Epilithic Algae from Seven Megaliths in the Vicinity of Topolovgrad (Haskovo District, Southeast Bulgaria)
Abstract
1. Introduction
2. Materials and Methods
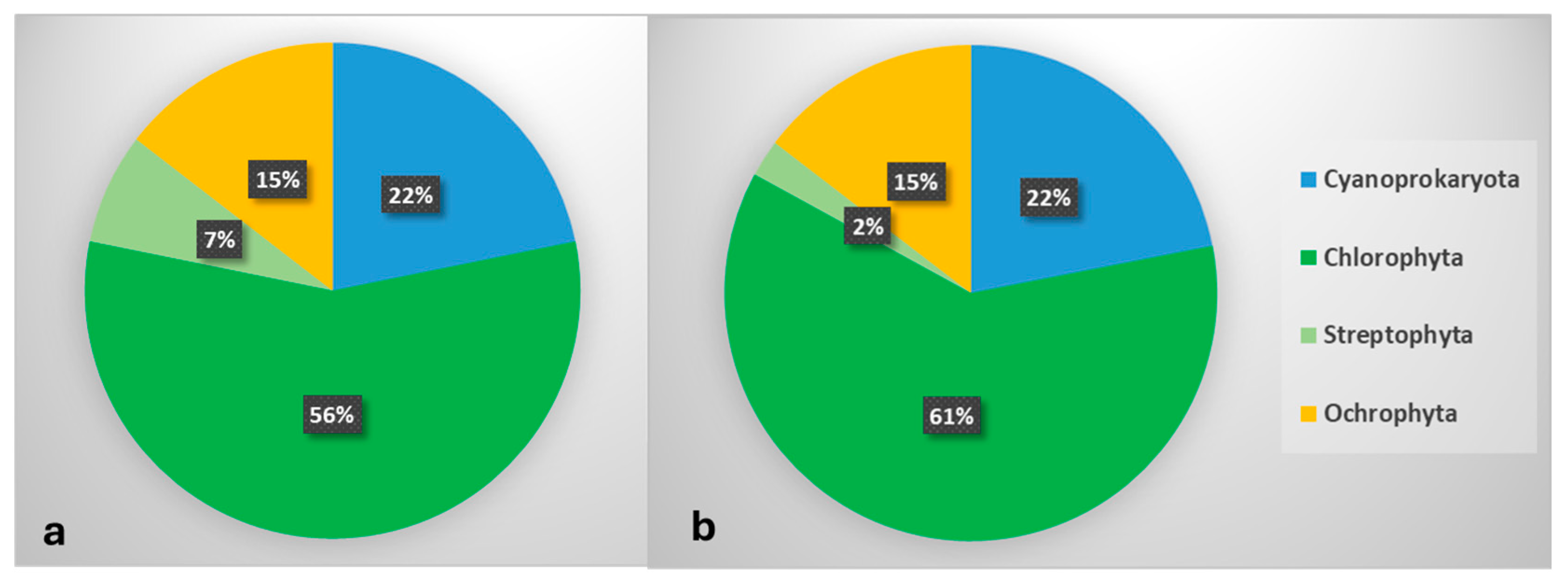
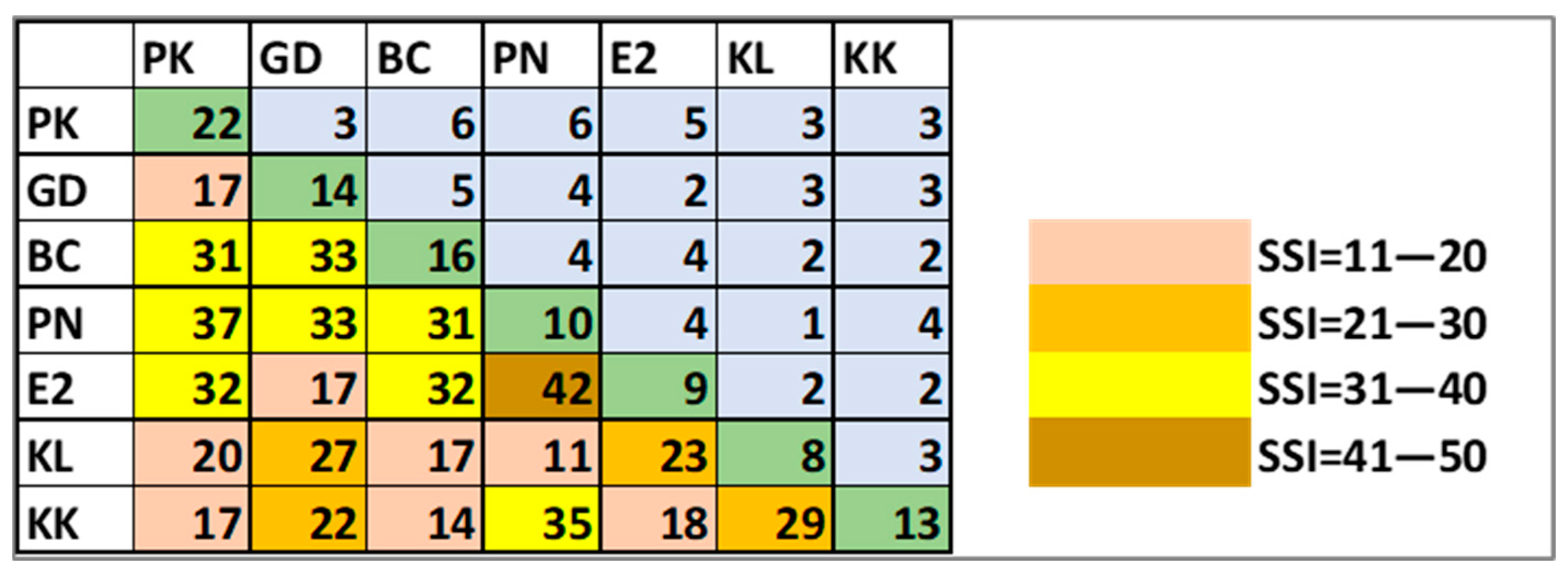
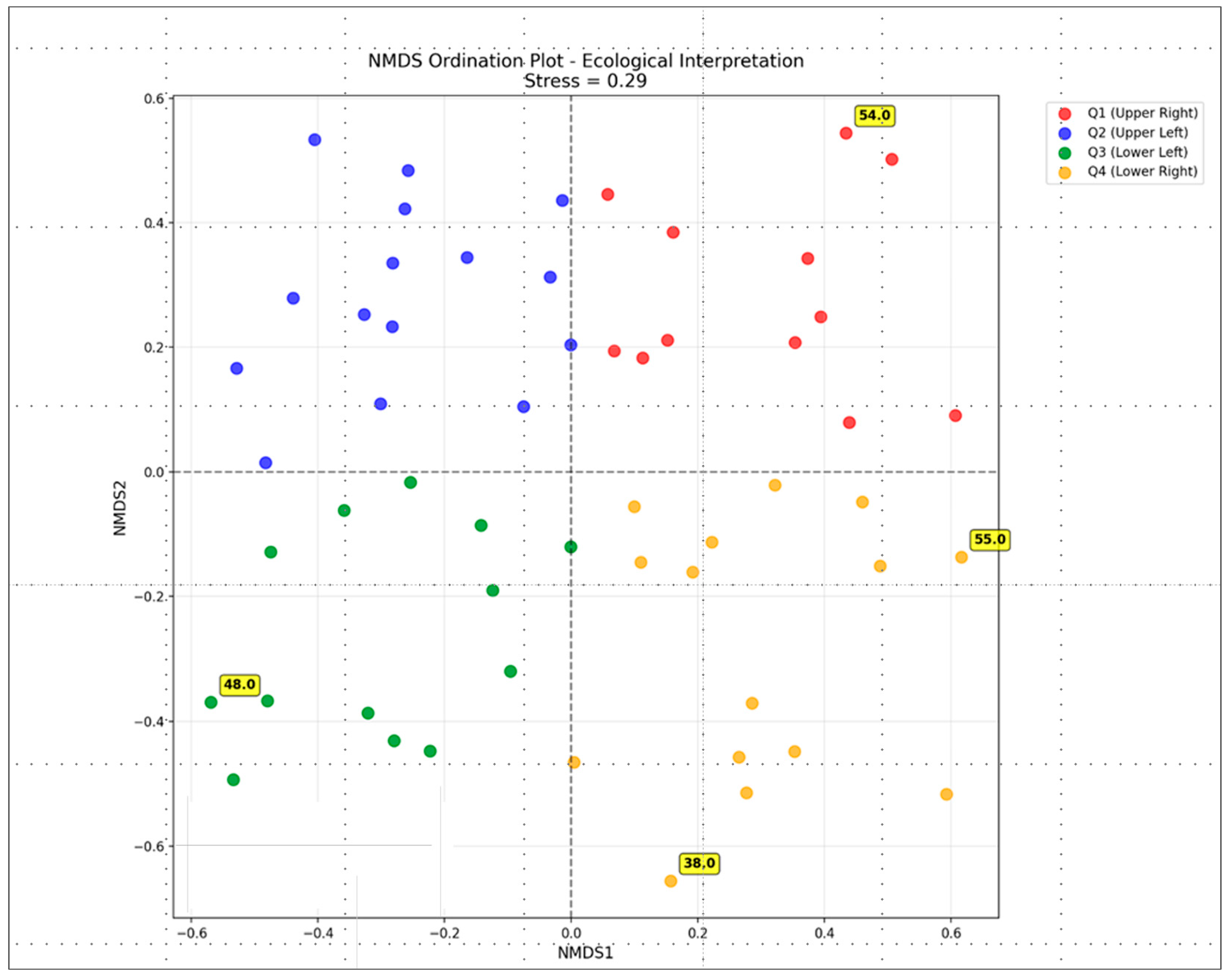
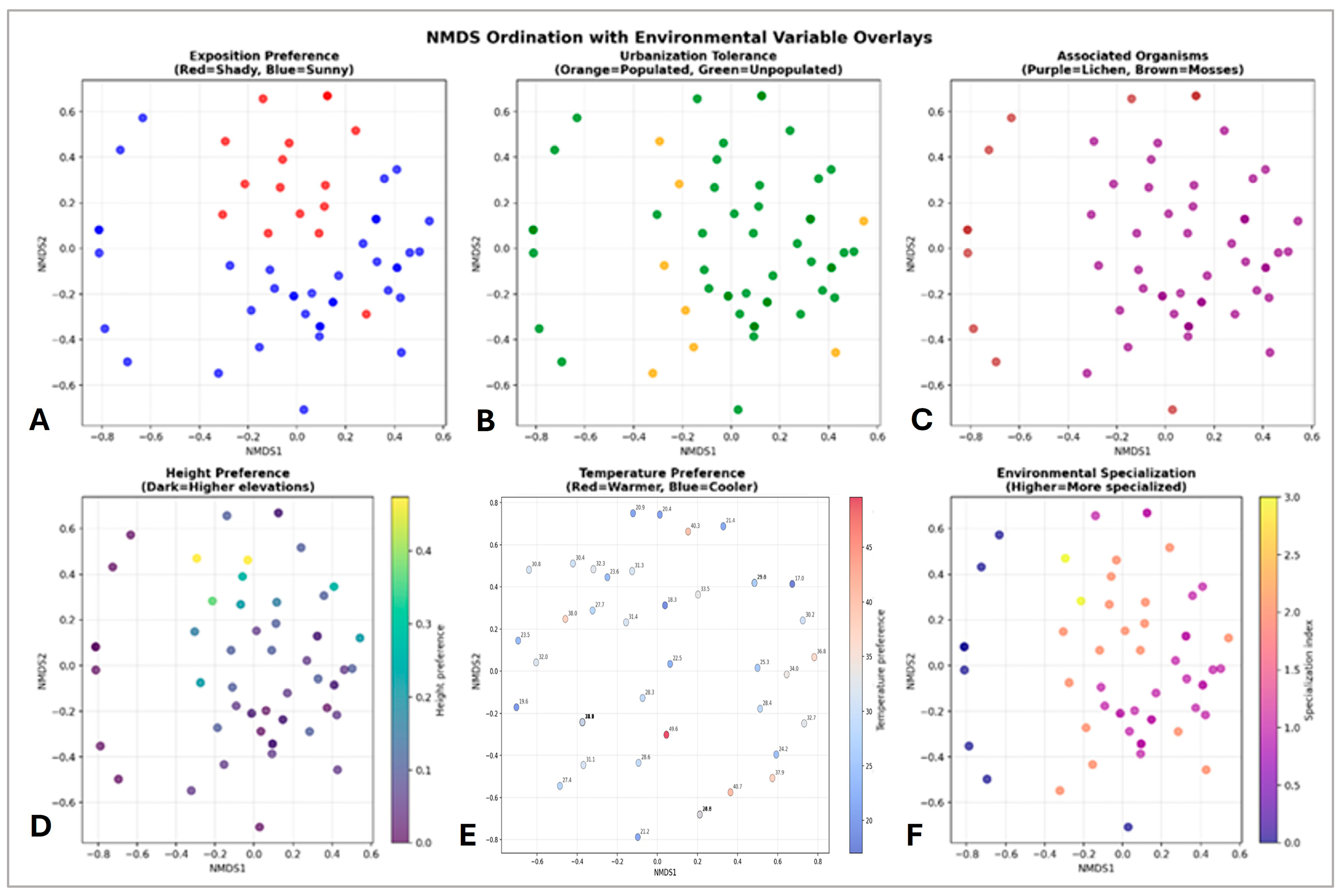
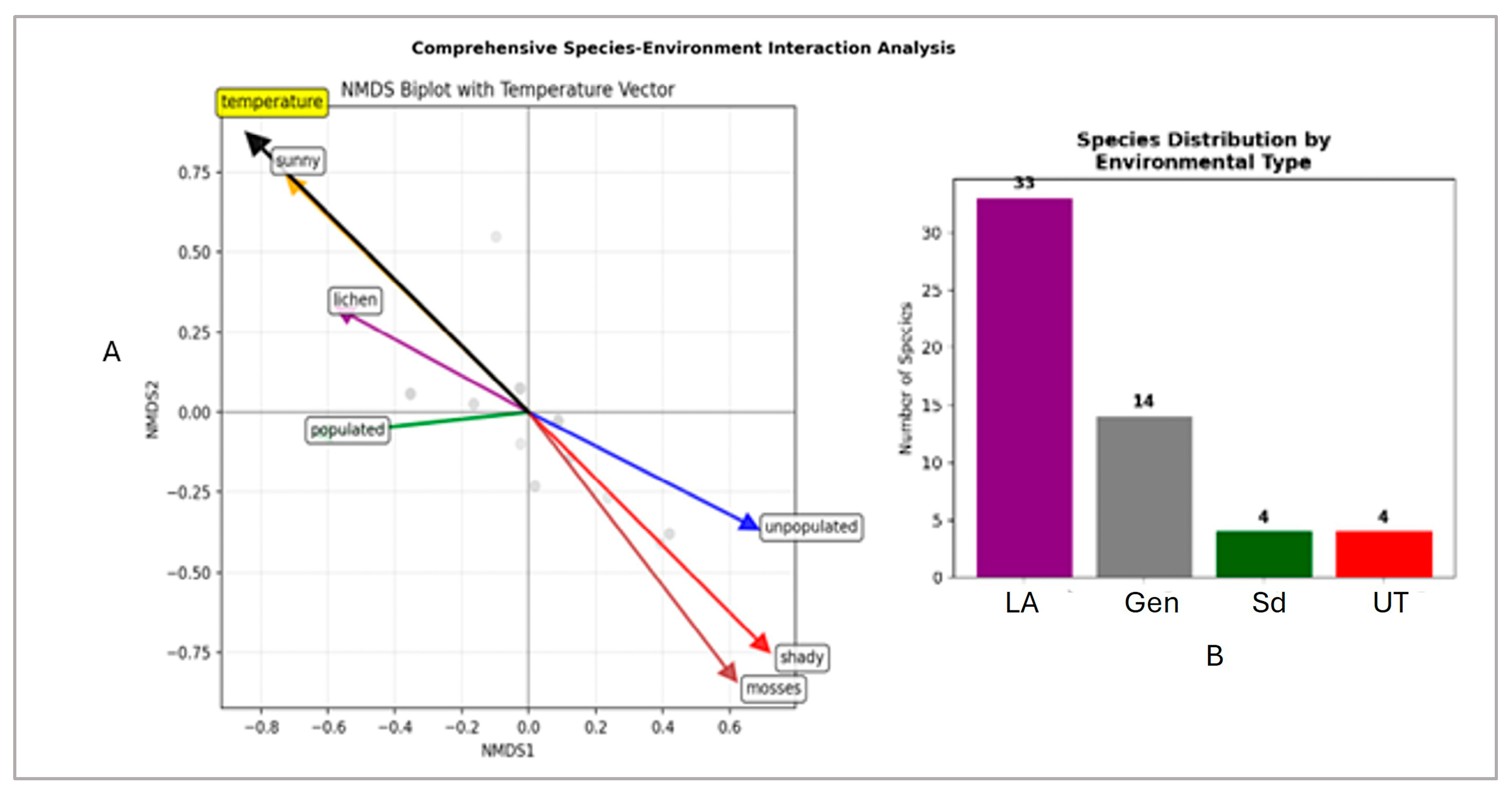
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft- und Flechtenalgen; Springer Spektrum: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Rindi, F. Diversity, distribution, and ecology of green algae in terrestrial environments. Prog. Phycol. Res. 2007, 22, 1–49. [Google Scholar]
- Petersen, J.B. Studier over danske aerophile alger. Mem. Acad. R. Sci. Lett. Danmark 1915, 7, 269–380. [Google Scholar]
- Petersen, J.B. Studies on the Biology and Taxonomy of Soil Algae; Dansk Botanisk Arkiv: Kbenhavn, Denmark, 1935; Volume 8, pp. 1–180. [Google Scholar]
- Fritsch, F. A general considerations of the subaërial and fresh-water algal flora of Ceylon. A contribution to the study of tropical algal ecology. Part I.—Subaërial algae and algae of the inland fresh-waters. Proc. R. Soc. Lond. B Biol. Sci. 1907, 79, 197–254. [Google Scholar] [CrossRef]
- Diels, L. Die Algen-Vegetation der Südtiroler Dolomitriffe. Ein Beitrag zur Ökologie der der Lithophyten. Ber. Deutsch. Bot. Ges. 1914, 32, 502–526. [Google Scholar]
- Jaag, O. Untersuchungen über die Vegetation und Biologie der Algen des nackten Gestins in den Alpen, im Jura und im schweizerischen Mitteland. Beitr. Krypt.-Fl. Schweiz. 1945, 9, 1–560. [Google Scholar]
- Ettl, H.; Gärtner, G. Syllabus der Boden-, Luft- und Flechtenalgen; Gustav Fischer: Stuttgart, Germany, 1995. [Google Scholar]
- Round, F.E. The Ecology of Algae; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Nienow, J.A. Ecology of subaerial algae. Nova Hedw. Beih. 1996, 112, 537–552. [Google Scholar]
- Temniskova, D.; Stoyneva, M. Algology; Pensoft: Sofia, Bulgaria, 2011. [Google Scholar]
- Holzinger, A.; Karsten, U. Desiccation tolerance and resistance in green algae: A bio-physiological perspective. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef]
- de los Ríos, A.; Wierzchos, J.; Sancho, L.; Ascaso, C. Exploring the physiological state of continental Antarctic endolithic microorganisms by microscopy. FEMS Microbiol. Ecol. 2004, 50, 143–152. [Google Scholar] [CrossRef]
- Walker, J.; Spear, J.; Pace, N. Geobiology of a microbial endolithic community in the Yellowstone geothermal environment. Nature 2005, 434, 1011–1014. [Google Scholar] [CrossRef]
- Friedmann, E.; Ocampo-Friedmann, R. The antarctic cryptoendolithic ecosystem: Relevance to exobiology. Origins Life Evol. Biosphere 1984, 14, 771–776. [Google Scholar] [CrossRef]
- de Vera, J.P.; Alawi, M.; Backhaus, T.; Baqué, M.; Billi, D.; Böttger, U.; Berger, T.; Bohmeier, M.; Cockell, C.; Demets, R.; et al. Limits of Life and the Habitability of Mars: The ESA Space Experiment BIOMEX on the ISS. Astrobiology 2019, 19, 145–157. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Gärtner, G.; Uzunov, B.; Androv, M.; Ivanov, K. Soil Algae Symbioses: Raising the Curtain of an Ancient Play. In Soil Algae; Mishra, A., Varma, A., Eds.; Springer: Singapore, 2025; pp. 37–116. [Google Scholar] [CrossRef]
- Gustavs, L.; Eggert, A.; Michalik, D.; Karsten, U. Physiological and biochemical responses of aeroterrestrial green algae (Trebouxiophyceae, Chlorophyta) to osmotic and matric stress. Protoplasma 2011, 248, 127–137. [Google Scholar] [CrossRef]
- Lakatos, M.; Strieth, D. Terrestrial Microalgae: Novel Concepts for Biotechnology and Applications. In Progress in Botany; Cánovas, F., Lüttge, U., Matyssek, R., Eds.; Springer: Cham, Switzerland, 2017; Volume 79. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Enigmatic Microalgae from Aeroterrestrial and Extreme Habitats in Cosmetics: The Potential of the Untapped Natural Sources. Cosmetics 2020, 7, 27. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.; Uzunov, B.; Gärtner, G. Aeroterrestrial and Extremophilic Microalgae as Promising Sources for Lipids and Lipid Nanoparticles in Dermal Cosmetics. Cosmetics 2022, 9, 11. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based BioeconomyFront. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef]
- Rojas-Villalta, D.; Rojas-Rodríguez, D.; Villanueva-Ilama, M.; Guillén-Watson, R.; Murillo-Vega, F.; Gómez-Espinoza, O.; Núñez-Montero, K. Exploring Extremotolerant and Extremophilic Microalgae: New Frontiers in Sustainable Biotechnological Applications. Biology 2024, 13, 712. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.V. Biodeterioration of Stone in Tropical Environments: An Overview; Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Zurita, Y.P.; Cultrone, G.; Castillo, P.S.; Sebastián, E.; Bolívar, F.C. Microalgae associated with deteriorated stonework of the fountain of Bibatauín in Granada, Spain. Int. Biodeterior. Biodegrad. 2005, 55, 55–61. [Google Scholar] [CrossRef]
- Hofbauer, W.K.; Gärtner, G. Microbial Life on Façades; Springer Spectrum: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Kamaljit, K.; Pandey, S. Biodeterioration Study of the Prehistoric Megalithic Rock Structures of Manipur: A Case Study. J. Bioremediat. Biodegr. 2020, 11, 470. [Google Scholar]
- Crispim, C.; Gaylarde, C. Cyanobacteria and Biodeterioration of Cultural Heritage: A Review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: An overview. Microbiology 2009, 155, 3476–3490. [Google Scholar] [CrossRef]
- Gutarowska, B.; Celikkol-Aydin, S.; Bonifay, V.; Otlewska, A.; Aydin, E.; Oldham, A.; Brauer, J.; Duncan, K.; Adamiak, J.; Sunner, J.; et al. Metabolomic and high-throughput sequencing analysis—Modern approach for the assessment of biodeterioration of materials from historic buildings. Front. Microbiol. 2015, 6, 979. [Google Scholar] [CrossRef]
- Soares, F.; Portugal, A.; Trovão, J.; Coelho, C.; Mesquita, N.; Pinheiro, A.C.; Gil, F.; Catarino, L.; Cardoso, S.M.; Tiago, I. Structural diversity of photoautotrophic populations within the UNESCO site ‘Old Cathedral of Coimbra’ (Portugal), using a combined approach. Int. Biodeterior. Biodegrad. 2019, 140, 9–20. [Google Scholar] [CrossRef]
- Rindi, F.; Menzel, D.; López-Bautista, J.M. The biodiversity and biogeography of terrestrial green algae: A review. Cryptogam. Algol. 2011, 32, 209–223. [Google Scholar]
- Anagnostidis, K.; Economou-Amilli, A.; Roussomoustakaki, M. Epilithic and chasmolithic microflora (Cyanophyta, Bacillariophyta) from marbles of the Parthenon (Acropolis-Athens, Greece). Nova Hedw. 1983, 38, 227–287. [Google Scholar]
- Ortega-Calvo, J.; Hernández-Mariné, M.; Saiz-Jiménez, C. Cyanobacteria and algae on historic buildings and monuments. In Recent Advances in Biodeterioration and Biodegradation; Garg, K.L., Garg, N., Mukerji, K.G., Eds.; Naya Prokash: Calcutta, India, 1993; pp. 173–203. [Google Scholar]
- Albertano, P.; Kovácik, L.; Grilli-Caiola, M. Preliminary investigations on epilithic cyanophytes from a Roman Necropolis. Arch. Hydrobiol./Suppl. Algol. Stud. 1994, 75, 71–74. [Google Scholar] [CrossRef]
- Noguerol-Seoane, A.; Rifón-Lastra, A. Epilithic phycoflora on monuments. A survey of San Esteban de Ribas de Sil monastery (Ourense, NW Spain). Cryptogam. Algol. 1997, 18, 351–361. [Google Scholar] [CrossRef]
- Adhikary, S.P. Survival in darkness and heterotrophic growth of epilithic cyanobacteria from temples of India. Algol. Stud. 2002, 105, 141–155. [Google Scholar]
- Gärtner, G.; Stoyneva, M.P. First Study of Aerophytic Cryptogams on Monuments in Bulgaria. Ber. Naturwiss. Med. Ver. Innsbr. 2003, 90, 73–82. [Google Scholar]
- Polo, A.; Gulotta, D.; Santo, N.; Di Benedetto, C.; Fascio, U.; Toniolo, L.; Villa, F.; Cappitelli, F. Importance of subaerial biofilms and airborne microflora in the deterioration of stonework: A molecular study. Biofouling 2012, 28, 1093–1106. [Google Scholar] [CrossRef][Green Version]
- Chimienti, G.; Piredda, R.; Pepe, G.; van der Werf, I.; Sabbatini, L.; Crecchio, C.; Ricciuti, P.; D’Erchia, A.; Manzari, C.; Pesole, G. Profile of microbial communities on carbonate stones of the medieval church of San Leonardo di Siponto (Italy) by Illumina-based deep sequencing. Appl. Microbiol. Biotechnol. 2016, 100, 8537–8548. [Google Scholar] [CrossRef]
- Vázquez-Nion, D.; Rodríguez-Castro, J.; López-Rodríguez, M.; Fernández-Silva, I.; Prieto, B. Subaerial biofilms on granitic historic buildings: Microbial diversity and development of phototrophic multi-species cultures. Biofouling 2016, 32, 657–669. [Google Scholar] [CrossRef]
- Ogawa, A.; Celikkol-Aydin, S.; Gaylarde, C.; Baptista-Neto, J.; Beech, I. Microbial communities on painted wet and dry external surfaces of a historic fortress in Niterói, Brazil. Int. Biodeterior. Biodegrad. 2017, 123, 164–173. [Google Scholar] [CrossRef]
- Uher, B. Spatial distribution of cyanobacteria and algae from the tombstone in a historic cemetery in Bratislava, Slovakia. Fottea 2009, 9, 81–92. [Google Scholar] [CrossRef]
- Uher, B.; Aboal, M.; Kovacik, L. Epilithic and chasmoendolithic phycoflora of monuments and buildings in South-Eastern Spain. Cryptogam. Algol. 2005, 26, 275–308. [Google Scholar]
- Stoyneva-Gärtner, M.; Androv, M.; Uzunov, B.; Ivanov, K.; Gärtner, G. Algal Biodiversity of Nine Megaliths in South-East Bulgaria. Life 2024, 14, 948. [Google Scholar] [CrossRef]
- Krzemińska, A.E.; Dzikowska, A.; Zaręba, A.D.; Jarosz, K.R.; Widawski, K.; Łach, J.S. The Significance of Megalithic Monuments in the Process of Place Identity Creation and in Tourism Development. Open Geosci. 2018, 10, 504–516. [Google Scholar] [CrossRef]
- Wunderlich, M. Megalithic monuments and social structures. Comparative studies on recent and Funnel Beaker societies. In Scales of Transformation in Prehistoric and Archaic Societies; Sidestone Press: Leiden, The Netherlands, 2019. [Google Scholar]
- Cassar, J.; Cefai, S.; Grima, R.; Stroud, K. Sheltering archaeological sites in Malta: Lessons learnt. Herit. Sci. 2018, 6, 36. [Google Scholar] [CrossRef]
- Roy, A.; Gogoi, N.; Yasmin, F.; Faroog, M. The use of algae for environmental sustainability: Trends and future prospects. Environ. Sci. Pollut. Res. 2022, 29, 40373–40383. [Google Scholar] [CrossRef]
- Hosny, S.; Elshobary, M.E.; El-Sheekh, M.M. Unleashing the power of microalgae: A pioneering path to sustainability and achieving the sustainable development goals. Environ. Sci. Pollut. Res. 2025, 32, 17312–17342. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgae biorefinery concepts and state-of-the-art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Ralph, P.J.; Pernice, M. Save the planet with green industries using algae. PLoS Biol. 2023, 21, e3002061. [Google Scholar] [CrossRef]
- Transliteration Act in the State Gazette, No. 19 of 13 March 2009, Last Changed in the State Gazette No. 98 of 13 December 2019. Available online: https://lex.bg/en/laws/ldoc/2135623667 (accessed on 18 June 2024).
- Gärtner, G.; Stoyneva, M.P.; Mancheva, A.D.; Uzunov, B.A. A new method in collection and cultivation of aerophytic and endolithic algae. Ber. Nat. Med. Ver. Innsbr. 2010, 96, 27–34. [Google Scholar]
- Uzunov, B.; Stoyneva, M.P.; Gärtner, G. Review of the studies on aero-terrestrial cyanoprokaryotes and algae in Bulgaria with a Checklist of the recorded species. II. Phytol. Balc. 2008, 14, 11–18. [Google Scholar]
- Mojumdar, A.; Behera, H.T.; Ray, L. Lithobiontic Ecology: Stone Encrusting Microbes and their Environment. In Environmental and Agricultural Microbiology: Applications for Sustainability; Mishra, B.B., Nayak, S.K., Mohapatra, S., Samantaray, D., Eds.; Wiley: New York, NY, USA, 2021; pp. 341–360. [Google Scholar]
- Andersen, R.A. (Ed.) Algal Culturing Techniques; Elsevier: New York, NY, USA; Academic Press: New York, NY, USA, 2005. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 1. Teil: Chroococcales. In Süßwasserflora von Mitteleuropa. Bd. 19/1; Ettl, H., Gärtner, G., Heynig, G., Mollenhauer, D., Eds.; Gustav Fischer: Jena, Germany; Stuttgart, Germany; Lübeck, Germany, 1999. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Teil: Oscillatoriales. In Süßwasserflora von Mitteleuropa. Bd. 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier Spektrum Akad. Verl.: Heidelberg, Germany; München, Germany, 2005. [Google Scholar]
- Komárek, J. Cyanoprokaryota. 3rd Part: Heterocytous Genera. In Süßwasserflora von Mitteleuropa, Bd. 19/3; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier Spektrum Akad. Verl.: Heidelberg, Germany, 2014. [Google Scholar]
- Škaloud, P.; Rindi, F.; Boedeker, C.; Leliaert, F. Chlorophyta: Ulvophyceae. In Süßwasserflora von Mitteleuropa/ Freshwater Flora of Central Europe, Bd 13; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin, Germany, 2018. [Google Scholar]
- Ettl, H. Xanthophyceae. 1 Teil. In Süßwasserflora von Mitteleuropa, Bd 3; Ettl, H., Gerloff, J., Heynig, H., Eds.; Gustav Fisher Verlag: Stuutgart, Germany; New York, NY, USA, 1978. [Google Scholar]
- Ettl, H.; Gärtner, G. Chlorophyta II. Tetrasporales, Chlorococcales, Gloeodendrales. In Süßwasserflora von Mitteleuropa, Bd 10; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuutgart, Germany; New York, NY, USA, 1988. [Google Scholar]
- Ettl, H.; Gärtner, G. Chlorophyta Chlorophyta I—Phytomonadina. In Süsswasserflora von Mitteleuropa, Bd. 9; Ettl, H., Gerloff, J., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuutgart, Germany, 1983. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae (Grünalgen). Ordnung: Chlorococcales. In Das Phytoplankton des Süßwassers, 7/1; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1983; pp. 1–1044. [Google Scholar]
- Hindák, F. Studies on the chlorococcal algae (Chlorophyceae). II; Biol. Práce SAV: Bratislava, Czechoslovakia, 1980; Volume 26, pp. 1–196. [Google Scholar]
- Hindák, F. Studies on the chlorococcal algae (Chlorophyceae). III; Biol. Práce SAV: Bratislava, Czechoslovakia, 1984; Volume 30, pp. 1–310. [Google Scholar]
- Hindák, F. Studies on the chlorococcal algae (Chlorophyceae). IV; Biol. Práce SAV: Bratislava, Czechoslovakia, 1988; Volume 34, pp. 1–264. [Google Scholar]
- John, D.; Brooks, A.; Whitton, B. The Freshwater Algal Flora of the British Isles. An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- John, D.; Whitton, B.; Brook, A. (Eds.) . The Freshwater Algal Flora of the British Isles, 2nd ed.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Wehr, J.; Sheath, R. (Eds.) Freshwater Algae of North America, Ecology and Classification; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2003. [Google Scholar]
- Wehr, J.; Sheath, R.; Kociolek, P. (Eds.) Freshwater Algae of North America, Ecology and Classification, 2nd ed.; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Gandhi, K.N.; Gravendyck, J.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Klopper, R.R.; Knapp, S.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Madrid Code); Chicago University Press: Chicago, IL, USA, 2025. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2024; Available online: https://www.algaebase.org (accessed on 25 July 2025).
- Stoyneva, M.P. Contribution to the Studies of the Biodiversity of Hydro- and Aerobiontic Prokaryotic and Eukaryotic Algae in Bulgaria. Ph.D. Thesis, Sofia University “St. Kliment Ohridski”, Sofia, Bulgaria, 2014. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Isheva, T.; Uzunov, B.; Dimitrova, P. Red List of Bulgarian Algae. II. Microalgae. Ann. Sof. Univ. Fac. Biol. Book 2 Bot. 2016, 100, 15–55. Available online: http://hdl.handle.net/10506/1389 (accessed on 15 July 2025). [CrossRef]
- Sørensen, T. A Method of Establishing Groups of Equal Amplitude in Plant Sociology Based on Similarity of Species and Its Application to Analyses of the Vegetation on Danish Commons; Biologiske Skrifter; Kongelige Danske Videnskabernes Selskab: København, Danmark, 1948; Volume 5, pp. 1–34. [Google Scholar]
- Gärtner, G.; Stoyneva-Gärtner, M.; Uzunov, B. Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review. Toxins 2021, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, W.K. Toxic or Otherwise Harmful Algae and the Built Environment. Toxins 2021, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Stoyneva-Gärtner, M.P.; Gärtner, G.; Uzunov, B. Toxic Compounds of Free-Living and Symbiotic Soil Algae. In Soil Algae; Mishra, A., Varma, A., Eds.; Springer: Singapore, 2025; pp. 305–354. [Google Scholar] [CrossRef]
- Evangelista, V.; Barsanti, L.; Frassanito, A.M.; Passarelli, V.; Gualtieri, P. (Eds.) Algal Toxins: Nature, Occurrence, Effect and Detection; NATO Advanced Study Institute on Sensor Systems for Biological Threats: The Algal Toxins Case, Pisa, Italy, 30 September–11 October 2007; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Meriluoto, J.; Spoof, L.; Codd, J. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar]
- Di Leo, G.; Sardanelli, F. Statistical significance: P value, 0.05 threshold, and applications to radiomics—Reasons for a conservative approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Baker, J.D. Applied Multivariate Statistics in R; University of Washington Pressbooks. Available online: https://uw.pressbooks.pub/appliedmultivariatestatistics/chapter/nmds/ (accessed on 4 September 2025).
- Dexter, E.; Rollwagen-Bollens, G.; Bollens, S.M. The trouble with stress: A flexible method for the evaluation of nonmetric multidimensional scaling. Limnol. Oceanogr. Methods 2018, 16, 434–443. [Google Scholar] [CrossRef]
- Androv, M. Checklist of lithophytic algae in Bulgaria (1905–2024). Ann. Sof. Univ. Fac. Biol. Book 2 Bot. 2024, 108, 29–59. [Google Scholar] [CrossRef]
- Ivanov, K.R.; Androv, M.I. Algal diversity on the granite monument in front of the Faculty of Biology of Sofia University. Ann. Sof. Univ. Fac. Biol. Book 2 Bot. 2023, 107, 114–123. [Google Scholar] [CrossRef]
- Grbić, M.L.; Jević, J.V.; Simić, G.S.; Krizmanić, J.; Stupar, M. Biofilm forming Cyanobacteria, Algae and Fungi on two historic monumnets in Belgrade, Serbia. Arch. Biol. Sci. 2010, 62, 625–631. [Google Scholar] [CrossRef]
- Fuentes, E.; Carballeira, R.; Prieto, B. Role of Exposure on the Microbial Consortiums on Historical Rural Granite Buildings. Appl. Sci. 2021, 11, 3786. [Google Scholar] [CrossRef]
- Mikhailyuk, T.I. Terrestrial lithophilic algae in a granite canyon of the Teteriv River (Ukraine). Biologia 2008, 63, 824–830. [Google Scholar] [CrossRef]
- Mikhailyuk, T.I. Terrestrial Algae from the Granite Outcrops of River Valleys of the Ukraine. InterJAlgae 2013, 15, 313–332. [Google Scholar] [CrossRef]
- Aptroot, A.; Berg, M.P. Collembola help lichens in competition with algae. Lichenologist 2004, 36, 167–169. [Google Scholar] [CrossRef]
- Romão, P.M.S.; Rattazzi, A. Biodeterioration on megalithic monuments. Study of lichens’ colonization on Tapadão and Zambujeiro dolmens (southern Portugal). Int. Biodeterior. Biodegrad. 1996, 37, 23–35. [Google Scholar] [CrossRef]
- Stoyneva, M.P.; Gärtner, G. Remarkable and newly recorded aeroterrestric Cyanoprokaryotes and algae in Bulgaria. In Proceedings of the IV Balkan Botanical Congress, Sofia, Bulgaria, 20–26 June 2006; Ivanova, D., Ed.; BAS: Sofia, Bulgaria, 2006; pp. 122–127. [Google Scholar]
- Stoyneva, M.; Mancheva, A.; Gärtner, G.; Uzunov, B. Are the algae from the uncommon Belogradchik rocks common ones? In Proceedings of the VII National Botanical Conference, Sofia, Bulgaria, 29–30 September 2011; Petrova, A., Ed.; Bulgarian Botanical Society: Sofia, Bulgaria, 2012; pp. 265–269. [Google Scholar]
- Mancheva, A. Aerophytic Algae from the Rock Phenomenon and Nature Monument Belogradchishki Skali. Ph.D. Thesis, Sofia University “St. Kliment Ohridski”, Sofia, Bulgaria, 2013. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Ivanov, P.; Zidarova, R.; Isheva, T.; Uzunov, B.A. A new method for assessment of the Red list threat status of microalgae. Ann. Sof. Univ. Fac. Biol. Book 2 Bot. 2016, 100, 5–14. [Google Scholar] [CrossRef]
- Pröschold, T.; Darienko, T. The green puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New generic and species concept among this widely distributed genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- Van, A.T.; Sommer, V.; Glaser, K. The Ecophysiological Performance and Traits of Genera within the Stichococcus-like Clade (Trebouxiophyceae) under Matric and Osmotic Stress. Microorganisms 2021, 9, 1816. [Google Scholar] [CrossRef]
- Neustupa, J. Soil algae from marlstone-substratum based biotopes in the Nature park Džbán (Central Bohemia, Czech Republic) with special attention to the natural treeless localities. Algol. Stud. 2001, 101, 109–120. [Google Scholar] [CrossRef]
- Saadaouia, I.; Cherifa, M.; Rasheeda, R.; Bounnita, T.; Al Jabria, H.; Sayadia, S.; Ben Hamadoub, R.; Manning, S.R. Mychonastes homosphaera (Chlorophyceae): A promising feedstock for high quality feed production in the arid environment. Algal Res. 2020, 5, 102021. [Google Scholar] [CrossRef]
- Sahu, V.; Toppo, K.; Suseela, M.R.; Asthana, A.K. Allelopathic effect of Stichococcus bacillaris Nageli (Green Alga) on the growth of two bryophytes. Arch. Bryol. 2013, 162, 1–4. [Google Scholar]
- Trenberth, K.E. Changes in Precipitation with Climate Change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef]
- Bolan, S.; Padhye, L.P.; Jasemizad, T.; Govarthanan, M.; Karmegam, N.; Wijesekara, H.; Amarasiri, D.; Hou, D.; Zhou, P.; Biswal, B.K.; et al. Impacts of climate change on the fate of contaminants through extreme weather events. Sci. Tot. Environ. 2024, 909, 168388. [Google Scholar] [CrossRef]
- Sangma, C.B.K.; Sultana, S. Algae as Indicators of Climate Change. In Climate Change and Microbes, Impacts and Vulnerability, 1st ed.; Parray, J.A., Bandh, S.A., Shameem, N., Eds.; Apple Academic Press: New York, NY, USA, 2022; pp. 142–173. [Google Scholar] [CrossRef]
- Karsten, U.; Holzinger, A. Light, temperature and desiccation effects on photosynthetic activity, and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microb. Ecol. 2012, 63, 51–63. [Google Scholar] [CrossRef]
- Rindi, F.; Guiry, M.D.; López-Bautista, J.M. Distribution, morphology and phylogeny of Klebsormidium (Klebsormidiales, Charophyceae) in urban environments in Europe. J. Phycol. 2008, 44, 1529–1540. [Google Scholar] [CrossRef]
- Ryšánek, D.; Elster, J.; Kováčik, L.; Škaloud, P. Diversity and dispersal capacities of a terrestrial algal genus Klebsormidium (Streptophyta) in polar regions. FEMS Microbiol. Ecol. 2016, 92, fiw039. [Google Scholar] [CrossRef]
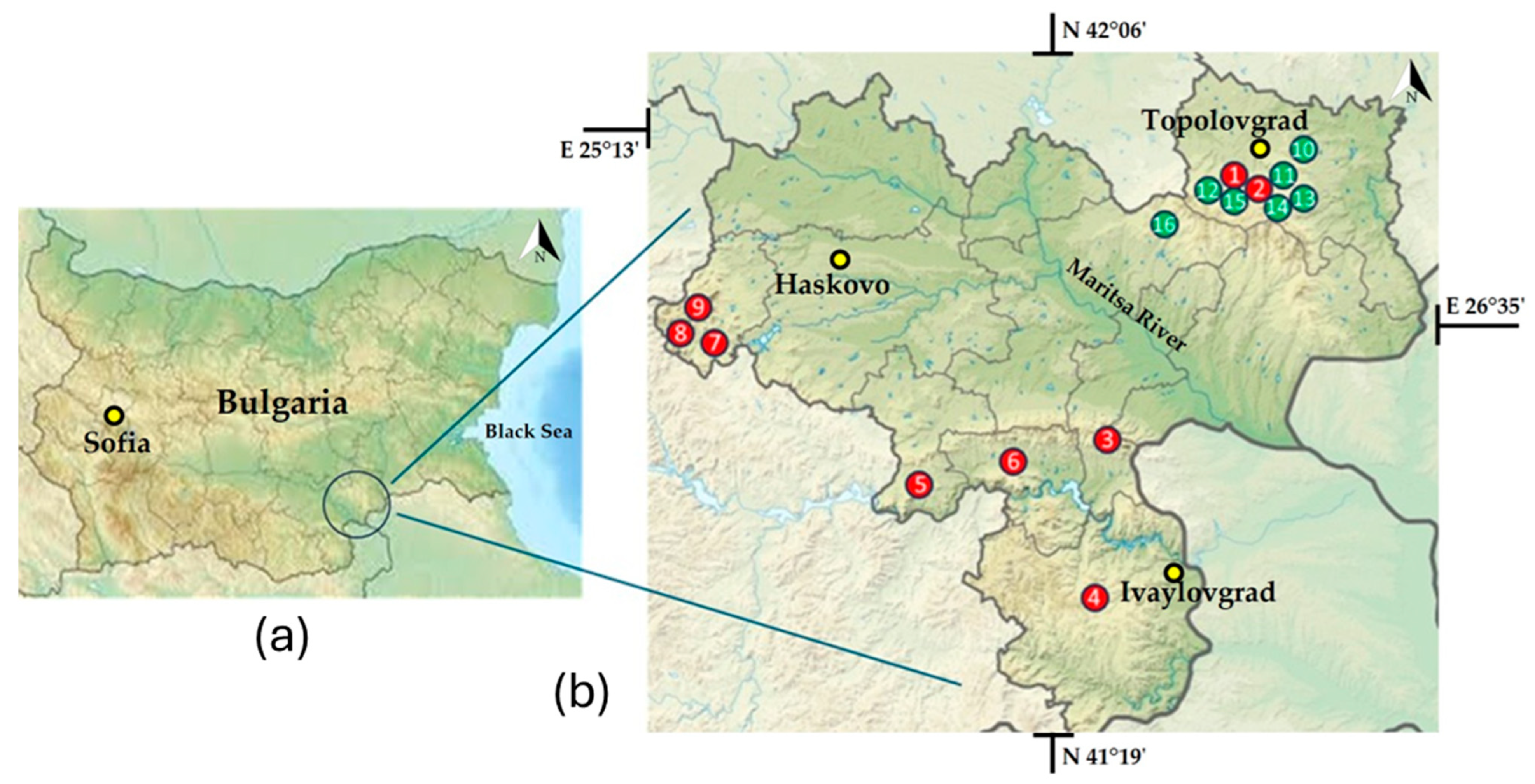





| Megalith | Abb | GC | TR * | Exp | HR | DAO | ED | NCS |
|---|---|---|---|---|---|---|---|---|
| Paleokastro | PK | 42°04′46.8″ N, 26°18′05.6″ E | 31.4–40.3 | Sunny | 25–260 | Lichens | N, E, S, SW | 7 |
| Gaydarov Dolap | GD | 42°02′48.2″ N 26°14′14.3″ E | 27.4–32.3 | Sunny | 20–60 | Lichens | E, SW, W | 4 |
| Blaga Cherkva | BC | 41°57′43.9″ N 26°08′13.9″ E | 20.4–23.6 | Sunny | 25–90 | Lichens | E, S, W | 4 |
| Ponichkata | PN | 42°03′09.8″ N 26°16′03.8″ E | 25.3–31.3 | Shady | 35–65 | Lichens | E, W | 2 |
| Evdzhika 2 | E2 | 42°02′38.5″ N 26°15′30.6″ E | 17–18.3 | Shady | 8–10 | Mosses | N, S | 2 |
| Kalinkin Kamuk | KL | 42°03′14.6″ N 26°15′12.7″ E | 23.5–34.8 | Sunny | 160–180 | Lichens | E, SW, W | 3 |
| Kamenna Kushta | KK | 42°02′20.6″ N 26°10′16.0″ E | 19.6–40.7 | Sunny | 20–75 | Lichens | E, S, W | 4 |
| Species/Megaliths | PK | GD | BC | PN | E2 | KL | KK | RLBM | PT | PRLFB | GW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CYANOPROKARYOTA | |||||||||||
| Aphanocapsa sp. 1 | x | + | M | ||||||||
| Aphanocapsa sp. 2 | x | x | + | M | |||||||
| Aphanothece sp. 1 | x | x | x | x | + | M | |||||
| Aphanothece sp. 2 | x | x | + | M | |||||||
| * Aphanothece sp. 3 | x | x | + | ||||||||
| Gloeobacter violaceus Rippka, J. B. Waterbury et Cohen-Bazire | x | x | M | ||||||||
| Leibleinia epiphytica (Hieronymus) Compère | x | M 1 | |||||||||
| Leptolyngbya compacta Komárek | x | + 2 | M 3 | ||||||||
| * Phormidium calcareum Kützing ex Gomont | x | ||||||||||
| * Pseudophormidium spelaeoides (Cado) Anagnostidis | x | ||||||||||
| * Synechocystis primigenia N. L. Gardner | x | ||||||||||
| Trichocoleus delicatulus (West & G. S. West) Anagnostidis | x | O | |||||||||
| CHLOROPHYTA | |||||||||||
| Apatococcus lobatus (Chodat) Petersen | x | M, O | G,R | ||||||||
| Chlorella miniata (Kützing) Oltmanns | x | x | x | O | |||||||
| Chlorella vulgaris Beijerinck | x | M, O | G | ||||||||
| Chloroidium ellipsoideum (Gerneck) Darienko, Gustavs, Mudimu, Menendez, Schumann, Karsten, Friedl et Proschold | x | x | M, O | R | |||||||
| Choricystis parasitica (Brandt) Pröschold et Darienko | x | M, O | |||||||||
| Coelastrella aeroterrestrica Tschaikner, Gärtner et Kofler | x | x | CR | ||||||||
| Coelastrella striolata Chodat | x | ||||||||||
| Coenobotrys gloeobotrydiformis (Reisigl) Kostikov, Darienko, Lukesová et Hoffmann | x | M, O | |||||||||
| Desmococcus olivaceus (Persoon ex Acharius) Laundon | x | x | M, O | G,R | |||||||
| Deuterostichococcus tetrallantoideus (Kol) Pröschold et Darienko | x | M | |||||||||
| * Diplosphaera chodatii Bialosuknia | x | ||||||||||
| Edaphochlorella mirabilis (Andreeva) Darienko et Pröschold | x | x | x | x | M, O | ||||||
| Elliptochloris reniformis Darienko et Pröschold | x | x | O | ||||||||
| * Lobosphaera incisa (Reisigl) Karsten, Friedl, Schumann, Hoyer et Lembcke | x | ||||||||||
| Lobosphaeropsis lobophora (Andreeva) Ettl et Gärtner | x | M, O | |||||||||
| * Macrochloris radiosa Ettl & Gärtner | x | ||||||||||
| Muriella decolor Vischer | x | M | |||||||||
| * Muriella magna F. E. Fritsch et R. P. John | x | ||||||||||
| Muriella terrestris J. B. Petersen | x | x | M | ||||||||
| Mychonastes homosphaera (Skuja) Kalina et Puncochárová | x | x | x | x | x | x | M, O | G,R | |||
| Neocystis brevis (Vischer) Kostikov et Hoffmann | x | x | x | M | |||||||
| Parachlorella kessleri (Fott et Nováková) Krienitz, Hegewald, Hepperle, Huss, Rohr et Wolf | x | M | |||||||||
| Pseudodictyochloris multinucleata (Broady) Ettl & Gärtner | x | M, O | |||||||||
| * Pseudomuriella engadinensis (Kol et F. Chodat) Fuciková, Rada et L. A. Lewis | x | x | |||||||||
| Pseudostichococcus monallantoides var. exiguus (Gerneck) Pröschold et Darienko | x | M,O | |||||||||
| Sphaerococcomyxa olivacea (Petersen) Kostikov, Darienko, Lukesová et Hoffmann. | x | M | |||||||||
| Stichococcus bacillaris Nägeli | x | x | x | x | x | x | x | M, O | G,R | ||
| Stichococcus minutus Grintzesco et Ș. Péterfi | x | M, O | |||||||||
| Stichococcus mirabilis Lagerheim | x | x | M | ||||||||
| Tetracystis pulchra R. M. Brown et Bold | x | M | |||||||||
| * Ulothrix implexa (Kützing) Kützing | x | ||||||||||
| STREPTOPHYTA | |||||||||||
| Klebsormidium crenulatum (Kützing) Lokhorst | x | M | R | ||||||||
| Klebsormidium dissectum (Gay) Ettl et Gärtner | x | x | M, O | ||||||||
| Klebsormidium flaccidum (Kützing) Silva, Mattox et Blackwell | x | x | M, O | G,R | |||||||
| * Klebsormidium montanum (Hansgirg) Shin Watanabe ex Molinari et Guiry | x | ||||||||||
| OCHROPHYTA | |||||||||||
| * Ellipsoidion parvum H. Reisigl | x | ||||||||||
| Ellipsoidion perminimum Pascher | x | x | x | M | |||||||
| Gloeobotrys ellipsoideus Pascher | x | O | |||||||||
| Navicula sp. s.l. | x | M, O | |||||||||
| * Nephrodiella minor Pascher | x | ||||||||||
| Pleurochloris commutata Pascher | x | M | |||||||||
| Vischeria helvetica (Vischer et Pascher) D. J. Hibberd | x | x | |||||||||
| Vischeria stellata (Chodat) Pascher | x | EN | M, O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyneva-Gärtner, M.P.; Androv, M.I.; Uzunov, B.A.; Ivanov, K.R.; Gärtner, G. Epilithic Algae from Seven Megaliths in the Vicinity of Topolovgrad (Haskovo District, Southeast Bulgaria). Life 2025, 15, 1451. https://doi.org/10.3390/life15091451
Stoyneva-Gärtner MP, Androv MI, Uzunov BA, Ivanov KR, Gärtner G. Epilithic Algae from Seven Megaliths in the Vicinity of Topolovgrad (Haskovo District, Southeast Bulgaria). Life. 2025; 15(9):1451. https://doi.org/10.3390/life15091451
Chicago/Turabian StyleStoyneva-Gärtner, Maya Petrova, Miroslav Ivov Androv, Blagoy Angelov Uzunov, Kristian Rosenov Ivanov, and Georg Gärtner. 2025. "Epilithic Algae from Seven Megaliths in the Vicinity of Topolovgrad (Haskovo District, Southeast Bulgaria)" Life 15, no. 9: 1451. https://doi.org/10.3390/life15091451
APA StyleStoyneva-Gärtner, M. P., Androv, M. I., Uzunov, B. A., Ivanov, K. R., & Gärtner, G. (2025). Epilithic Algae from Seven Megaliths in the Vicinity of Topolovgrad (Haskovo District, Southeast Bulgaria). Life, 15(9), 1451. https://doi.org/10.3390/life15091451








