Biomarkers in Pediatric Neuropsychiatric Systemic Lupus Erythematosus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Systematic Literature Review
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Collection and Synthesis
2.5. Risk of Bias Assessment
- Selection: This domain assesses the representativeness of the study groups, the clarity of case/control/exposed/unexposed definitions, and whether the outcome was present at the start of the study (for cohorts) or if the exposure was ascertained by a validated tool (for cross-sectional). A maximum of 4 stars are given for cohort and case-control studies and a maximum of 5 stars for cross-sectional studies.
- Comparability: This domain evaluates whether the study adequately controlled for confounding factors between the groups compared, either through design (e.g., matching) or statistical analysis (e.g., multivariate regression). A maximum of 2 stars are awarded, one for controlling the most important factor and another for any additional factors. If no explicit control for confounders is stated, 0 stars are given.
- Outcome/Exposure: This domain assesses how outcomes (for cohort/cross-sectional) or exposures (for case-control) were ascertained and the adequacy of follow-up (for cohorts); a maximum of 3 stars are awarded for cohort and case-control studies and a maximum of 2 stars for cross-sectional.
2.6. Statistical Analysis
3. Results
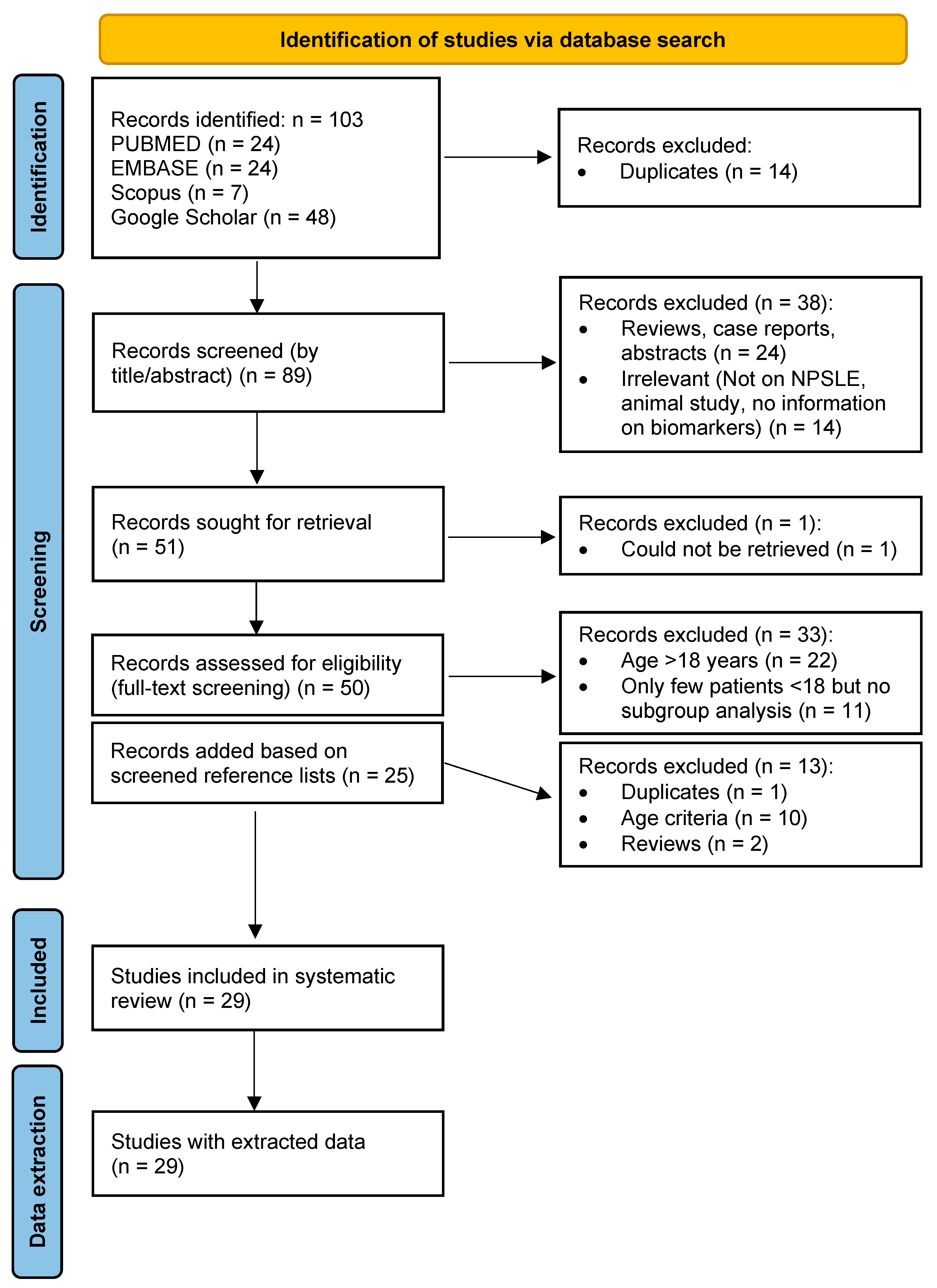
3.1. Search Results
3.2. Study Characteristics
3.3. Patient and Disease Characteristics
3.4. Biomarker Associations with Neuropsychiatric Symptoms
3.5. Risk of Bias and Study Quality
4. Discussion
5. Limitations and Future Perspective
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| AHRQ | Agency for Healthcare Research and Quality |
| ANA | anti-nuclear antibodies |
| AQP4 | aquaporin 4 |
| AUC | area under the curve |
| BBB | blood-brain barrier |
| b2GP1 | b2 glycoprotein 1 |
| CHO/Cr | choline to creatine |
| CI | Confidence interval |
| CL | cardiolipin |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| CVD | cerebrovascular disease |
| dsDNA | double-stranded DNA |
| DTI | diffusion tensor imaging |
| ENA | extractable nuclear antigen |
| ELISA | enzyme-linked immunosorbent assay |
| FCGR3A | Fc gamma receptor 3A |
| GRADE | Grading of Recommendations, Assessment, Development, and Evaluation |
| IFN-α | interferon-alpha |
| IL | interleukin |
| MRI | magnetic resonance imaging |
| MRS | magnetic resonance spectrometry |
| NAA/Cr | N-acetylaspartate to creatine |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NOS | Newcastle–Ottawa Scale |
| NP | neuropsychiatric |
| NPSLE | neuropsychiatric systemic lupus erythematosus |
| NR2 | N-methyl-D-aspartate receptor 2 |
| OR | odds ratio |
| pNPSLE | pediatric-onset neuropsychiatric systemic lupus erythematosus |
| PL | phospholipid |
| PNS | peripheral nervous system |
| pSLE | pediatric-onset systemic lupus erythematosus |
| RibP | ribosomal P |
| RNP | ribonucleoprotein |
| SLE | systemic lupus erythematosus |
| Sm | Smith |
| SNP | single nucleotide polymorphism |
| SSA/B | Sjogren’s syndrome A/B |
| TNF | tumor necrosis factor |
References
- The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999, 42, 599–608. [CrossRef]
- Rubinstein, T.B.; Putterman, C.; Goilav, B. Biomarkers for CNS involvement in pediatric lupus. Biomarkers Med. 2015, 9, 545–558. [Google Scholar] [CrossRef]
- Jayasinghe, M.; Rashidi, F.; Gadelmawla, A.F.; Pitton Rissardo, J.; Rashidi, M.; Elendu, C.C.; Fornari Caprara, A.L.; Khalil, I.; Hmedat, K.I.; Atef, M.; et al. Neurological manifestations of systemic lupus erythematosus: A comprehensive review. Cureus 2025, 17, e79569. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, S.; Pereira, D.R.; Julio, P.R.; Reis, F.; Rittner, L.; Marini, R. Neuropsychiatric manifestations in childhood-onset systemic lupus erythematosus. Lancet Child Adolesc. Health 2022, 6, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Natoli, V.; Charras, A.; Hahn, G.; Hedrich, C.M. Neuropsychiatric involvement in juvenile-onset systemic lupus erythematosus (jSLE). Mol. Cell Pediatr. 2023, 10, 5. [Google Scholar] [CrossRef]
- Lindblom, J.; Mohan, C.; Parodis, I. Biomarkers in neuropsychiatric systemic lupus erythematosus: A systematic literature review of the last decade. Brain Sci. 2022, 12, 192. [Google Scholar] [CrossRef]
- Bărbulescu, A.L.; Sandu, R.E.; Vreju, A.F.; Ciurea, P.L.; Criveanu, C.; Firulescu, S.C.; Chisălău, A.B.; Pârvănescu, C.D.; Ciobanu, D.A.; Radu, M.; et al. Neuroinflammation in systemic lupus erythematosus—A review. Rom. J. Morphol. Embryol. 2019, 60, 781–786. [Google Scholar]
- Kammeyer, R.; Chapman, K.; Furniss, A.; Hsieh, E.; Fuhlbrigge, R.; Ogbu, E.A.; Boackle, S.; Zell, J.; Nair, K.V.; Borko, T.L.; et al. Blood-based biomarkers of neuronal and glial injury in active major neuropsychiatric systemic lupus erythematosus. Lupus 2024, 33, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Landman, A.J.E.M.C.; Don, E.E.; Vissers, G.; Ket, H.C.J.; Oudijk, M.A.; de Groot, C.J.M.; Huirne, J.A.F.; de Boer, M.A. Modified Newcastle Ottawa quality assessment scale and AHRQ standards. PLoS ONE 2022, 17, e0269478. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W.; Kunz, R.; Craig, J.; Montori, V.M.; et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef]
- Frittoli, R.B.; Pereira, D.R.; Lapa, A.T.; Postal, M.; Sinicato, N.A.; Fernandes, P.T.; Cendes, F.; Castellano, G.; Rittner, L.; Marini, R.; et al. Axonal dysfunction is associated with interferon-γ levels in childhood-onset systemic lupus erythematosus: A multivoxel magnetic resonance spectroscopy study. Rheumatology 2022, 61, 1529–1537. [Google Scholar] [CrossRef]
- Labouret, M.; Trebossen, V.; Ntorkou, A.; Bartoli, S.; Aubart, M.; Auvin, S.; Bader-Meunier, B.; Baudouin, V.; Corseri, O.; Dingulu, G.; et al. Juvenile neuropsychiatric systemic lupus erythematosus: A specific clinical phenotype and proposal of a probability score. Lupus 2024, 33, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Labouret, M.; Costi, S.; Bondet, V.; Trebossen, V.; Le Roux, E.; Ntorkou, A.; Bartoli, S.; Auvin, S.; Bader-Meunier, B.; Baudouin, V.; et al. Juvenile neuropsychiatric systemic lupus erythematosus: Identification of novel central neuroinflammation biomarkers. J. Clin. Immunol. 2023, 43, 615–624. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, M.W.; Gitelman, D.R.; Klein-Gitelman, M.S.; Sagcal-Gironella, A.C.; Zelko, F.; Beebe, D.; Parrish, T.; Hummel, J.; Ying, J.; Brunner, H.I. Functional neuronal network activity differs with cognitive dysfunction in childhood-onset systemic lupus erythematosus. Arthritis Res. Ther. 2013, 15, R40. [Google Scholar] [CrossRef] [PubMed]
- Gitelman, D.R.; Klein-Gitelman, M.S.; Ying, J.; Sagcal-Gironella, A.C.; Zelko, F.; Beebe, D.W.; Difrancesco, M.; Parrish, T.; Hummel, J.; Beckwith, T.; et al. Brain morphometric changes associated with childhood-onset systemic lupus erythematosus and neurocognitive deficit. Arthritis Rheum. 2013, 65, 2190–2200. [Google Scholar] [CrossRef]
- Jones, J.T.; DiFrancesco, M.; Zaal, A.I.; Klein-Gitelman, M.S.; Gitelman, D.; Ying, J.; Brunner, H.I. Childhood-onset lupus with clinical neurocognitive dysfunction shows lower streamline density and pairwise connectivity on diffusion tensor imaging. Lupus 2015, 24, 705–710. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Dong, J.; Li, H.; Wang, J.; Yao, Y.; Yang, Q.R. Predictors for neuropsychiatric development in Chinese adolescents with systemic lupus erythematosus. Rheumatol. Int. 2012, 32, 2681–2686. [Google Scholar] [CrossRef]
- Lapa, A.T.; Postal, M.; Sinicato, N.A.; Bellini, B.S.; Fernandes, P.T.; Marini, R.; Appenzeller, S. S100b is associated with cognitive impairment in childhood-onset systemic lupus erythematosus patients. Lupus 2017, 26, 478–483. [Google Scholar] [CrossRef]
- Press, J.; Palayew, K.; Laxer, R.M.; Elkon, K.; Eddy, A.; Rakoff, D.; Silverman, E.D. Antiribosomal P antibodies in pediatric patients with systemic lupus erythematosus and psychosis. Arthritis Rheum. 1996, 39, 671–676. [Google Scholar] [CrossRef]
- Rana, A.; Minz, R.; Aggarwal, R.; Anand, S.; Pasricha, N.; Singh, S. Gene expression of cytokines (TNF-α, IFN-γ), serum profiles of IL-17 and IL-23 in paediatric systemic lupus erythematosus. Lupus 2012, 21, 1105–1112. [Google Scholar]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Bao, S.; Huang, H.; Jin, Y.; Ding, F.; Yang, Z.; Xu, X.; Liu, C.; Lu, J.; Jin, Y. Autoantibody-based subgroups and longitudinal seroconversion in juvenile-onset systemic lupus erythematosus. Lupus Sci. Med. 2023, 10, e000834. [Google Scholar]
- Brunner, H.I.; Klein-Gitelman, M.S.; Zelko, F.; Beebe, D.W.; Foell, D.; Lee, J.; Zaal, A.; Jones, J.; Roebuck-Spencer, T.; Ying, J. Blood-based candidate biomarkers of the presence of neuropsychiatric systemic lupus erythematosus in children. Lupus Sci. Med. 2014, 1, e000038. [Google Scholar] [CrossRef] [PubMed]
- Fathy, H.A.; Alkady, M.M.; Tawfik, M.S. Tumor necrosis factor receptor 2 and anti-ribosomal P antibodies as biomarkers in juvenile neuropsychiatric systemic lupus erythematosus. J. Radiat. Res. Appl. Sci. 2022, 15, 232–237. [Google Scholar] [CrossRef]
- Giani, T.; Smith, E.M.D.; Al-Abadi, E.; Armon, K.; Bailey, K.; Ciurtin, C.; Davidson, J.; Gardner-Medwin, J.; Haslam, K.; Hawley, D.P.; et al. Neuropsychiatric involvement in juvenile-onset systemic lupus erythematosus: Data from the UK Juvenile-onset systemic lupus erythematosus cohort study. Lupus 2021, 30, 1955–1965. [Google Scholar] [CrossRef]
- Jurencák, R.; Fritzler, M.; Tyrrell, P.; Hiraki, L.; Benseler, S.; Silverman, E. Autoantibodies in pediatric systemic lupus erythematosus: Ethnic grouping, cluster analysis, and clinical correlations. J. Rheumatol. 2009, 36, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Harel, L.; Sandborg, C.; Lee, T.; von Scheven, E. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus and association with antiphospholipid antibodies. J. Rheumatol. 2006, 33, 1873–1877. [Google Scholar] [PubMed]
- Khajezadeh, M.A.; Zamani, G.; Moazzami, B.; Nagahi, Z.; Mousavi-Torshizi, M.; Ziaee, V. Neuropsychiatric involvement in juvenile-onset systemic lupus erythematosus. Neurol. Res. Int. 2018, 2018, 2548142. [Google Scholar] [CrossRef]
- Liphaus, B.L.; Silva, S.C.; Palmeira, P.; Silva, C.A.; Goldenstein-Schainberg, C.; Carneiro-Sampaio, M. Reduced expressions of apoptosis-related proteins TRAIL, Bcl-2, and TNFR1 in NK cells of juvenile-onset systemic lupus erythematosus patients: Relations with disease activity, nephritis, and neuropsychiatric involvement. Front. Immunol. 2024, 15, 1327255. [Google Scholar] [CrossRef]
- Moraitis, E.; Stathopoulos, Y.; Hong, Y.; Al-Obaidi, M.; Mankad, K.; Hacohen, Y.; Sen, D.; Hemingway, C.; Eleftheriou, D. Aquaporin-4 IgG antibody-related disorders in patients with juvenile systemic lupus erythematosus. Lupus 2019, 28, 1243–1249. [Google Scholar]
- Mostafa, G.A.; Ibrahim, D.H.; Shehab, A.A.; Mohammed, A.K. The role of measurement of serum autoantibodies in prediction of pediatric neuropsychiatric systemic lupus erythematosus. J. Neuroimmunol. 2010, 227, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nowling, T.K.; Kral, M.; Wolf, B.; Gilkeson, G.; Ruth, N.M. Formal neurocognitive function and anti-N-methyl-D-aspartate receptor antibodies in paediatric lupus. Lupus Sci. Med. 2021, 8, e000462. [Google Scholar] [CrossRef]
- Rahman, H.M.; Hashim, H.M.; Karam, R.A. Psychiatric disorders in juvenile systemic lupus erythematosus: Prevalence and association with autoantibodies. Middle East. Curr. Psychiatry 2012, 19, 48–55. [Google Scholar]
- Singh, S.; Gupta, M.K.; Ahluwalia, J.; Singh, P.; Malhi, P. Neuropsychiatric manifestations and antiphospholipid antibodies in pediatric onset lupus: 14 years of experience from a tertiary center of North India. Rheumatol. Int. 2009, 29, 1455–1461. [Google Scholar] [CrossRef]
- Valões, C.C.; Molinari, B.C.; Pitta, A.C.; Gormezano, N.W.; Farhat, S.C.; Kozu, K.; Sallum, A.M.; Appenzeller, S.; Sakamoto, A.P.; Terreri, M.T.; et al. Anti-ribosomal P antibody: A multicenter study in childhood-onset systemic lupus erythematosus patients. Lupus 2017, 26, 484–489. [Google Scholar]
- Yu, H.H.; Lee, J.H.; Wang, L.C.; Yang, Y.H.; Chiang, B.L. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus: A 20-year study. Lupus 2006, 15, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Zuniga Zambrano, Y.C.; Guevara Ramos, J.D.; Penagos Vargas, N.E.; Benitez Ramirez, D.C.; Ramirez Rodriguez, S.M.; Vargas Niño, A.C.; Izquierdo Bello, A.H. Risk factors for neuropsychiatric manifestations in children with systemic lupus erythematosus: Case-control study. Pediatr. Neurol. 2014, 51, 403–409. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, Y.S.; Hammed, A.M.E.; El-Sayed, R.M. Aquaporin-4 IgG antibodies: Predictors of positivity and their relationship with neuropsychiatric disorders and white matter lesions in Juvenile systemic lupus erythematosus. Pediatr. Rheumatol. 2023, 21, 47. [Google Scholar] [CrossRef]
- Soliman, H.; El-Kafafy, E.; Abdelaziz, M. Hyperprolactinemia and its correlation with disease activity and neuropsychiatric manifestations in children with systemic lupus erythematosus. Pediatr. Rheumatol. 2023, 21, 128. [Google Scholar] [CrossRef]
- Ye, C.; Chen, L.; Zhang, L.; Zheng, Y.; Liu, X.; Huang, Z.; Tang, K.; Jiang, X.; Chen, P. IL-17A, IL-23R, FCGR3A are associated with neuropsychiatric systemic lupus erythematosus susceptibility in pediatric patients with lupus nephritis. Cytokine 2025, 188, 156874. [Google Scholar] [CrossRef] [PubMed]
- Miyaue, N.; Yamanishi, Y.; Ito, Y.; Ando, R.; Nagai, M. CSF Neopterin levels are elevated in various neurological diseases and aging. J. Clin. Med. 2024, 13, 4542. [Google Scholar] [CrossRef]
- Mahmoud, R.A.; El-Gendi, H.I.; Ahmed, H.H. Serum neopterin, tumor necrosis factor-alpha and soluble tumor necrosis factor receptor II (p75) levels and disease activity in Egyptian female patients with systemic lupus erythematosus. Clin. Biochem. 2005, 38, 134–141. [Google Scholar]
- Tay, S.H.; Fairhurst, A.M.; Mak, A. Clinical utility of circulating anti-N-methyl-d-aspartate receptor subunits NR2A/B antibody for the diagnosis of neuropsychiatric syndromes in systemic lupus erythematosus and Sjögren’s syndrome: An updated meta-analysis. Autoimmun. Rev. 2017, 16, 114–122. [Google Scholar] [PubMed]
- Mike, E.V.; Makinde, H.M.; Gulinello, M.; Vanarsa, K.; Herlitz, L.; Gadhvi, G.; Winter, D.R.; Mohan, C.; Hanly, J.G.; Mok, C.C.; et al. Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. J. Autoimmun. 2019, 96, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Emerson, J.S.; Gruenewald, S.M.; Gomes, L.; Lin, M.W.; Swaminathan, S. The conundrum of neuropsychiatric systemic lupus erythematosus: Current and novel approaches to diagnosis. Front. Neurol. 2023, 14, 1111769. [Google Scholar] [CrossRef]
- Patel, V. The challenge of neuropsychiatric systemic lupus erythematosus: From symptoms to therapeutic strategies. Diagnostics 2024, 14, 1186. [Google Scholar] [CrossRef]
- Muscal, E.; Brey, R.L. Neurologic manifestations of systemic lupus erythematosus in children and adults. Neurol. Clin. 2010, 28, 61–73. [Google Scholar] [CrossRef]
- Mikdashi, J.; Handwerger, B. Predictors of neuropsychiatric damage in systemic lupus erythematosus: Data from the Maryland lupus cohort. Rheumatology 2004, 43, 1555–1560. [Google Scholar] [CrossRef]
- Sanna, G.; Bertolaccini, M.L.; Cuadrado, M.J.; Laing, H.; Khamashta, M.A.; Mathieu, A.; Hughes, G.R. Neuropsychiatric manifestations in systemic lupus erythematosus: Prevalence and association with antiphospholipid antibodies. J. Rheumatol. 2003, 30, 985–992. [Google Scholar]
- Ho, R.C.; Ong, H.; Thiaghu, C.; Lu, Y.; Ho, C.S.; Zhang, M.W. Genetic variants that are associated with neuropsychiatric systemic lupus erythematosus. J. Rheumatol. 2016, 43, 541–551. [Google Scholar] [CrossRef]
- Imani, D.; Rezaei, R.; Poorsheikhani, A.; Alizadeh, S.; Mahmoudi, M. Association of IL-23R gene rs7517847 T>G SNP and susceptibility to systemic lupus erythematosus: A systematic review and meta-analysis. Rheumatol. Res. 2018, 3, 13–20. [Google Scholar] [CrossRef]
- Padhi, S.; Sarangi, S.; Nayak, N.; Barik, D.; Pati, A.; Panda, A.K. Interleukin 17A rs2275913 polymorphism is associated with susceptibility to systemic lupus erythematosus: A meta and trial sequential analysis. Lupus 2022, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Magro-Checa, C.; Steup-Beekman, G.M.; Huizinga, T.W.; van Buchem, M.A.; Ronen, I. Laboratory and neuroimaging biomarkers in neuropsychiatric systemic lupus erythematosus: Where do we stand, where to go? Front. Med. 2018, 5, 340. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, N.; Toledano, P.; Calvo, A.; Roura, E.; Sarbu, M.I.; Espinosa, G.; Lladó, X.; Cervera, R.; Bargalló, N. Advanced MRI techniques: Biomarkers in neuropsychiatric lupus. Lupus 2017, 26, 510–516. [Google Scholar] [CrossRef] [PubMed]
| Biomarker(s) Analyzed | Key Findings (References) | Clinical Utility Assessment |
|---|---|---|
| Autoantibodies | ||
| Anti-RibP | Early diagnosis of pNPSLE; Screening utility: AUC > 0.7, 86.7% sensitivity, 54% specificity Low sensitivity (0.38) and questionable specificity for detecting psychosis among pSLE patients. | |
| Anti-dsDNA | Conflicting results across studies, low clinical utility in pNPSLE (if any) | |
| Anti-NR2 |
| 86.7% sensitivity, 51% specificity for cognitive impairment in pSLE patients |
| ANA |
| Low utility for pNPSLE |
| Anti-PL | Anti-PL (all):
| Anti-CL: Insight for pathogenic mechanism in pSLE. Low utility as biomarker for pNPSLE Anti-b2GP1: Low utility, mostly no associations reported Lupus anticoagulant: Low diagnostic value, repeated testing limitations; potential use as a risk predictor |
| anti-ENA | No utility | |
| Anti-ganglioside M1 | High predictive value for pNPSLE and cognitive dysfunction; 100% predictive value when combined with anti-RibP | |
| Autoantibody Clusters | ||
| Cluster 1: anti-dsDNA, low prevalence of other autoantibodies; Cluster 2: anti-SSA/SSB, anti-dsDNA, anti-chromatin, anti-RibP, anti-U1RNP, anti-Sm; Cluster 3: anti-dsDNA, anti-U1RNP, anti-Sm |
| Complete autoantibody profile may help predict the clinical course of pNPSLE |
| Cluster 1: anti-Sm/RNPpos Cluster 2: anti-Sm/RNPneg |
| Clustered autoantibodies linked to pNPSLE |
| Neuronal or brain injury markers | ||
| Anti-neuronal |
| Potential value in predicting psychiatric symptoms. High predictive value (100%) if combined with anti-PL and anti-RibP |
| NGAL |
| 92% specificity; very low sensitivity (26.7%) (not useful alone) |
| NMDAR |
| No utility as a biomarker in pNPSLE |
| S100A8/9 |
| Considered clinically irrelevant |
| S100B |
| Low utility, inconsistent results |
| AQP4-IgG | Potential role in patient screening and identification of patients at risk for neurological involvement | |
| CSF neopterin |
| Diagnostic and activity biomarker for pNPSLE Sensitivity: 95%, Specificity: 85% |
| Set of 5 biomarkers (S100A8/9, S100B, NGAL, anti-NR2, anti-RibP) |
| 100% sensitivity, 76% specificity for NCD diagnosis |
| Cytokines/Chemokines | ||
| sTNFR2 |
| No utility for pNPSLE |
| Pan-IFN-α |
| Possible diagnostic utility for CSF levels |
| Peripheral IFN-γ and TNF-α, IL-17, IL-23 |
| Unknown; needs to be assessed further |
| IFN-γ, TNF-α, IL-4, -5, -6, -10, -12, and -17 |
| No utility for pNPSLE |
| NK cell populations |
| No utility for pNPSLE |
| Other | ||
| Prolactin |
| Unknown; more studies required |
| Genetic markers | ||
| SNPs FCGR3A rs396991, IL-17RA rs2895332, and IL-23R rs10889677 |
| Potential screening utility but conflicting literature results; more studies required |
| Neuroimaging markers | ||
| MRS metabolites |
| NAA/Cr and CHO/Cr may be useful biomarkers for pNPSLE |
| MRI (gray/white matter) |
| Morphometric parameters could serve as outcome measures of pNPSLE studies on etiology and treatment |
| Brain activation during fMRI tasks and during performing FNTs |
| fMRI as candidate imaging biomarker for pSLE-associated NCD |
| Diffusion tensor imaging (streamline density, pairwise connectivity) |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapountzi, E.; Kotanidou, E.P.; Tsinopoulou, V.-R.; Tatsiopoulou, P.; Dafoulis, V.; Athanasopoulou, L.; Fotis, L.; Galli-Tsinopoulou, A. Biomarkers in Pediatric Neuropsychiatric Systemic Lupus Erythematosus: A Systematic Review. Life 2025, 15, 1445. https://doi.org/10.3390/life15091445
Sapountzi E, Kotanidou EP, Tsinopoulou V-R, Tatsiopoulou P, Dafoulis V, Athanasopoulou L, Fotis L, Galli-Tsinopoulou A. Biomarkers in Pediatric Neuropsychiatric Systemic Lupus Erythematosus: A Systematic Review. Life. 2025; 15(9):1445. https://doi.org/10.3390/life15091445
Chicago/Turabian StyleSapountzi, Evdoxia, Eleni P. Kotanidou, Vasiliki-Rengina Tsinopoulou, Paraskevi Tatsiopoulou, Vaios Dafoulis, Lilian Athanasopoulou, Lampros Fotis, and Assimina Galli-Tsinopoulou. 2025. "Biomarkers in Pediatric Neuropsychiatric Systemic Lupus Erythematosus: A Systematic Review" Life 15, no. 9: 1445. https://doi.org/10.3390/life15091445
APA StyleSapountzi, E., Kotanidou, E. P., Tsinopoulou, V.-R., Tatsiopoulou, P., Dafoulis, V., Athanasopoulou, L., Fotis, L., & Galli-Tsinopoulou, A. (2025). Biomarkers in Pediatric Neuropsychiatric Systemic Lupus Erythematosus: A Systematic Review. Life, 15(9), 1445. https://doi.org/10.3390/life15091445








