Sarcoidosis: A Population-Based Study 1976–2013. Ocul. Immunol. Inflamm. 2017, 27, 389–395. [Google Scholar] [CrossRef]
- Norn, M. Quantitative tear ferning: Clinical investigations. Acta Ophthalmol. 1994, 72, 369–372. [Google Scholar] [CrossRef]
- Felberg, S.; Cordeiro, H.; Sato, E.H.; Martini Filho, D.; NIshiwaki-Dantas, M.C.; Endo, R.M.; Dantas, P.E.C. Reprodutibilidade na classificação do teste de cristalização do filme lacrimal em pacientes com síndrome de Sjögren. Arq. Bras. De Oftalmol. 2008, 71, 228–233. [Google Scholar] [CrossRef][Green Version]
- Masmali, A.M.; Purslow, C.; Murphy, P.J. The tear ferning test: A simple clinical technique to evaluate the ocular tear film. Clin. Exp. Optom. 2014, 97, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.A.; Aldawood, M.A.; Badawood, Y.S.; El-Hiti, G.A.; Masmali, A.M. A comparative study of the quality of non-stimulated and stimulated tears in normal eye male subjects using the tear ferning test. Clin. Optom. 2019, 11, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Fagehi, R.; El-Hiti, G.A.; Alqarni, B.M.; Alanazi, M.A.; Masmali, A.M.; Almubrad, T. Improvement in Tear Ferning Patterns of Sheep Tears After Addition of Various Electrolyte Solutions. Front. Med. 2021, 8, 721969. [Google Scholar] [CrossRef]
- Almutleb, E.S.; El-Hiti, G.A.; Al-Dawas, H.A.; Alanzi, M.K.; Alquwayi, M.; Alotaibi, A.G.; Baashen, M.A.; Altoaimi, B.H.; Alanazi, S.A.; Masmali, A.M. Effect of monovalent electrolyte solutions on the human tear ferning pattern. PLoS ONE 2023, 18, e0280853. [Google Scholar] [CrossRef]
- Kannan, R.; Das, S.; Shetty, R.; Zhou, L.; Ghosh, A.; Deshpande, V. Tear proteomics in dry eye disease. Indian J. Ophthalmol. 2023, 71, 1203–1214. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef]
- Nebbioso, M.; Sacchetti, M.; Bianchi, G.; Zicari, A.M.; Duse, M.; Del Regno, P.; Lambiase, A. Tear Ferning Test and Pathological Effects on Ocular Surface before and after Topical Cyclosporine in Vernal Keratoconjunctivitis Patients. J. Ophthalmol. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Rolando, M.; Baldi, F.; Zingirian, M. Effects of hyperosmolarity on tear mucus ferning. Fortschritte Der Ophthalmol. Z. Der Dtsch. Ophthalmol. Ges. 1986, 83, 644–646. [Google Scholar]
- Kiyat, P.; Palamar, M.; Gerceker Turk, B. Dry eye and Meibomian gland dysfunction evaluation in sarcoidosis patients. Eur. J. Ophthalmol. 2021, 32, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, S. Biochemistry of human tear film: A review. Exp. Eye Res. 2022, 220, 109101. [Google Scholar] [CrossRef]
- Tabbara, K.F.; Okumoto, M. Ocular Ferning Test. Ophthalmology 1982, 89, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Norn, M. Ferning in conjunctival-cytologic preparations. Crystallisation in stained semiquantitative pipette samples of conjunctival fluid. Acta Ophthalmol. 1987, 65, 118–122. [Google Scholar] [CrossRef]
- Masmali, A.M.; Maeni, Y.A.; El-Hiti, G.A.; Murphy, P.J.; Almubrad, T. Investigation of Ocular Tear Ferning in Controlled and Uncontrolled Diabetic Subjects. Eye Contact Lens Sci. Clin. Pract. 2018, 44, S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Masmali, A.M.; Al-Shehri, A.; Alanazi, S.A.; Abusharaha, A.; Fagehi, R.; El-Hiti, G.A. Assessment of Tear Film Quality among Smokers Using Tear Ferning Patterns. J. Ophthalmol. 2016, 2016, 8154315. [Google Scholar] [CrossRef] [PubMed]
- Ganea, C.V.; Sandu, C.A.; Bogdănici, C.G.; Bogdănici, C.M. Management Strategies for Dry Eye Syndrome in Patients with Obesity—A Literature Review. Life 2025, 15, 1102. [Google Scholar] [CrossRef]
- Alanazi, S.A.; Badawood, Y.S.; Aldawood, M.A.; El-Hiti, G.A.; Masmali, A.M. Effect of Refresh Plus® preservative-free lubricant eyedrops on tear ferning patterns in dry eye and normal eye subjects. Clin. Ophthalmol. 2019, 13, 1011–1017. [Google Scholar] [CrossRef]

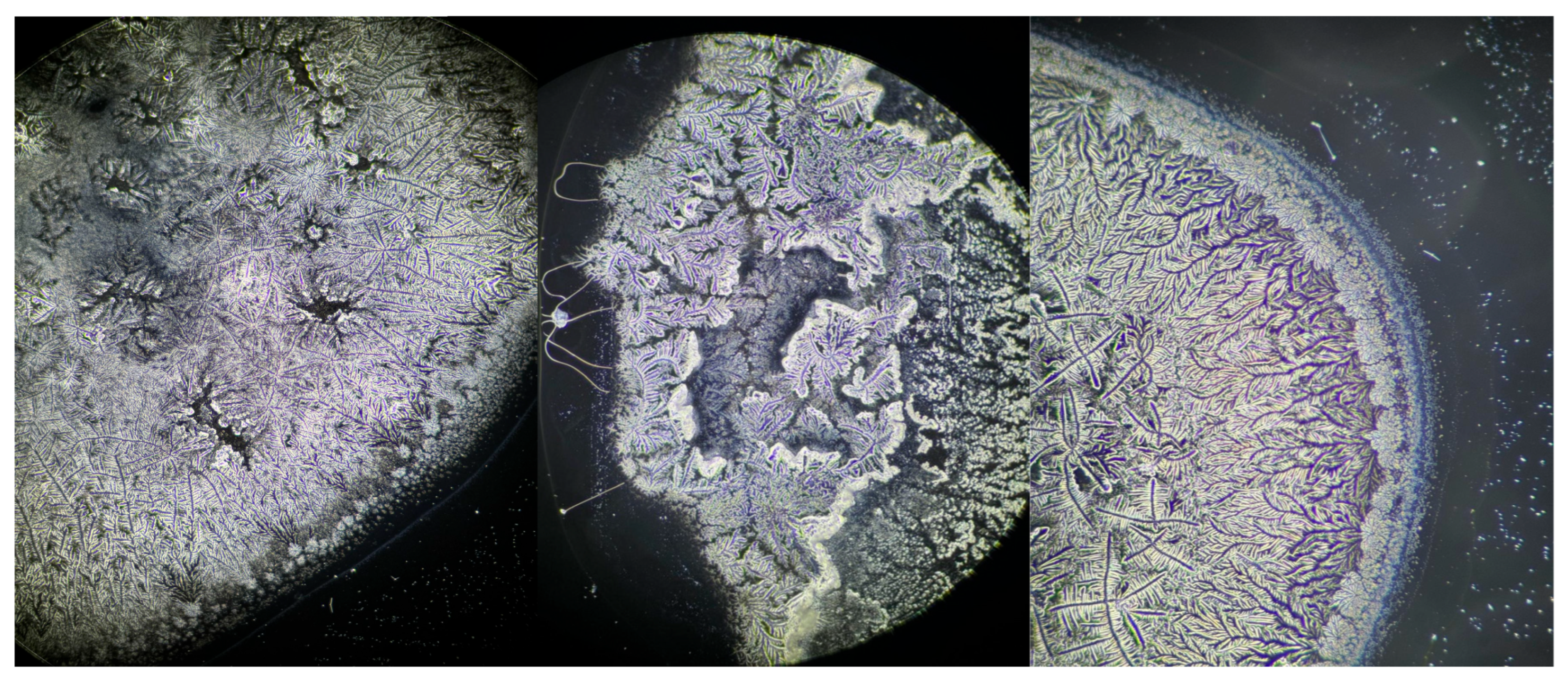
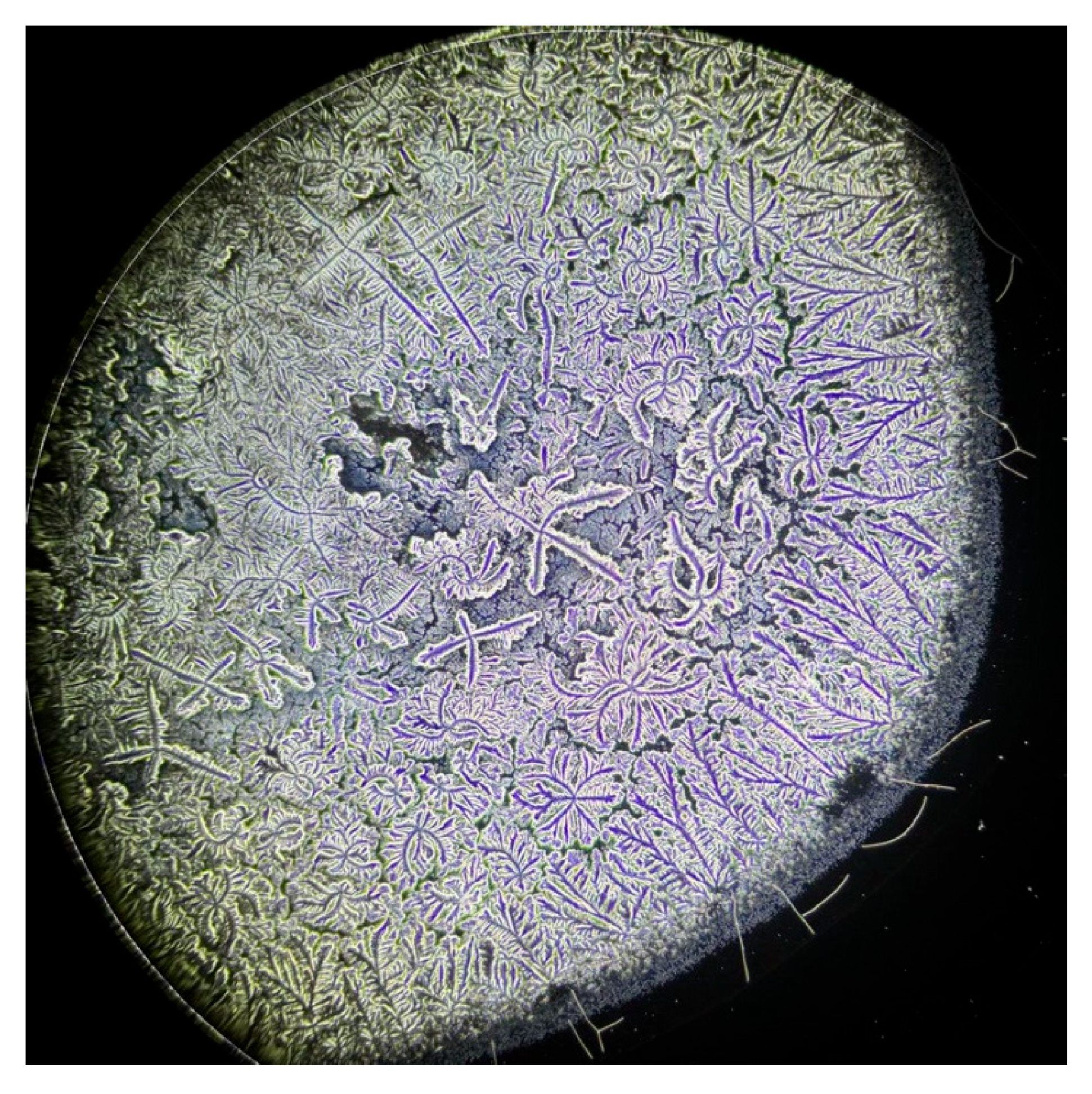

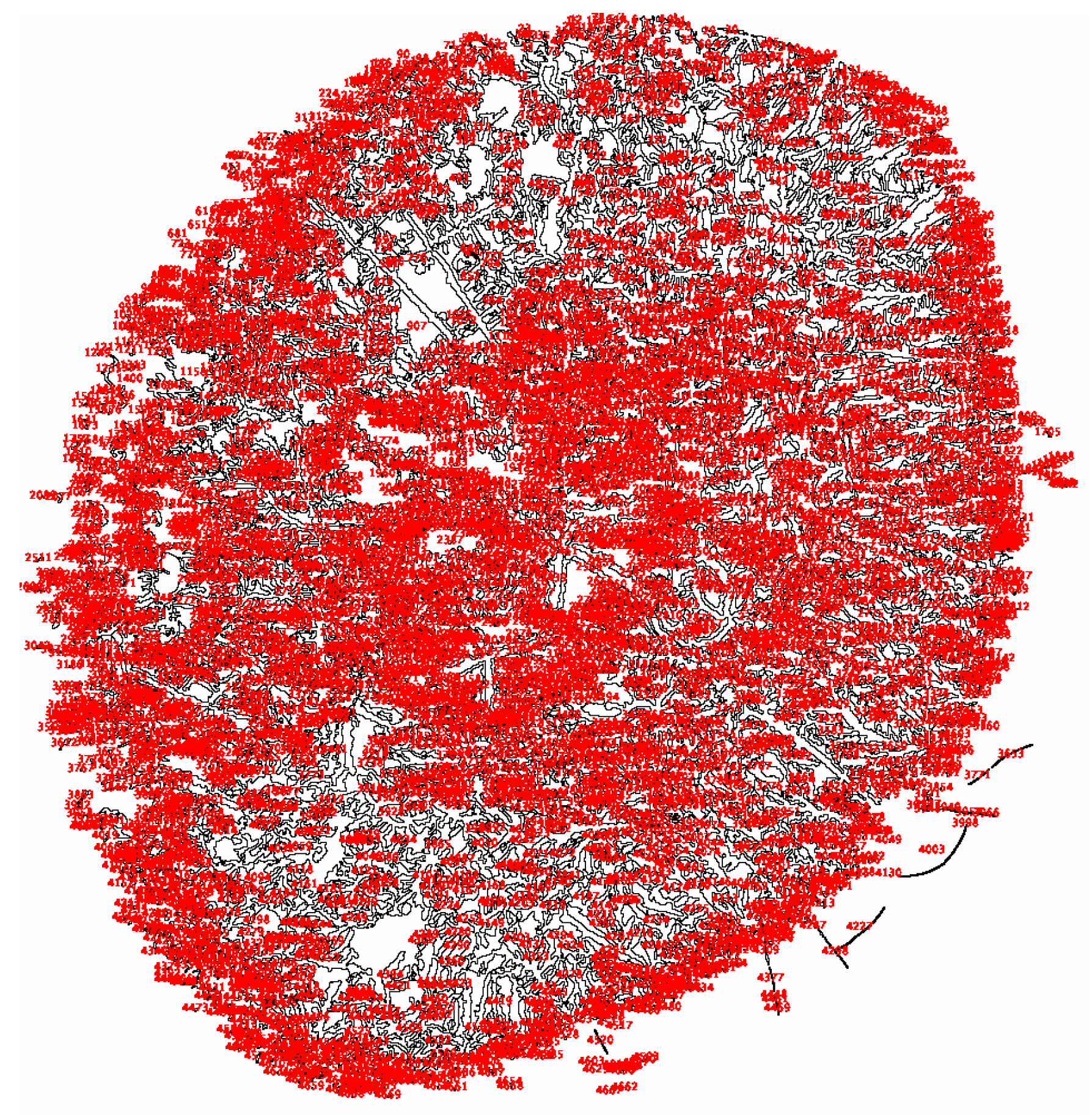
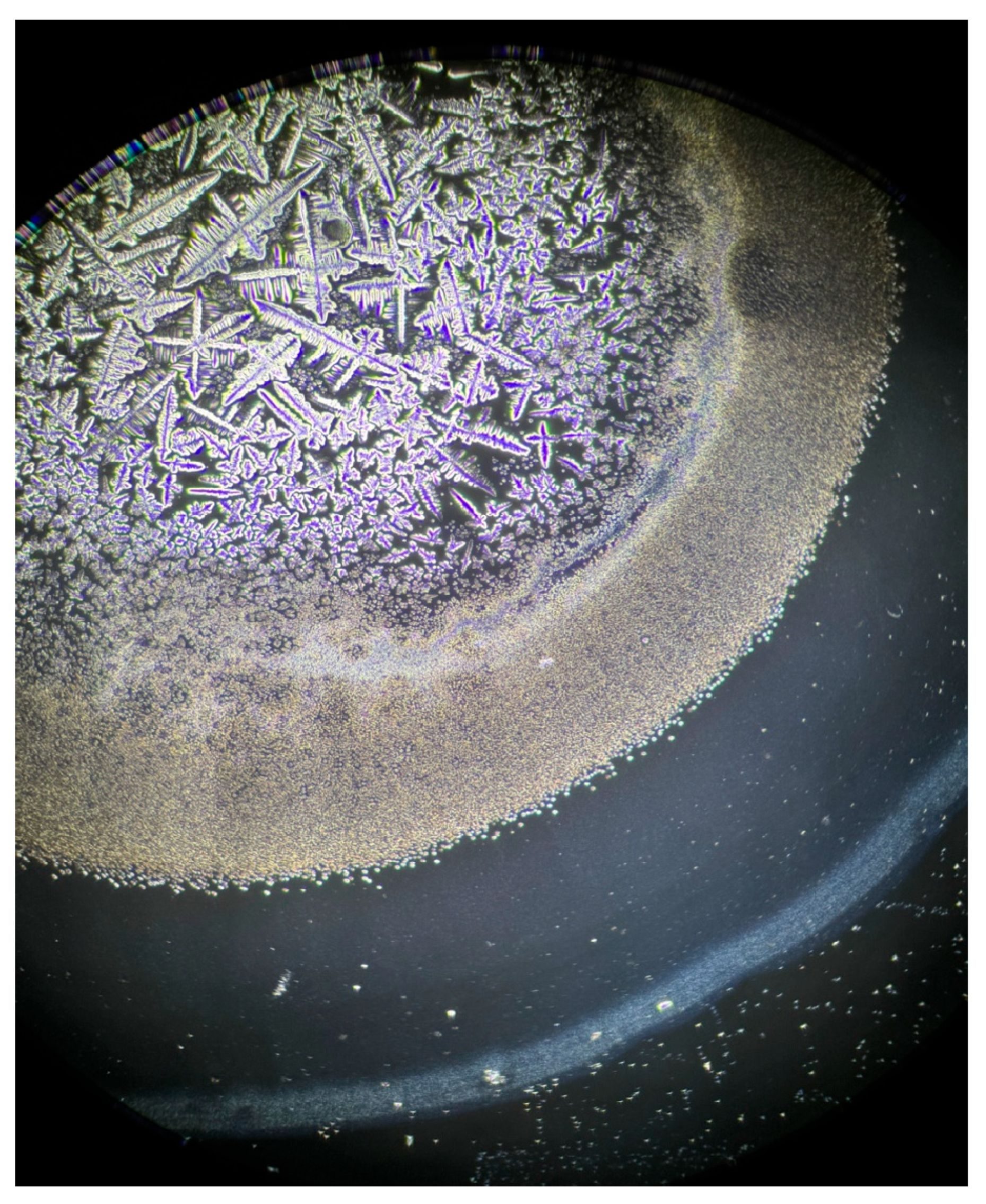
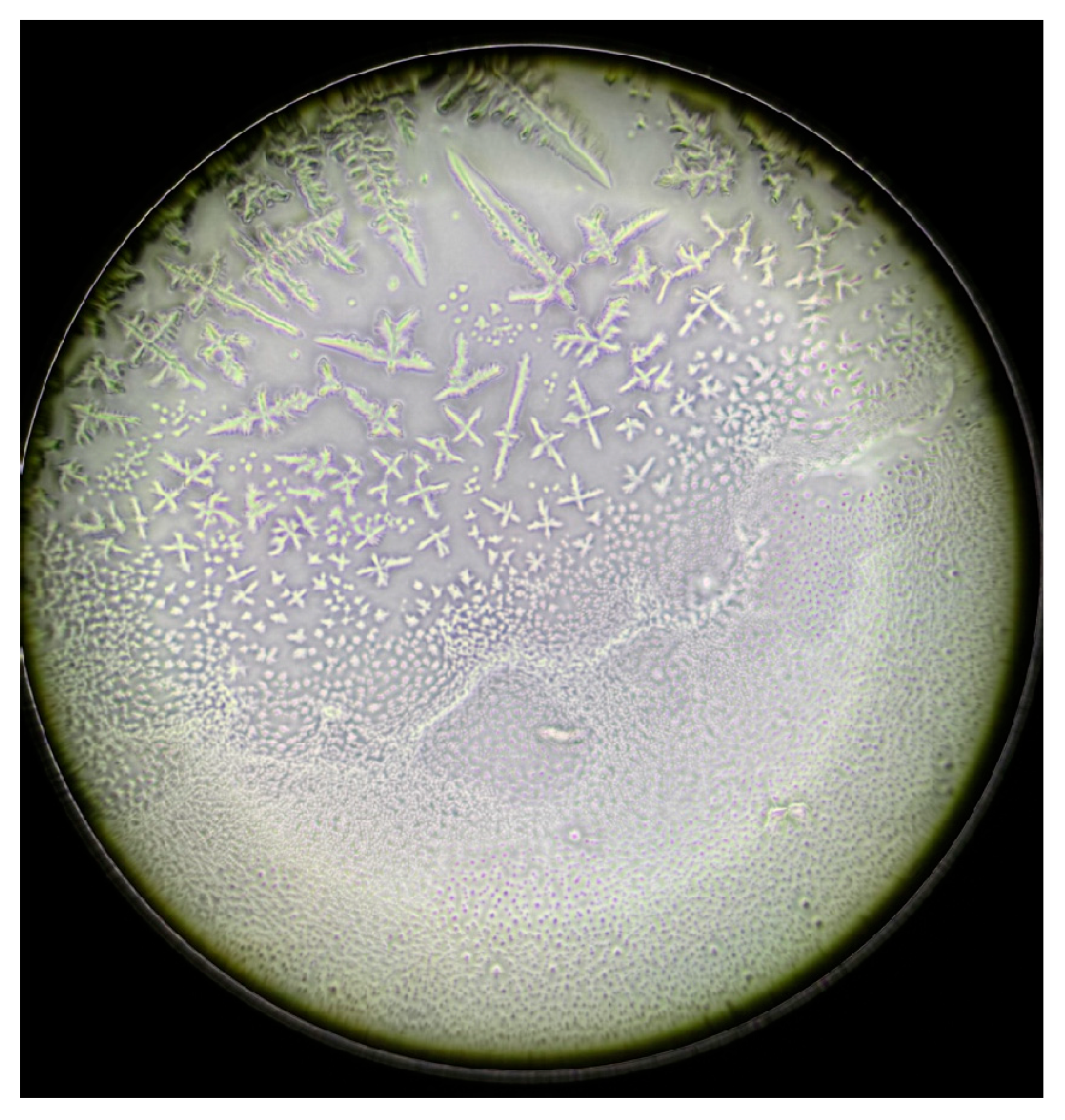


| Characteristics | S-DED Group (n = 10) | DED Group (n = 10) | Control Group (n = 10) | p * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | % | n | Mean ± SD | % | n | Mean ± SD | % | ||
| Gender | 0.436 | |||||||||
| Male | 3 | 30.0 | 1 | 10.0 | 3 | 30.0 | ||||
| Female | 7 | 70.0 | 9 | 90.0 | 7 | 70.0 | ||||
| Age | 0.010 | |||||||||
| ≤40 years | 3 | 30.0 | 4 | 40.0 | 9 | 90.0 | ||||
| >40 years | 8 | 70.0 | 6 | 60.0 | 1 | 10.0 | ||||
| Schirmer Value | 14.4 ± 12.67 | 9.7 ± 7.09 | 24 ± 5.5 | |||||||
| Brightness 155 | 7618.8 ± 3408.59 | 2990 ± 1402.02 | 4198 ± 2169.12 | |||||||
| Brightness 205 | 13,028.8 ± 4189 | 8260 ± 2177.78 | 9065.1 ± 2214.48 | |||||||
| FT | 11,704.3 ± 3745.07 | 5887.2 ± 1909.06 | 5449.6 ± 1721.01 | |||||||
| Compared Groups | Mean Difference ± Std. Error Difference | p |
|---|---|---|
| S-DED vs. DED | 5817.1 ± 1329.29 | p < 0.0005 |
| S-DED vs. Normal | 6254.7 ± 1303.36 | p < 0.0005 |
| DED vs. Normal | 437.6 ± 812.8 | p = 0.597 |
| Group | N | Mean | Standard Deviation | Standard Error of Mean | Min | Max | t (Independent Samples Test) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total | 30 | 7680 | 3849 | 702.6 | 3322 | 17,784 | 17.728 | 0.001 |
| Control | 10 | 5450 | 1721 | 544.2 | 3322 | 7950 | ||
| S-DED | 10 | 11,704 (a) | 3745 | 1184.3 | 3791 | 17,784 | ||
| DED | 10 | 5887 (ns) (a) | 1909 | 606.7 | 3529 | 10,533 |
| Compared Groups | Mean Difference ± Std. Error Difference | p |
|---|---|---|
| S-DED vs. DED | 4628.8 ± 1165.51 | p = 0.001 |
| S-DED vs. Normal | 3420.4 ± 1277.64 | p = 0.015 |
| Normal vs. DED | 1208.4 ± 816.75 | p = 0.159 |
| Group | Model | R | Adjusted R2 | Std. Error | Coefficient B | t | p-Value |
|---|---|---|---|---|---|---|---|
| S-DED | Sex | 0.020 | 0.125 | 0.001 | 272 | 0.104 | 0.920 |
| Sex, Age | 0.197 | 0.236 | 0.039 | 35 | 0.530 | 0.613 | |
| DED | Sex | 0.438 | 0.091 | 0.192 | 1611 | 1.061 | 0.324 |
| Sex, Age | 0.500 | 0.035 | 0.058 | 35 | 0.736 | 0.486 |
| Compared Groups | Mean Difference ± Std. Error Difference | p |
|---|---|---|
| SAR vs. DED | 4768.8 ± 1493 | p = 0.005 |
| SAR vs. Normal | 3963.7 ± 1498.39 | p = 0.016 |
| Normal vs. DED | 805.1 ± 982.17 | p = 0.423 |
| Group | Model | R | Adjusted R2 | Std. Error | Coefficient B | t | p-Value |
|---|---|---|---|---|---|---|---|
| S-DED | Sex | 0.630 | 0.321 | 0.397 | 2079 | 2.231 | 0.061 |
| Sex, Age | 0.646 | 0.251 | 0.020 | 65 | 0.494 | 0.636 | |
| DED | Sex | 0.438 | 0.091 | 0.192 | 1611 | 1.061 | 0.324 |
| Sex, Age | 0.500 | 0.035 | 0.058 | 35 | 0.736 | 0.486 |
| Classification | S-DED Group (n = 10) | DED Group (n = 10) | Control Group (n = 10) | p-Value * | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| MASMALI | |||||||
| Grade 0 | 0 | 0.0 | 4 | 40.0 | 2 | 20.0 | 0.036 |
| Grade 1 | 3 | 30.0 | 3 | 30.0 | 6 | 60.0 | |
| Grade 2 | 7 | 70.0 | 3 | 30.0 | 2 | 20.0 | |
| ROLANDO | |||||||
| Normal | 3 | 30.0 | 7 | 70.0 | 8 | 80.0 | 0.040 |
| Pathologic | 7 | 70.0 | 3 | 30.0 | 2 | 20.0 | |
| SCHIRMER | |||||||
| Severe | 2 | 20.0 | 2 | 20.0 | 0 | 0.0 | 0.001 |
| Moderate | 3 | 30.0 | 5 | 30.0 | 0 | 0.0 | |
| Mild | 2 | 20.0 | 2 | 20.0 | 0 | 0.0 | |
| Normal | 3 | 30.0 | 1 | 30.0 | 10 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu, C.A.; Ganea, C.V.; Donica, V.C.; Alexa, A.I.; Sandu, I.A.; Bilha, M.I.; Bogdănici, C.M. The Clinical Value of the Ferning Test in Monitoring Dry Eye Syndrome in Patients with Sarcoidosis. Life 2025, 15, 1464. https://doi.org/10.3390/life15091464
Sandu CA, Ganea CV, Donica VC, Alexa AI, Sandu IA, Bilha MI, Bogdănici CM. The Clinical Value of the Ferning Test in Monitoring Dry Eye Syndrome in Patients with Sarcoidosis. Life. 2025; 15(9):1464. https://doi.org/10.3390/life15091464
Chicago/Turabian StyleSandu, Călina Anda, Cosmin Victor Ganea, Vlad Constantin Donica, Anisia Iuliana Alexa, Ioana Alexandra Sandu, Madalina Ioana Bilha, and Camelia Margareta Bogdănici. 2025. "The Clinical Value of the Ferning Test in Monitoring Dry Eye Syndrome in Patients with Sarcoidosis" Life 15, no. 9: 1464. https://doi.org/10.3390/life15091464
APA StyleSandu, C. A., Ganea, C. V., Donica, V. C., Alexa, A. I., Sandu, I. A., Bilha, M. I., & Bogdănici, C. M. (2025). The Clinical Value of the Ferning Test in Monitoring Dry Eye Syndrome in Patients with Sarcoidosis. Life, 15(9), 1464. https://doi.org/10.3390/life15091464








