Cutaneous and Lymphangitic Infection Caused by Purpureocillium lilacinum in Immunocompromised Patients: A Case Report with a Narrative Review of the Literature
Abstract
1. Introduction
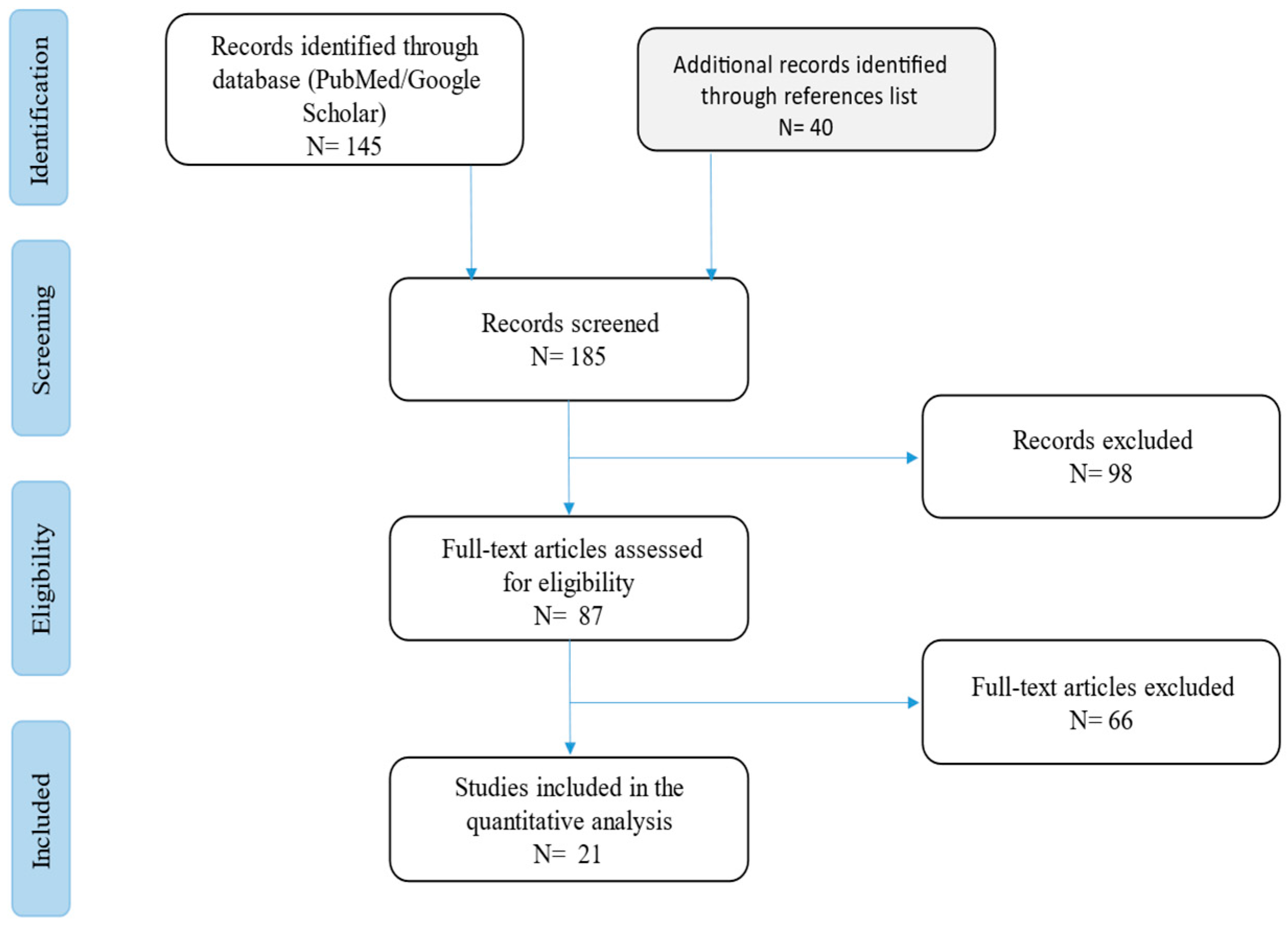
2. Materials and Methods
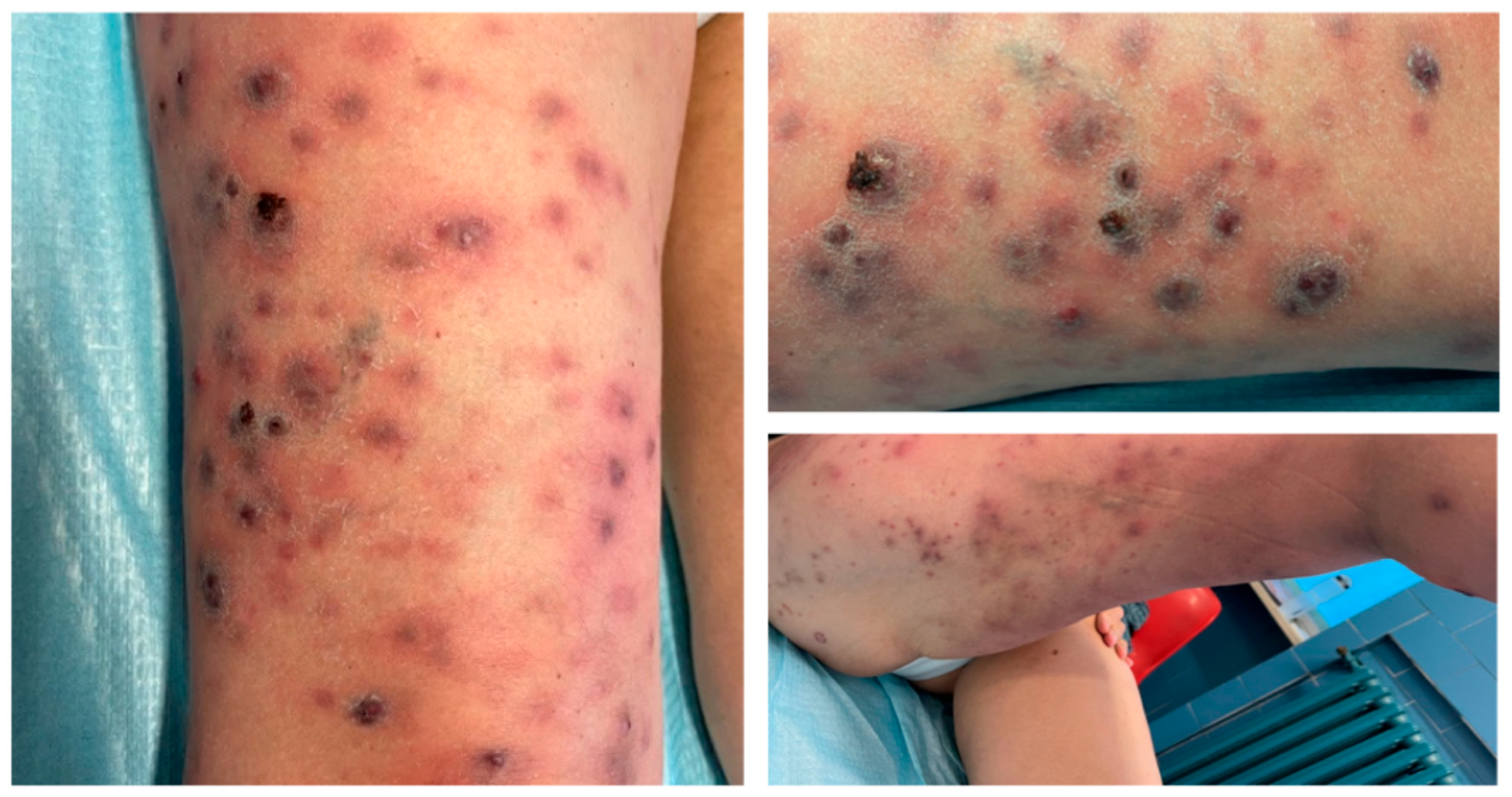
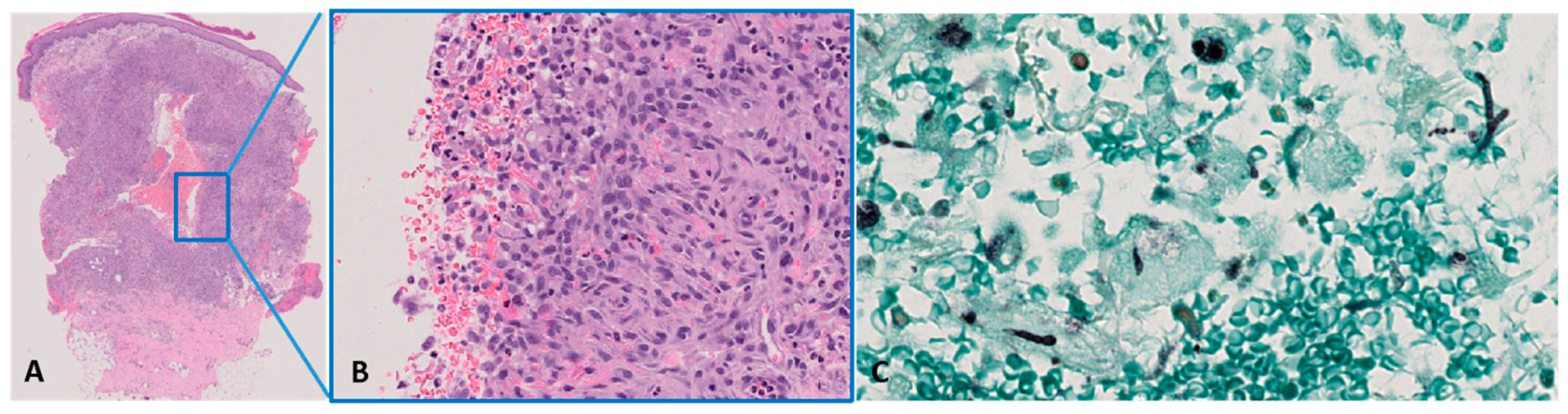
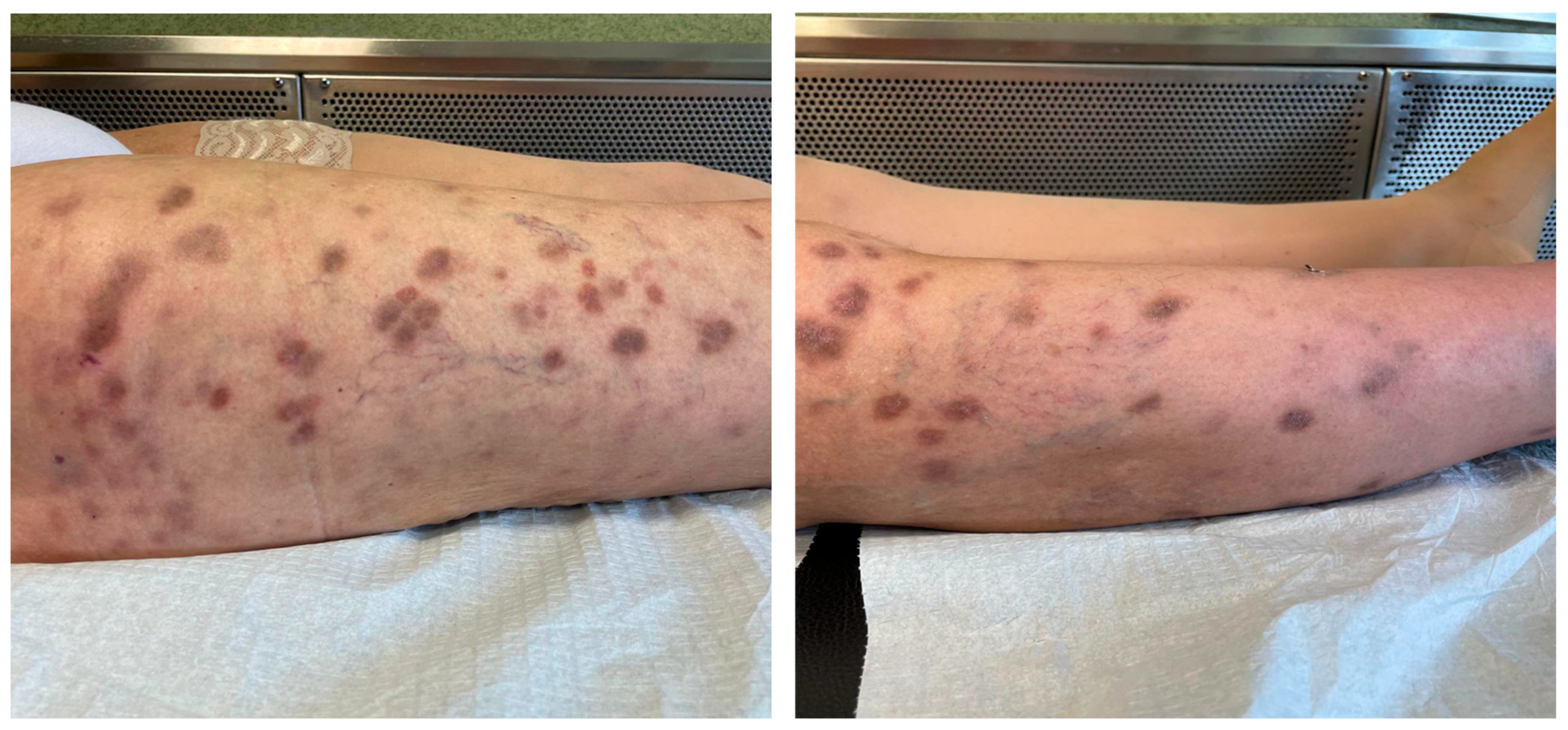
3. Case Report
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a New Genus for the Medically Important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Nnadi, N.E.; Carter, D.A. Climate Change and the Emergence of Fungal Pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- Heutte, N.; André, V.; Dubos Arvis, C.; Bouchart, V.; Lemarié, F.; Legendre, P.; Votier, E.; Louis, M.Y.; Madelaine, S.; Séguin, V.; et al. Assessment of multi-contaminant exposure in a cancer treatment center: A 2-year monitoring of molds, mycotoxins, endotoxins, and glucans in bioaerosols. Environ. Monit. Assess. 2017, 189, 31. [Google Scholar] [CrossRef] [PubMed]
- Sprute, R.; Salmanton-García, J.; Sal, E.; Malaj, X.; Ráčil, Z.; Ruiz De Alegría Puig, C.; Falces-Romero, I.; Barać, A.; Desoubeaux, G.; Kindo, A.J.; et al. Invasive Infections with Purpureocillium lilacinum: Clinical Characteristics and Outcome of 101 Cases from FungiScope® and the Literature. J. Antimicrob. Chemother. 2021, 76, 1593–1603. [Google Scholar] [CrossRef]
- Saghrouni, F.; Saidi, W.; Ben Said, Z.; Gheith, S.; Ben Said, M.; Ranque, S.; Denguezli, M. Cutaneous Hyalohyphomycosis Caused by Purpureocillium lilacinum in an Immunocompetent Patient: Case Report and Review. Med. Mycol. 2013, 51, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Abolghasemi, S.; Tabarsi, P.; Adimi, P.; Kiani, A.; Dolatshahi, S.; Mansouri, D. Pulmonary Paecilomyces in a diabetic patient. Tanaffos 2015, 14, 268–271. [Google Scholar] [PubMed] [PubMed Central]
- Khalique, Z.; Hatipoğlu, S.; Rosendahl, U.; Mohiaddin, R. Unusual Complicated Fungal Endocarditis in a Patient with Vascular Ehlers-Danlos Syndrome. Ann. Thorac. Surg. 2019, 107, e269–e271. [Google Scholar] [CrossRef]
- Chew, R.; Dorman, A.; Woods, M.L. Purpureocillium lilacinum Keratitis: A Case Series and Review of the Literature. Can. J. Ophthalmol. 2016, 51, 382–385. [Google Scholar] [CrossRef]
- Castelli, M.V.; Alastruey-Izquierdo, A.; Cuesta, I.; Monzon, A.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Susceptibility Testing and Molecular Classification of Paecilomyces spp. Antimicrob. Agents Chemother. 2008, 52, 2926–2928. [Google Scholar] [CrossRef]
- Drogari-Apiranthitou, M.; Mantopoulou, F.D.; Skiada, A.; Kanioura, L.; Grammatikou, M.; Vrioni, G.; Mitroussia-Ziouva, A.; Tsakris, A.; Petrikkos, G. In Vitro Antifungal Susceptibility of Filamentous Fungi Causing Rare Infections: Synergy Testing of Amphotericin B, Posaconazole and Anidulafungin in Pairs. J. Antimicrob. Chemother. 2012, 67, 1937–1940. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Corbeddu, M.; Ferreli, C.; Cappai, R.; Ferraguti, P.; Atzori, L.; Pilloni, L.; Rongioletti, F. Fatal Hyalohyphomycosis with Cutaneous Involvement Caused by Purpureocillium Lilacinum in an Immunocompromised Patient with Bullous Pemphigoid. Acta Biomed. 2021, 92, e2021139. [Google Scholar] [CrossRef]
- Desoubeaux, G.; García, D.; Bailly, E.; Augereau, O.; Bacle, G.; De Muret, A.; Bernard, L.; Cano-Lira, J.F.; Garcia-Hermoso, D.; Chandenier, J. Subcutaneous Phaeohyphomycosis Due to Phialemoniopsis Ocularis Successfully Treated by Voriconazole. Med. Mycol. Case Rep. 2014, 5, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.C.; Goyal, S.; Davis, M.D.P.; Walsh, J.S. Cutaneous Hyalohyphomycosis Caused by Paecilomyces lilacinus: Report of Three Cases and Review of the Literature. Int. J. Dermatol. 2004, 43, 648–653. [Google Scholar] [CrossRef]
- Van Schooneveld, T.; Freifeld, A.; Lesiak, B.; Kalil, A.; Sutton, D.A.; Iwen, P.C. Paecilomyces lilacinus Infection in a Liver Transplant Patient: Case Report and Review of the Literature. Transpl. Infect. Dis. 2008, 10, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Demitsu, T.; Nagashima, K.; Okabe, T.; Morisawa, Y.; Ishikawa, N.; Yagisawa, T.; Fukuta, H.; Ohtsuki, M.; Kano, R.; Harada, K. Subcutaneous Hyalohyphomycosis Due to Purpureocillium lilacinum in an Immunocompromised Patient after Renal Transplantation. J. Dermatol. 2017, 44, 725–726. [Google Scholar] [CrossRef]
- Nagamoto, E.; Fujisawa, A.; Yoshino, Y.; Yoshitsugu, K.; Odo, M.; Watanabe, H.; Igata, T.; Noguchi, H. Case of Paecilomyces lilacinus Infection Occurring in Necrotizing Fasciitis-Associated Skin Ulcers on the Face and Surrounding a Tracheotomy Stoma. Med. Mycol. J. 2014, 55, E21–E27. [Google Scholar] [CrossRef]
- Kitami, Y.; Kagawa, S.; Iijima, M. [A Case of Cutaneous Paecilomyces lilacinus Infection on the Face]. Nihon Ishinkin Gakkai Zasshi 2005, 46, 267–272. [Google Scholar] [CrossRef][Green Version]
- Keshtkar-Jahromi, M.; McTighe, A.H.; Segalman, K.A.; Fothergill, A.W.; Campbell, W.N. Unusual Case of Cutaneous and Synovial Paecilomyces lilacinus Infection of Hand Successfully Treated with Voriconazole and Review of Published Literature. Mycopathologia 2012, 174, 255–258. [Google Scholar] [CrossRef]
- Albert, R.; Lemaignen, A.; Desoubeaux, G.; Bailly, E.; Bernard, L.; Lacasse, M. Chronic Subcutaneous Infection of Purpureocillium lilacinum in an Immunocompromised Patient: Case Report and Review of the Literature. Med. Mycol. Case Rep. 2022, 38, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Hilmarsdottir, I.; Thorsteinsson, S.B.; Asmundsson, P.; Bodvarsson, M.; Arnadottir, M. Cutaneous Infection Caused by Paecilomyces lilacinus in a Renal Transplant Patient: Treatment with Voriconazole. Scand. J. Infect. Dis. 2000, 32, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Accetta, J.; Powell, E.; Boh, E.; Bull, L.; Kadi, A.; Luk, A. Isavuconazonium for the Treatment of Purpureocillium lilacinum Infection in a Patient with Pyoderma Gangrenosum. Med. Mycol. Case Rep. 2020, 29, 18–21. [Google Scholar] [CrossRef]
- Trinh, S.A.; Angarone, M.P. Purpureocillium lilacinum Tattoo-Related Skin Infection in a Kidney Transplant Recipient. Transpl. Infect. Dis. 2017, 19, e12689. [Google Scholar] [CrossRef]
- Lavergne, R.A.; Cassaing, S.; Nocera, T.; Pauwels, C.; Cointault, O.; Basse, G.; Lavayssière, L.; Berry, A.; Kamar, N.; Lamant, L.; et al. Simultaneous Cutaneous Infection Due to Paecilomyces lilacinus and Alternaria in a Heart Transplant Patient. Transpl. Infect. Dis. 2012, 14, E156–E160. [Google Scholar] [CrossRef]
- Ezzedine, K.; Belin, E.; Guillet, S.; D’Almeida, M.; Droitcourt, C.; Accocebery, I.; Milpied, B.; Jouary, T.; Malvy, D.; Taieb, A. Cutaneous Hyphomycosis Due to Paecilomyces lilacinus. Acta Derm. Venereol. 2012, 92, 156–157. [Google Scholar] [CrossRef]
- Ounissi, M.; Abderrahim, E.; Trabelsi, S.; Khaled, S.; Bezzine, H.; Ben Hamida, F.; Hedri, H.; Ben Abdallah, T.; Ben Maïz, H.; Kheder, A. Hyalohyphomycosis Caused by Paecilomyces lilacinus after Kidney Transplantation. Transplant. Proc. 2009, 41, 2917–2919. [Google Scholar] [CrossRef]
- Martin, C.A.; Roberts, S.; Greenberg, R.N. Voriconazole Treatment of Disseminated Paecilomyces Infection in a Patient with Acquired Immunodeficiency Syndrome. Clin. Infect. Dis. 2002, 35, 78–81. [Google Scholar] [CrossRef]
- Sotello, D.; Cappel, M.; Huff, T.; Meza, D.; Alvarez, S.; Libertin, C.R. Cutaneous Fungal Infection in an Immunocompromised Host. JMM Case Rep. 2017, 4, e005101. [Google Scholar] [CrossRef]
- Xia, X.J.; Liu, Z.H. Cutaneous Hyalophomycosis Caused by Purpureocillium lilacinum in an Autoimmune Hemolytic Anemia Patient with Previous Pulmonary Tuberculosis and Cutaneous Cryptococcosis Infection. Mycopathologia 2021, 186, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Lemaigre, C.; Suarez, F.; Martellosio, J.P.; Barbarin, C.; Brunet, K.; Chomel, J.C.; Hainaut, E.; Rammaert, B.; Roblot, F.; Torregrosa-Diaz, J.M. Late Onset of Chronic Granulomatous Disease Revealed by Paecilomyces lilacinus Cutaneous Infection. J. Clin. Immunol. 2022, 42, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, F.; Lu, X.; Liu, H.; Zhang, F. Case Report: Cutaneous Mycosis Caused by Purpureocillium lilacinum. Am. J. Trop. Med. Hyg. 2023, 108, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Lin, S.R.; Hung, S.J. Successful Treatment of Recurrent Cutaneous Purpureocillium lilacinum (Paecilomyces lilacinus) Infection with Posaconazole and Surgical Debridement. Acta Derm. Venereol. 2019, 99, 1313–1314. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Hoenigl, M.; Thompson, G.R., 3rd; Carvalho, A.; Jenks, J.D. Let’s talk about sex characteristics—As a risk factor for invasive fungal diseases. Mycoses 2022, 65, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Alexander, B.D. Antifungal activities of SCY-078 (MK-3118) and standard antifungal agents against clinical non-Aspergillus mold isolates. Antimicrob. Agents Chemother. 2015, 59, 4308–4311. [Google Scholar] [CrossRef]
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.A.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M.; et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 2011, 55, 4652–4658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Age Sex | Comorbidities | Hobby | Skin Lesions | Pain | Site | Diffusion | Therapy | Outcome | REF | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79, M | Diabetes mellitus; Pemphigus vulgaris | Gardening | Erythematous pustule, warm, single | no | Hand, monolateral | Bilateral leg and ankle cellulitis, encephalic | Itraconazole 200 mg bid Voriconazole 6 mg/kg every 12 h | Dead | [12] |
| 2 | 84, M | Steroid therapy; Diabetes mellitus | NA | Swelling and bursitis of the first metatarsophalangeal joint | yes | Lower extremity, monolateral | No | Surgery plus voriconazole 200 mg bid | Recovered | [13] |
| 3 | 65, M | Steroid therapy for temporal arteritis | NA | Erythematous and oedematous area with papules and pustules | no | Lower extremity, monolateral | Cellulitis | Itraconazole 200 bid plus surgery | Recovered | [14] |
| 4 | 56, M | Liver transplant | NA | Erythematous, tender single nodule | no | Knee, monolateral | Multiple nodules following lymphatic distribution | Voriconazole 200 mg bid alone and in association with Terbinafine Voriconazole 300 mg bid | Recovered | [15] |
| 5 | 72, M | Renal transplant | NA | Erythematous nodule | no | Forearms, monolateral | Multiple reddish nodules | Itraconazole 200 mg daily Voriconazole 400 mg/day and Terbinafine 250 mg/day | Recovered | [16] |
| 6 | 28, M | Bone marrow transplant; Previous Pseudomonas aeruginosa necrotising fasciitis on the face | None | Erythematous papules and pustules, crusted at the margin of previous ulcers | no | Face and site of closed tracheostomy | Multiple necrotising ulcers | Itraconazole 200 bid Micafungin 50 mg/day LAMPHB 150 mg/day Voriconazole 400 mg/day | Recovered | [17] |
| 7 | 74, F | Immunosuppressed therapy for autoimmune haemolytic anaemia | NA | Erythematous brownish plaque, single | yes | Hemiface | Multiple papules and abscesses around the plaque on the hemiface | Griseofulvin 500 mg/daily Itraconazole 200 mg/day for 11 weeks, then 400 mg daily, 7 days a month | Recovered | [18] |
| 8 | 71, F | Steroid therapy for giant cell arteritis | NA | Erythematous nodule | no | Elbow, monolateral | NA | Micafungin 50 mg/day Itraconazole (dosage not available) | NA | [19] |
| 9 | 52, M | Renal transplant | Gardening | Oedematous and erythematous area | no | Lower extremity, monolateral | Cellulitis with multiple fistula orifices | Posaconazole 300 mg/day Surgery for local relapse | Recovered | [20] |
| 10 | 59, M | Renal transplant | NA | Erythematous papules, pustules and small ulcers | yes | Foot and interdigital space, monolateral | Multiple lesions on foot and leg, sepsis due to Candida spp. and Gram-negative bacteria | Itraconazole 400–600 mg/day Voriconazole 400 mg/day LAMPHB | Dead | [21] |
| 11 | 50, M | CLL; previous pyoderma gangrenosum | NA | Purpuric nodules and a necrotic ulcer | yes | Lower extremity, monolateral | Extended necrotic ulcer | LAMPHB 6 mg/kg/day Micafungin 150 mg/day Voriconazole 400 mg bid Isavuconazole IV 372 mg/day plus surgery | Recovered | [22] |
| 12 | 33, M | Renal transplant | Recent tattoo | Erythematous papules, pustules clustered over tattoo | no | Left forearm and shin, right ankle | None | Voriconazole 400 mg/day | Recovered | [23] |
| 13 | 63, M | Heart transplantation; Diabetes mellitus | NA | Erythematous nodules | yes | Leg and elbow | Multiple necrotic ulcers | Voriconazole 400 mg/day Intralesional Voriconazole (1 mL) followed by oral treatment with Terbinafine 250 mg a day the first month, then 125 mg/day | Recovered | [24] |
| 14 | 60, M | Pulse steroid therapy for rheumatoid arthritis | NA | Erythematous nodules with pseudo-verrucous aspect | NA | Hemiface | Multiple necrotic ulcers on face and hand | Voriconazole 400 mg/day Posaconazole 400 mg/bid | NA | [25] |
| 15 | 48, F | Renal transplant | NA | Erythematous oedematous area | yes | Lower extremity, monolateral | Extensive cellulitis with multiple ulcers | Itraconazole 400 mg/day Voriconazole (dosage non reported) | Recovered | [26] |
| 16 | 40, M | AIDS | NA | Erythematous nodules | yes | Lower extremity, monolateral | Multiple ulcerated nodules on entire leg and body | Itraconazole 200 mg bid LAMPHB 5 mg/kg/day Voriconazole 200–300 mg/bid | Dead | [27] |
| 17 | 69, M | Liver transplant recipient | None, lives with a dog | Erythematous nodules with pseudo-verrucous aspect | yes | Hand, monolateral | Multiple nodules tracking on arm and axilla | Voriconazole 400 mg/day | Recovered | [28] |
| 18 | 53, M | Steroid therapy for autoimmune haemolytic anaemia | None | Erythematous pustule and ulcerated nodule | no | Neck | None | Itraconazole 400 mg/day Posaconazole 300 mg/die | Recovered | [29] |
| 19 | 67, F | X-linked chronic granulomatous disease | Sheep farmer | Erythematous nodules with pustules | yes | Upper extremity (thumb) | None | Voriconazole 400 mg/day | Recovered | [30] |
| 20 | 51, F | Steroid therapy for nephrotic syndrome | NA | Recurrent papules, pustules, and ulceration | NA | Finger and forearm, monolateral | None | Voriconazole (dosage not available) | Recovered | [31] |
| 21 | NA, M | Steroid therapy for Evans’ Syndrome | Farmer | Erythematous papules and pustules | yes | Forearm, monolateral | Multiple ulcerated lesions | Voriconazole 400 mg/day then Posaconazole 300 mg/day with surgery | Recovered | [32] |
| n (%) | |
|---|---|
| Patient age | |
| Years, mean (min-max) | 59 (28–84) years |
| Sex | |
| Male | 16 (76.2) |
| Female | 6 (27.2) |
| Race/ethnicity | |
| Caucasian | 9 (40.9) |
| Black/African American | 6 (27.2) |
| Asiatic | 7 (31.8) |
| Immunosuppression | |
| Steroids | 9/22 (40.9) |
| Solid transplant | 9/22 (40.9) |
| Haematological condition | 3/22 (13.6) |
| HIV/AIDS | 1/22 (0.5) |
| Hobbies | |
| Unknown/Other | 15/22 (68) |
| Farmer | 3/22 (13.6) |
| Gardening | 2/22 (9) |
| Skin lesions | |
| Papules/pustules | 9/22 (40.9) |
| Nodules | 10/22 (45,4) |
| Erythematous purpuric plaques | 3/22, 13.6) |
| Other | 1/22 (0.5) |
| Localization | |
| Upper extremities | 7/22 (31.8) |
| Lower extremities | 10/22 (45.4) |
| Pain associated | |
| Yes | 10/22 (45,4) |
| No | 9/22 (40.9) |
| Local diffusion | 11/22 (50) |
| Systemic diffusion/secondary foci | 5/22 (22.7) |
| Treatment | |
| Azoles | 22/22 (100) |
| Terbinafine | 3/22 (13.6) |
| Echinocandins | 3/22 (13.6) |
| Liposomal amphotericin | 4/22 (18.1) |
| Griseofulvin | 1/22 (0.5) |
| Surgical debridement | 2/22 (9) |
| Mortality | 3/22 (13.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupia, T.; Sarda, C.; Canta, F.; Casarotto, M.; Accardo, G.; Roccuzzo, G.; Macagno, N.; Gelato, F.; Senetta, R.; Ottobrelli, A.; et al. Cutaneous and Lymphangitic Infection Caused by Purpureocillium lilacinum in Immunocompromised Patients: A Case Report with a Narrative Review of the Literature. Life 2025, 15, 1453. https://doi.org/10.3390/life15091453
Lupia T, Sarda C, Canta F, Casarotto M, Accardo G, Roccuzzo G, Macagno N, Gelato F, Senetta R, Ottobrelli A, et al. Cutaneous and Lymphangitic Infection Caused by Purpureocillium lilacinum in Immunocompromised Patients: A Case Report with a Narrative Review of the Literature. Life. 2025; 15(9):1453. https://doi.org/10.3390/life15091453
Chicago/Turabian StyleLupia, Tommaso, Cristina Sarda, Francesca Canta, Marco Casarotto, Guido Accardo, Gabriele Roccuzzo, Nicole Macagno, Federica Gelato, Rebecca Senetta, Antonio Ottobrelli, and et al. 2025. "Cutaneous and Lymphangitic Infection Caused by Purpureocillium lilacinum in Immunocompromised Patients: A Case Report with a Narrative Review of the Literature" Life 15, no. 9: 1453. https://doi.org/10.3390/life15091453
APA StyleLupia, T., Sarda, C., Canta, F., Casarotto, M., Accardo, G., Roccuzzo, G., Macagno, N., Gelato, F., Senetta, R., Ottobrelli, A., De Rosa, F. G., Corcione, S., Ribero, S., Quaglino, P., & Fava, P. (2025). Cutaneous and Lymphangitic Infection Caused by Purpureocillium lilacinum in Immunocompromised Patients: A Case Report with a Narrative Review of the Literature. Life, 15(9), 1453. https://doi.org/10.3390/life15091453








