Effect of Different Exercise Modalities on Inflammatory Markers in Individuals with Depressive Disorder: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.1.1. Inclusion Criteria and Rationale
2.1.2. Exclusion Criteria
2.2. Search Strategy
2.3. Quality Assessment
2.4. Data Extraction
2.5. Data Synthesis and Analysis
3. Results
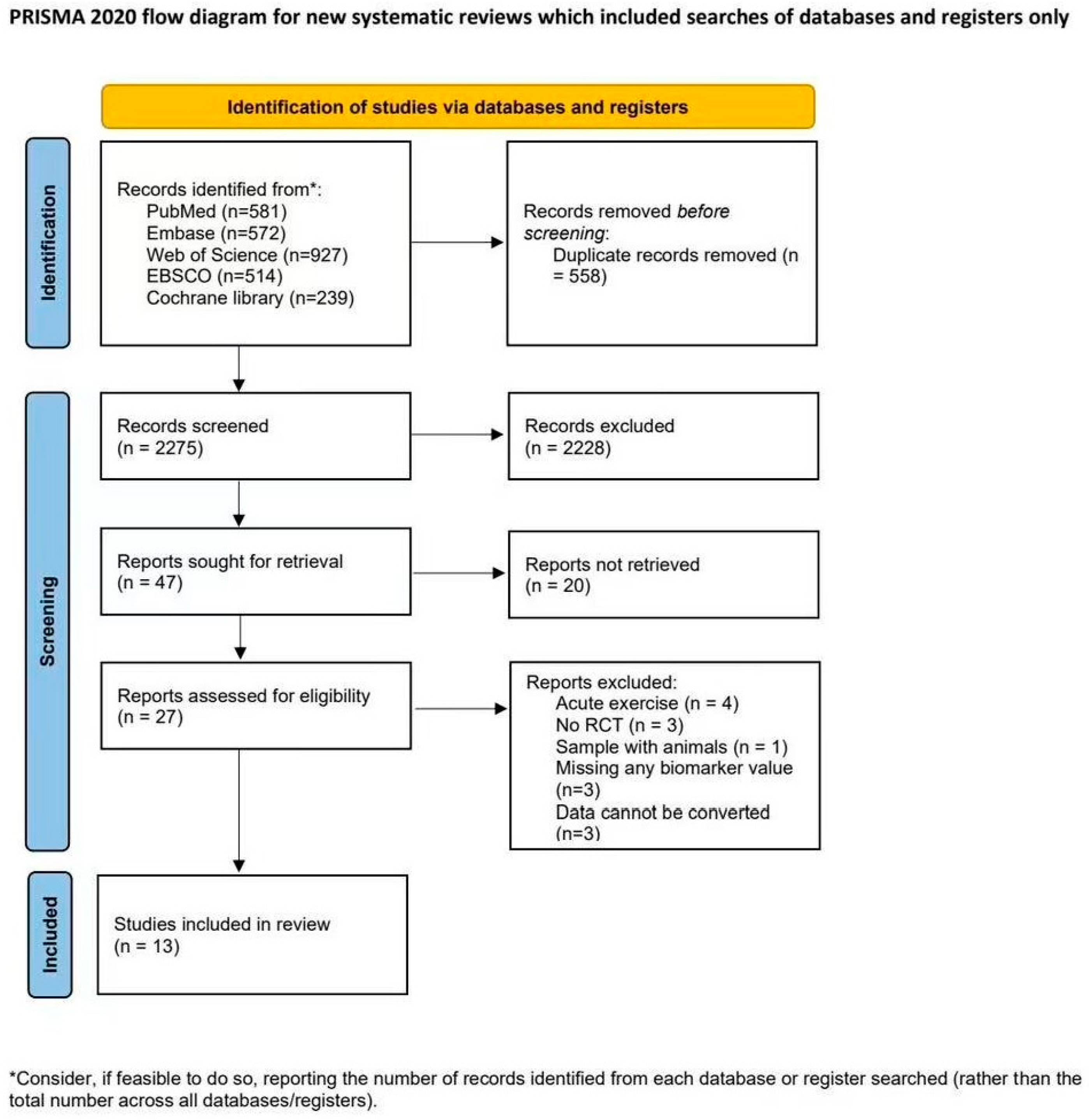
3.1. The Process of Study Selection
3.2. Characteristics of Included Studies
3.3. Description of Exercise Interventions
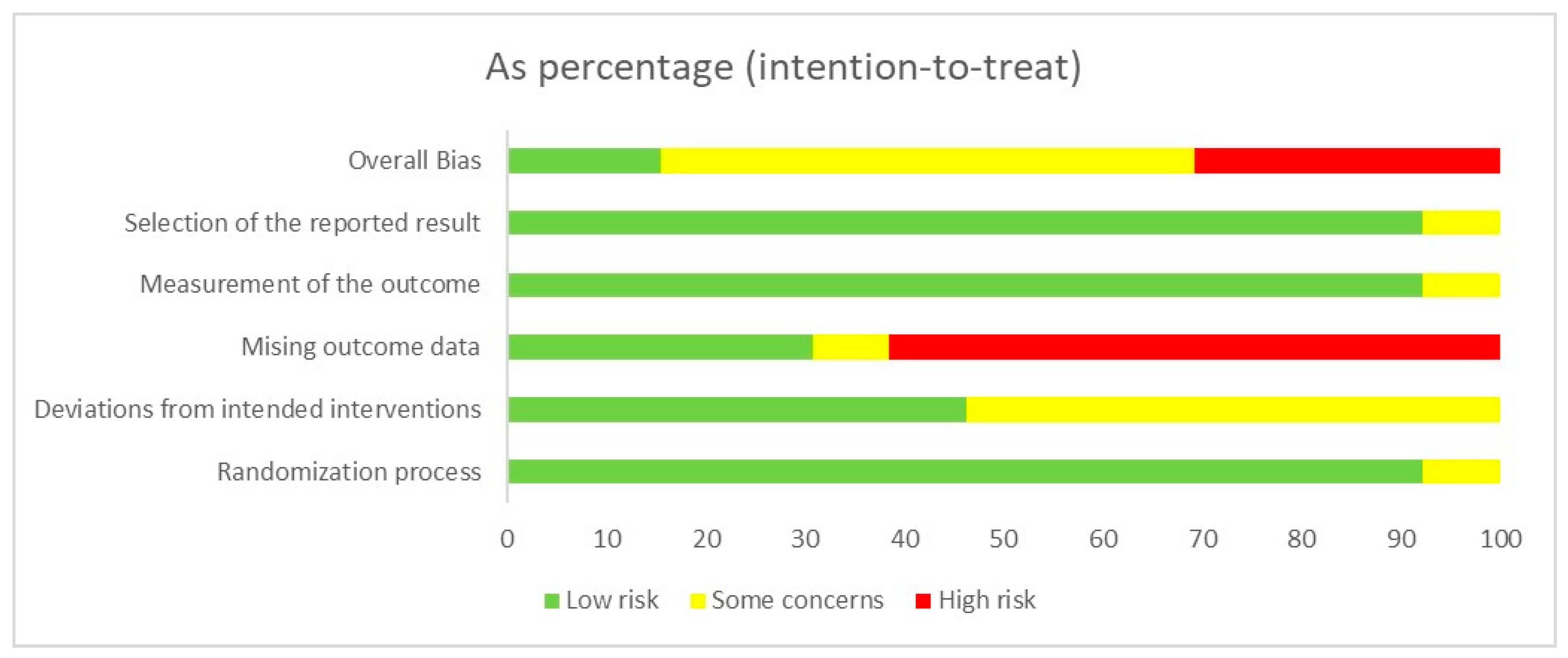
3.4. Risk of Bias and Quality Assessment
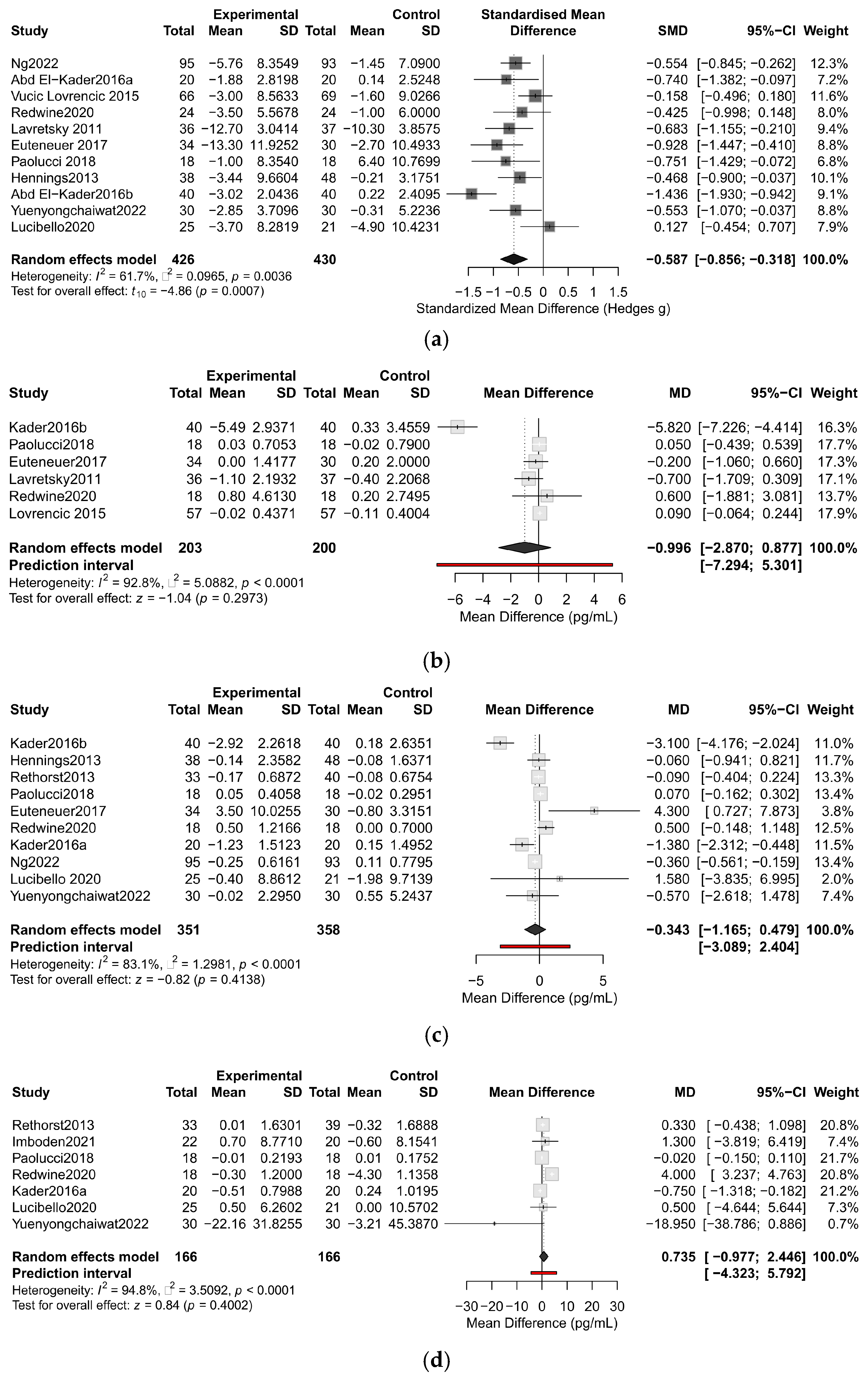
3.5. Primary Outcome
3.6. Subgroup Analysis
3.7. Meta-Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | Adenosine Monophosphate-Activated Protein Kinase |
| BCAT | Branched-Chain Amino Acid Transaminase |
| BCKDH | Branched-Chain α-Keto Acid Dehydrogenase Complex |
| BDNF | Brain-Derived Neurotrophic Factor |
| CCL3 | C-C Motif Chemokine Ligand 3 |
| CI | Confidence Interval |
| CRP | C-Reactive Protein |
| HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor alpha |
| TNF-β | Tumor Necrosis Factor beta (Lymphotoxin-α) |
References
- World Health Organization. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 28 August 2025).
- Chan, V.K.Y.; Leung, M.Y.M.; Chan, S.S.M.; Yang, D.; Knapp, M.; Luo, H.; Craig, D.; Chen, Y.; Bishai, D.M.; Wong, G.H.Y.; et al. Projecting the 10-year costs of care and mortality burden of depression until 2032: A Markov modelling study developed from real-world data. Lancet Reg. Health West. Pac. 2024, 45, 101026. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Association, A.P.; Force, D.-T. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.G.; van Pelt, J.; DeRijk, R.H.; Verhagen, J.C.M.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W.J.H. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity: Results from a Large Cohort Study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef]
- Schiepers, O.J.G.; Wichers, M.C.; Maes, M. Cytokines and major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 201–217. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lázaro, E.; Arregi, A.; Beitia, G.; Vegas, O.; Azpiroz, A.; Garmendia, L. Individual differences in chronically defeated male mice: Behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress 2011, 14, 537–548. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Lichtblau, N.; Minkwitz, J.; Chittka, T.; Thormann, J.; Kirkby, K.C.; Sander, C.; Mergl, R.; Faßhauer, M.; Stumvoll, M.; et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J. Psychiatr. Res. 2014, 55, 29–34. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, J.; Sun, Y.; Zhang, Y.; Shan, F.; Ge, J.; Xia, Q. Serum cytokines-based biomarkers in the diagnosis and monitoring of therapeutic response in patients with major depressive disorder. Int. Immunopharmacol. 2023, 118, 110108. [Google Scholar] [CrossRef]
- Corrigan, M.; O’Rourke, A.M.; Moran, B.; Fletcher, J.M.; Harkin, A. Inflammation in the pathogenesis of depression: A disorder of neuroimmune origin. Neuronal Signal. 2023, 7, NS20220054. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jia, M.; Cai, M.; Zhu, T.; Yang, J.-J.; Hashimoto, K. Central-peripheral neuroimmune dynamics in psychological stress and depression: Insights from current research. Mol. Psychiatry 2025, 30, 4881–4898. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des. 2005, 11, 973–984. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Immune regulation of central nervous system functions: From sickness responses to pathological pain. J. Intern. Med. 2005, 257, 139–155. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Blood-Brain Barrier Permeability in Aging and Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2014, 1, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Lewitus, G.M.; Cohen, H.; Schwartz, M. Reducing post-traumatic anxiety by immunization. Brain Behav. Immun. 2008, 22, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Filatova, E.V.; Shadrina, M.I.; Slominsky, P.A. Major Depression: One Brain, One Disease, One Set of Intertwined Processes. Cells 2021, 10, 1283. [Google Scholar] [CrossRef]
- Felger, J.C.; Li, Z.; Haroon, E.; Woolwine, B.J.; Jung, M.Y.; Hu, X.; Miller, A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 2016, 21, 1358–1365. [Google Scholar] [CrossRef]
- Parrott, J.M.; Redus, L.; Santana-Coelho, D.; Morales, J.; Gao, X.; O’Connor, J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry 2016, 6, e918. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, Z.; Sun, L.; Zhou, L.; Wang, G.; Xiao, L.; Wang, H. The Effects and Mechanisms of Exercise on the Treatment of Depression. Front. Psychiatry 2021, 12, 705559. [Google Scholar] [CrossRef]
- Netz, Y. Is the Comparison between Exercise and Pharmacologic Treatment of Depression in the Clinical Practice Guideline of the American College of Physicians Evidence-Based? Front. Pharmacol. 2017, 8, 257. [Google Scholar] [CrossRef]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef]
- Harris, E. Meta-Analysis: Exercise as Effective as Therapy for Treating Depression. JAMA 2024, 331, 908. [Google Scholar] [CrossRef] [PubMed]
- Noetel, M.; Sanders, T.; Gallardo-Gómez, D.; Taylor, P.; del Pozo Cruz, B.; van den Hoek, D.; Smith, J.J.; Mahoney, J.; Spathis, J.; Moresi, M.; et al. Effect of exercise for depression: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2024, 384, e075847. [Google Scholar] [CrossRef] [PubMed]
- Heissel, A.; Heinen, D.; Brokmeier, L.L.; Skarabis, N.; Kangas, M.; Vancampfort, D.; Stubbs, B.; Firth, J.; Ward, P.B.; Rosenbaum, S.; et al. Exercise as medicine for depressive symptoms? A systematic review and meta-analysis with meta-regression. Br. J. Sports Med. 2023, 57, 1049–1057. [Google Scholar] [CrossRef]
- Langston, P.K.; Mathis, D. Immunological regulation of skeletal muscle adaptation to exercise. Cell Metab. 2024, 36, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Liu, Y.; Zhang, Z. Effects of Exercise-Induced ROS on the Pathophysiological Functions of Skeletal Muscle. Oxid. Med. Cell Longev. 2021, 2021, 3846122. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L.; Jackson, M. Reactive oxygen species promote endurance exercise-induced adaptations in skeletal muscles. J. Sport. Health Sci. 2024, 13, 780–792. [Google Scholar] [CrossRef]
- Supruniuk, E.; Górski, J.; Chabowski, A. Endogenous and Exogenous Antioxidants in Skeletal Muscle Fatigue Development during Exercise. Antioxidants 2023, 12, 501. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Borrás, C.; Pallardó, F.V.; Sastre, J.; Ji, L.L.; Viña, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567, 113–120. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Fischer, C.P. Beneficial health effects of exercise—The role of IL-6 as a myokine. Trends Pharmacol. Sci. 2007, 28, 152–156. [Google Scholar] [CrossRef]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef]
- Ringleb, M.; Javelle, F.; Haunhorst, S.; Bloch, W.; Fennen, L.; Baumgart, S.; Drube, S.; Reuken, P.A.; Pletz, M.W.; Wagner, H.; et al. Acute resistance exercise-induced changes in IL-6, IL-10, and IL-1ra in healthy adults: A systematic review and meta-analysis. medRxiv 2023. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an energy allocator in muscle tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Gleeson, M. Immune function in sport and exercise. J. Appl. Physiol. 2007, 103, 693–699. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Liu, S.; Niu, Y.; Fu, L. Metabolic Adaptations to Exercise Training. J. Sci. Sport. Exerc. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Loukov, D.; Bowdish, D.M.E.; Heisz, J.J. Exercise reduces depression and inflammation but intensity matters. Biol. Psychol. 2018, 133, 79–84. [Google Scholar] [CrossRef]
- Euteneuer, F.; Dannehl, K.; Del Rey, A.; Engler, H.; Schedlowski, M.; Rief, W. Immunological effects of behavioral activation with exercise in major depression: An exploratory randomized controlled trial. Transl. Psychiatry 2017, 7, e1132. [Google Scholar] [CrossRef]
- Ng, S.M.; Yin, M.X.C.; Chan, J.S.M.; Chan, C.H.Y.; Fong, T.C.T.; Li, A.; So, K.F.; Yuen, L.P.; Chen, J.P.; Chung, K.F.; et al. Impact of mind-body intervention on proinflammatory cytokines interleukin 6 and 1β: A three-arm randomized controlled trial for persons with sleep disturbance and depression. Brain Behav. Immun. 2022, 99, 166–176. [Google Scholar] [CrossRef]
- Rethorst, C.D.; Toups, M.S.; Greer, T.L.; Nakonezny, P.A.; Carmody, T.J.; Grannemann, B.D.; Huebinger, R.M.; Barber, R.C.; Trivedi, M.H. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol. Psychiatry 2013, 18, 1119–1124. [Google Scholar] [CrossRef]
- Ren, J.; Xiao, H. Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life 2023, 13, 1505. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Tan, J.; Zhou, H.-H.; Cao, M.; Zou, Y. Long-term exercise training and inflammatory biomarkers in healthy subjects: A meta-analysis of randomized controlled trials. Front. Psychol. 2023, 14, 1253329. [Google Scholar] [CrossRef]
- Fernandes, B.M.; Scotti-Muzzi, E.; Soeiro-de-Souza, M.G. Effects of antidepressant drug therapy with or without physical exercise on inflammatory biomarkers in major depressive disorder: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2022, 78, 339–349. [Google Scholar] [CrossRef]
- Hartmann, T.E.; Robertson, C.V.; Miller, T.D.; Hunter, J.R.; Skein, M. Associations between exercise, inflammation and symptom severity in those with mental health disorders. Cytokine 2021, 146, 155648. [Google Scholar] [CrossRef]
- Carrera-Bastos, P.; Bottino, B.; Stults-Kolehmainen, M.; Schuch, F.B.; Mata-Ordoñez, F.; Müller, P.T.; Blanco, J.-R.; Boullosa, D. Inflammation and depression: An evolutionary framework for the role of physical activity and exercise. Front. Psychol. 2025, 16, 1554062. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands; American College of Sports Medicine: Indianapolis, IN, USA, 2018. [Google Scholar]
- Radua, J. PRISMA 2020—An updated checklist for systematic reviews and meta-analyses. Neurosci. Biobehav. Rev. 2021, 124, 324–325. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 14898. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Stewart, S.A.; Bagg, M.K.; Stanojevic, S.; Yamato, T.P.; Saragiotto, B.T. Some types of exercise are more effective than others in people with chronic low back pain: A network meta-analysis. J. Physiother. 2021, 67, 252–262. [Google Scholar] [CrossRef]
- Santos, T.M.; Gomes, P.S.C.; Oliveira, B.R.R.; Ribeiro, L.G.; Thompson, W.R. A New Strategy for the Implementation of an Aerobic Training Session. J. Strength. Cond. Res. 2012, 26, 87–93. [Google Scholar] [CrossRef]
- Blomstrand, P.; Tesan, D.; Nylander, E.M.; Ramstrand, N. Mind body exercise improves cognitive function more than aerobic- and resistance exercise in healthy adults aged 55 years and older—An umbrella review. Eur. Rev. Aging Phys. Act. 2023, 20, 15. [Google Scholar] [CrossRef]
- Coates, A.M.; Joyner, M.J.; Little, J.P.; Jones, A.M.; Gibala, M.J. A Perspective on High-Intensity Interval Training for Performance and Health. Sports Med. 2023, 53, 85–96. [Google Scholar] [CrossRef]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.J.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008, 3, CD005381. [Google Scholar] [CrossRef]
- Ye, M.; Wang, L.; Xiong, J.; Zheng, G. The effect of mind-body exercise on memory in older adults: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 1163–1173. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr. Health Sci. 2016, 16, 1045–1055. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; Al-Jiffri, O.H. Exercise alleviates depression related systemic inflammation in chronic obstructive pulmonary disease patients. Afr. Health Sci. 2016, 16, 1078–1088. [Google Scholar] [CrossRef]
- Lavretsky, H.; Alstein, L.L.; Olmstead, R.E.; Ercoli, L.M.; Riparetti-Brown, M.; Cyr, N.S.; Irwin, M.R. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: A randomized controlled trial. Am. J. Geriatr. Psychiatry 2011, 19, 839–850. [Google Scholar] [CrossRef]
- Redwine, L.S.; Pung, M.A.; Wilson, K.; Bangen, K.J.; Delano-Wood, L.; Hurwitz, B. An exploratory randomized sub-study of light-to-moderate intensity exercise on cognitive function, depression symptoms and inflammation in older adults with heart failure. J. Psychosom. Res. 2020, 128, 109883. [Google Scholar] [CrossRef]
- Imboden, C.; Gerber, M.; Beck, J.; Eckert, A.; Lejri, I.; Pühse, U.; Holsboer-Trachsler, E.; Hatzinger, M. Aerobic Exercise and Stretching as Add-On to Inpatient Treatment for Depression Have No Differential Effects on Stress-Axis Activity, Serum-BDNF, TNF-Alpha and Objective Sleep Measures. Brain Sci. 2021, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Yuenyongchaiwat, K.; Akekawatchai, C.; Khattiya, J. Effects of a Pedometer-Based Walking Home Program Plus Resistance Training on Inflammatory Cytokines and Depression in Thai Older People with Sarcopenia: A Three-Arm Randomized Controlled Trial. Clin. Gerontol. 2023, 46, 717–728. [Google Scholar] [CrossRef]
- Lucibello, K.M.; Paolucci, E.M.; Graham, J.D.; Heisz, J.J. A randomized control trial investigating high-intensity interval training and mental health: A novel non-responder phenotype related to anxiety in young adults. Ment. Health Phys. Act. 2020, 18, 100327. [Google Scholar] [CrossRef]
- Vučić Lovrenčić, M.; Pibernik-Okanović, M.; Šekerija, M.; Prašek, M.; Ajduković, D.; Kos, J.; Hermanns, N. Improvement in Depressive Symptoms Is Associated with Reduced Oxidative Damage and Inflammatory Response in Type 2 Diabetic Patients with Subsyndromal Depression: The Results of a Randomized Controlled Trial Comparing Psychoeducation, Physical Exercise, and Enhanced Treatment as Usual. Int. J. Endocrinol. 2015, 2015, 210406. [Google Scholar] [CrossRef]
- Hennings, A.; Schwarz, M.J.; Riemer, S.; Stapf, T.M.; Selberdinger, V.B.; Rief, W. Exercise affects symptom severity but not biological measures in depression and somatization—Results on IL-6, neopterin, tryptophan, kynurenine and 5-HIAA. Psychiatry Res. 2013, 210, 925–933. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Sabaghi, A.; Nedaei, E.; Yousofvand, N. Mitigating the Impact of High-Fat Diet: The Role of Metformin and Exercise Interventions in Alleviating Autism-Like Behaviors and Insulin Resistance in Mice. J. Sci. Sport. Exerc. 2025, 7, 230–237. [Google Scholar] [CrossRef]
- Su, X.; Xiao, Q.; Zhai, J.; Kong, Z.; Li, X. Effects of Exercise Interventions on Anxiety and Depression in Patients with Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trails. J. Sci. Sport. Exerc. 2025, 7, 97–111. [Google Scholar] [CrossRef]
- Ross, R.E.; VanDerwerker, C.J.; Saladin, M.E.; Gregory, C.M. The role of exercise in the treatment of depression: Biological underpinnings and clinical outcomes. Mol. Psychiatry 2023, 28, 298–328. [Google Scholar] [CrossRef]
- Harvey, S.B.; Øverland, S.; Hatch, S.L.; Wessely, S.; Mykletun, A.; Hotopf, M. Exercise and the Prevention of Depression: Results of the HUNT Cohort Study. Am. J. Psychiatry 2018, 175, 28–36. [Google Scholar] [CrossRef]
- Marlatt, M.W.; Potter, M.C.; Lucassen, P.J.; van Praag, H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev. Neurobiol. 2012, 72, 943–952. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Gourgouvelis, J.; Yielder, P.; Clarke, S.T.; Behbahani, H.; Murphy, B.A. Exercise Leads to Better Clinical Outcomes in Those Receiving Medication Plus Cognitive Behavioral Therapy for Major Depressive Disorder. Front. Psychiatry 2018, 9, 37. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Liu, L.; Tang, J.; Liang, X.; Li, Y.; Zhu, P.; Zhou, M.; Qin, L.; Deng, Y.; Li, J.; Wang, Y.; et al. Running exercise alleviates hippocampal neuroinflammation and shifts the balance of microglial M1/M2 polarization through adiponectin/AdipoR1 pathway activation in mice exposed to chronic unpredictable stress. Mol. Psychiatry 2024, 29, 2031–2042. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef]
- Guimarães, M.E.A.; Derhon, V.; Signori, L.U.; Seiffer, B.A.; Wolf, S.; Schuch, F.B. Acute and chronic effects of physical exercise in inflammatory biomarkers in people with depression: A systematic review with meta-analysis. J. Psychiatr. Res. 2024, 179, 26–32. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Leggate, M.; Nowell, M.A.; Jones, S.A.; Nimmo, M.A. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress. Chaperones 2010, 15, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Villar-Fincheira, P.; Paredes, A.J.; Hernández-Díaz, T.; Norambuena-Soto, I.; Cancino-Arenas, N.; Sanhueza-Olivares, F.; Contreras-Briceño, F.; Mandiola, J.; Bruneau, N.; García, L.; et al. Soluble Interleukin-6 Receptor Regulates Interleukin-6-Dependent Vascular Remodeling in Long-Distance Runners. Front. Physiol. 2021, 12, 722528. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Effects of Exhaustive Aerobic Exercise on Tryptophan-Kynurenine Metabolism in Trained Athletes. PLoS ONE 2016, 11, e0153617. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem. Res. 1998, 23, 635–644. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Han, Q.; Robinson, H.; Cai, T.; Tagle, D.A.; Li, J. Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol. Cell Biol. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Rose, G.L.; Skinner, T.L.; Mielke, G.I.; Schaumberg, M.A. The effect of exercise intensity on chronic inflammation: A systematic review and meta-analysis. J. Sci. Med. Sport. 2021, 24, 345–351. [Google Scholar] [CrossRef]
- Cabrera-Rivera, G.L.; Madera-Sandoval, R.L.; León-Pedroza, J.I.; Ferat-Osorio, E.; Salazar-Rios, E.; Hernández-Aceves, J.A.; Guadarrama-Aranda, U.; López-Macías, C.; Wong-Baeza, I.; Arriaga-Pizano, L.A. Increased TNF-α production in response to IL-6 in patients with systemic inflammation without infection. Clin. Exp. Immunol. 2022, 209, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalo, R.; De Paz, J.A.; Rodriguez-Miguelez, P.; Cuevas, M.J.; González-Gallego, J. Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J. Appl. Physiol. 2012, 112, 2011–2018. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Keller, P.; Plomgaard, P.; Wolsk-Petersen, E.; Febbraio, M. The metabolic role of IL-6 produced during exercise: Is IL-6 an exercise factor? Proc. Nutr. Soc. 2004, 63, 263–267. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar] [PubMed]
- Robson-Ansley, P.J.; Blannin, A.; Gleeson, M. Elevated plasma interleukin-6 levels in trained male triathletes following an acute period of intense interval training. Eur. J. Appl. Physiol. 2007, 99, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Markovitch, D.; Tyrrell, R.M.; Thompson, D. Acute moderate-intensity exercise in middle-aged men has neither an anti- nor proinflammatory effect. J. Appl. Physiol. 2008, 105, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.; Thomas, A.W.; Webb, R.; Hughes, M.G. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: The effect of exercise intensity and volume. Appl. Physiol. Nutr. Metab. 2016, 41, 803–808. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, L.L.; Feter, N.; Alt, R.; Rombaldi, A.J. Effects of exercise training on inflammatory, neurotrophic and immunological markers and neurotransmitters in people with depression: A systematic review and meta-analysis. J. Affect. Disord. 2023, 326, 73–82. [Google Scholar] [CrossRef]
| Author/Year/Country | Sample Size (n) | Mean Age (Years) | Intervention | Control Group Treatment | Biomarkers | Depressive Symptom Measure | Depression Severity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | Type of intervention | Intensity | Sessions per wk | Wks | ||||||
| Euteneuer 2017 [50] Germany | 34 | 30 | 37.3 ± 12.2 | Mixed exercise | Low–moderate | 4 | 16 | Wait-list | CRP/IL-6/IL-10 | BDI-Ⅱ | MDD |
| Lavretsky 2011 [71] America | 36 | 37 | 70 ± 7.2 | Mind–Body exercises | Low–moderate | 1 | 10 | Health education | CRP | HAMD/HAMA/ PSQI | MDD |
| Abd El-Kader & Al-Jiffri, 2016b [70] America | 40 | 40 | 34.17 ± 4.98 | Traditional aerobic exercises | Middle–high | 3 | 12 | Wait-list | IL-4/IL/6/CRP/TNF-β | BDI | Mild |
| Ng 2022 [51] Hong Kong | 95 | 93 | 55.49 ± 10.12 | Mind–Body exercises | Low–moderate | 3 | 12 | Wait-list | IL-6/IL-1β | PSQI/CES-D | Moderate–Severe |
| Paolucci 2018 [49] Canda | 19 | 18 | 21 ± 2 | HIIT | High–moderate | 3 | 6 | Wait-list | IL-6/TNF-α/CRP | BDI-Ⅱ/PSS/BAI | Moderate |
| Redwine 2020 [72] America | 24 | 24 | 65 ± 8.31 | Mind–Body Exercises | Moderate | 2 | 16 | Usual care (standard medical treatment) | IL-6/TNF-α/CRP | BDI | Mild |
| Vučić Lovrenčić 2015 [76] Croatia | 66 | 69 | 58.15 ± 5.53 | Mixed exercise | Low–moderate | 6 | 48 | Enhanced treatment | CRP | CES-D | Moderate–Severe |
| Abd El-Kader & Al-Jiffri, 2016a [69] America | 20 | 20 | 69.035 ± 5.96 | Traditional aerobic exercises | Moderate | 3 | 8 | Wait-list | TNF-α/ IL-6 | BDI system/ POMS/ RSES/ SF-36 | Mild |
| Hennings 2013 [77] Germany | 38 | 48 | 34.51 ± 12.84 | Active week x passive week (cross-over trial) | Moderate | 7 | 4 | Active week x passive week (cross-over trial) | IL-6 | BDI | MDD |
| Rethorst 2013 [52] America | 53 | 52 | 47.51 ± 9.44 | Mixed exercise | Moderate | 16KKW per week | 12 | 4KKW for 12 weeks | IL-6/TNF-α | HRSD/IDS-C/IDS-SR | MDD |
| Imboden 2021 [73] Switzerland | 22 | 20 | 39.9 ± 11.4 | Traditional aerobic exercises | Moderate | 3 | 6 | Stretching condition (active control) | TNF-α | HDRS17 | Moderate |
| Yuenyongchaiwat 2023 [74] Thailand | 30 | 30 | 71.7 ± 4.9 | Mixed exercise | Low | 5 | 12 | Usual care (live normally without intervention) | IL-6/TNF-α | TGDS | Mild |
| Lucibello 2020 [75] Canada | 25 | 21 | 19.8 ± 2.2 | HIIT | High | 3 | 9 | Placebo | IL-6/TNF-α | BDI-II | Mild–Moderate |
| CRP | IL-6 | TNF-α | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Interventions | Participants | Effect Metrics | Interventions | Participants | Effect Metrics | Interventions | Participants | Effect Metrics | |
| Type of training | |||||||||
| Aerobic | 3 | 189 | −2.04 [−5.88,1.80], I2 = 94.9%, p = 0.30 | 4 | 344 | −1.03 [−2.51,0.45], I2 = 91.7%, p = 0.17 | 3 | 118 | 1.55 [−1.73, 4.83], I2 = 97.9%, p = 0.35 |
| HIIT | 1 | 36 | 0.05 [−0.44,0.54], NA, p = 0.84 | 2 | 82 | 0.07 [−0.16,0.30], I2 = 0, p = 0.54 | 2 | 82 | −0.02 [−0.15, 0.11], I2 = 0, p = 0.77 |
| Mixed | 2 | 178 | 0.08 [−0.07,0.23], I2 = 0, p = 0.30 | 4 | 283 | −0.07 [−0.36,0.23], I2 = 49.9%, p = 0.65 | 2 | 132 | −6.66 [−24.82, 11.51], I2 = 72.4%, p = 0.47 |
| Intensity | |||||||||
| Low | 2 | 178 | 0.08 [−0.07, 0.23], I2 = 0, p = 0.30 | 4 | 385 | −0.23 [−0.50, 0.04], I2 = 64.3%, p = 0.09 | 2 | 132 | −6.66 [−24.82, 11.51], I2 = 72.4%, p = 0.47 |
| Moderate | 2 | 109 | −0.52 [−1.45, 0.42], I2 = 0, p = 0.28 | 3 | 162 | −0.28 [−1.36, 0.81], I2 = 81%, p = 0.618 | 3 | 118 | 1.55 [−1.73, 4.83], I2 = 97.9%, p = 0.354 |
| High | 2 | 116 | −2.85 [−8.60, 2.91], I2 = 98.3%, p = 0.33 | 3 | 162 | −0.99 [−3.63, 1.64], I2 = 93.8%, p = 0.50 | 2 | 82 | −0.02 [−0.15, 0.11], I2 = 0, p = 0.77 |
| Frequency | |||||||||
| ≤2/week | 2 | 109 | −0.52 [−1.45, 0.42], I2 = 0, p = 0.28 | 1 | 36 | 0.50 [−0.15,1.15], NA, p = 0.17 | 1 | 36 | 4.00 [3.24, 4.76], NA, p = 0.60 |
| 3–4/week | 3 | 180 | −1.94 [−5.66, 1.77], I2 = 96.7%, p = 0.31 | 6 | 454 | −0.36 [−2.08,1.35], I2 = 89.5%, p = 0.68 | 4 | 164 | −0.29 [−0.94,0.37], I2 = 52.8%, p < 0.001 |
| ≥5/week | 1 | 114 | 0.09 [−0.06,0.24], NA, p = 0.25 | 2 | 146 | −0.14 [−0.95,0.67], I2 = 0, p = 0.74 | 1 | 60 | −18.95 [38.79, 0.89], NA, p = 0.06 |
| Length (weeks) | |||||||||
| <8 | 1 | 36 | 0.05 [−0.44,0.54], NA, p = 0.84 | 2 | 122 | 0.06 [−0.16,0.29], I2 = 0, p = 0.59 | 2 | 78 | −0.02 [−0.15,0.11], I2 = 0, p = 0.72 |
| 8–12 | 1 | 73 | −0.70 [−1.71,0.31]. NA, p = 0.17 | 2 | 86 | −1.15 [−2.70,0.40], I2 = 10.3%, p = 0.15 | 2 | 86 | −0.73 [−1.30, −0.17], I2 = 0, p = 0.01 |
| ≥12 | 4 | 294 | −1.35 [−4.29,1.58], I2 = 95.6%, p = 0.37 | 6 | 501 | −0.24 [−1.70,1.22], I2 = 87.6%, p = 0.75 | 3 | 168 | 1.20 [−3.26,5.66], I2 = 95.9%, p = 0.60 |
| Age (years) | |||||||||
| <30 | 1 | 36 | 0.05 [−0.44,0.54], NA, p = 0.84 | 2 | 82 | 0.07 [−0.16,0.30], I2 = 0, p = 0.538 | 2 | 82 | −0.02 [−0.15,0.11], I2 = 0, p = 0.77 |
| 30–59 | 3 | 258 | −1.93 [−5.65,1.80], I2 = 97%, p = 0.31 | 5 | 491 | −0.26 [−2.04,1.53], I2 = 88.4%, p = 0.78 | 3 | 114 | 0.35 [−0.41, 1.11], I2 = 0, p = 0.37 |
| ≥60 | 2 | 109 | −0.52 [−1.45,0.42], I2 = 0, p = 0.28 | 3 | 136 | −0.43 [−1.71, 0.85], I2 = 81.3%, p = 0.513 | 3 | 136 | −0.19 [−6.73, 6.36], I2 = 98%, p = 0.96 |
| Depression severity | |||||||||
| Mild | 2 | 116 | −2.70 [−8.98, 3.60], I2 = 94.9%, p = 0.40 | 4 | 216 | −1.14 [−2.72, 0.44], I2 = 91.3%, p = 0.16 | 3 | 136 | −0.19 [−6.73, 6.36], I2 = 98%, p = 0.96 |
| Moderate | 2 | 150 | 0.09 [−0.06; 0.23], I2 = 0, p = 0.25 | 3 | 270 | −0.14 [−0.56, 0.28], I2 = 74.8%, p = 0.52 | 3 | 124 | −0.02 [−0.15, 0.11], I2 = 0, p = 0.78 |
| Severe | 2 | 137 | −0.41 [−1.07; 0.24], I2 = 0, p = 0.22 | 3 | 223 | −0.06 [−0.35, 0.24], I2 = 65.3%, p = 0.71 | 1 | 72 | 0.33 [−0.44,1.10], NA, p = 0.40 |
| Sex, % female | |||||||||
| >50% | 3 | 223 | 0.070 [−0.08, 0.22] I2 = 13.3%, p = 0.34 | 6 | 489 | −0.13 [−0.37, 0.11] I2 = 40.3%, p = 0.28 | 4 | 214 | −0.01 [−0.14, 0.12] I2 = 30.5%, p = 0.87 |
| ≤50% | 3 | 180 | −1.86 [−5.83, 2.11] I2 = 95.8%, p = 0.36 | 4 | 220 | −0.25 [−2.99, 2.49] I2 = 92.8%, p = 0.86 | 3 | 118 | 1.55 [−1.73, 4.83] I2 = 97.9%, p = 0.35 |
| Comorbidities | |||||||||
| Yes | 3 | 230 | −1.75 [−5.79, 2.30] I2 = 97%, p = 0.40 | 5 | 404 | −0.956 [−2.19, 0.28] I2 = 88.9%, p = 0.13 | 3 | 136 | −0.19 [−6.73, 6.36] I2 = 98%, p = 0.96 |
| No | 3 | 173 | −0.115 [−0.51, 0.28] I2 = 0, p = 0.57 | 5 | 305 | 0.02 [−0.16, 0.21] I2 = 38.6%, p = 0.80 | 4 | 196 | −0.01 [−0.14, 0.12] I2 = 0, p = 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhang, J.; Wang, X.; Liang, J.; Li, Y. Effect of Different Exercise Modalities on Inflammatory Markers in Individuals with Depressive Disorder: A Systematic Review and Meta-Analysis. Life 2025, 15, 1452. https://doi.org/10.3390/life15091452
Song J, Zhang J, Wang X, Liang J, Li Y. Effect of Different Exercise Modalities on Inflammatory Markers in Individuals with Depressive Disorder: A Systematic Review and Meta-Analysis. Life. 2025; 15(9):1452. https://doi.org/10.3390/life15091452
Chicago/Turabian StyleSong, Jie, Jinning Zhang, Xijin Wang, Jiaqi Liang, and Yan Li. 2025. "Effect of Different Exercise Modalities on Inflammatory Markers in Individuals with Depressive Disorder: A Systematic Review and Meta-Analysis" Life 15, no. 9: 1452. https://doi.org/10.3390/life15091452
APA StyleSong, J., Zhang, J., Wang, X., Liang, J., & Li, Y. (2025). Effect of Different Exercise Modalities on Inflammatory Markers in Individuals with Depressive Disorder: A Systematic Review and Meta-Analysis. Life, 15(9), 1452. https://doi.org/10.3390/life15091452






