Proarrhythmogenic Echocardiographic Markers in Metabolic Syndrome: A Cross-Sectional Study
Abstract
1. Introduction
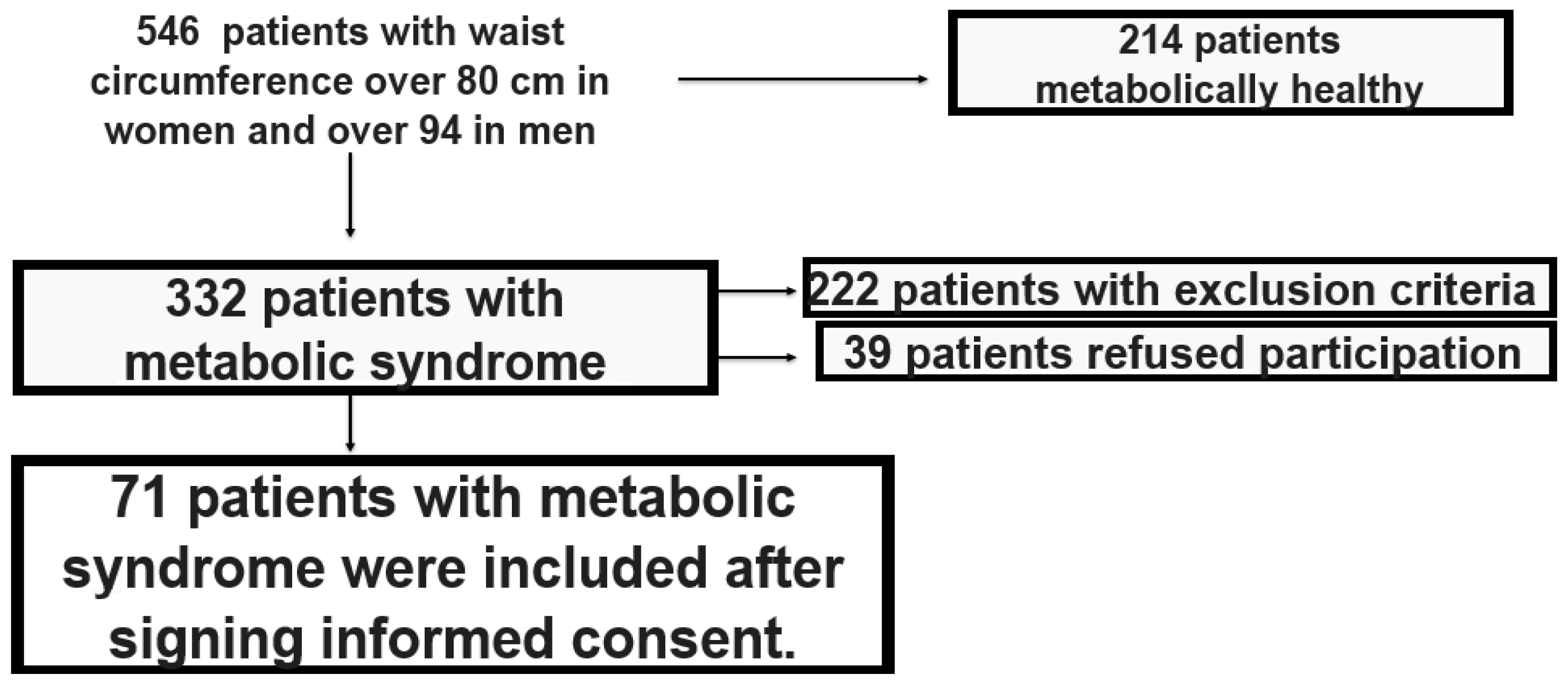
2. Materials and Methods
2.1. Ethical Approval
2.2. Laboratory Diagnostic Tests
2.3. Inflammatory Mediators and Adipokines
2.4. Adiposity-Related Markers
- Minimize translational motion of the heart by using lower frequency transducers (below 2.5 MHz); adjust gain, dynamic range, transmission, and side gain controls appropriately; frame rate ≥ 30/s; harmonic imaging
- Maximize endocardial border delineation-tissue harmonic imaging and contrast echocardiography
- Identify end diastole and end systole-using ventricular cavity size and mitral valve motion, not ECG (end diastole = maximum size and end systole = minimum size)
2.5. Statistical Methods
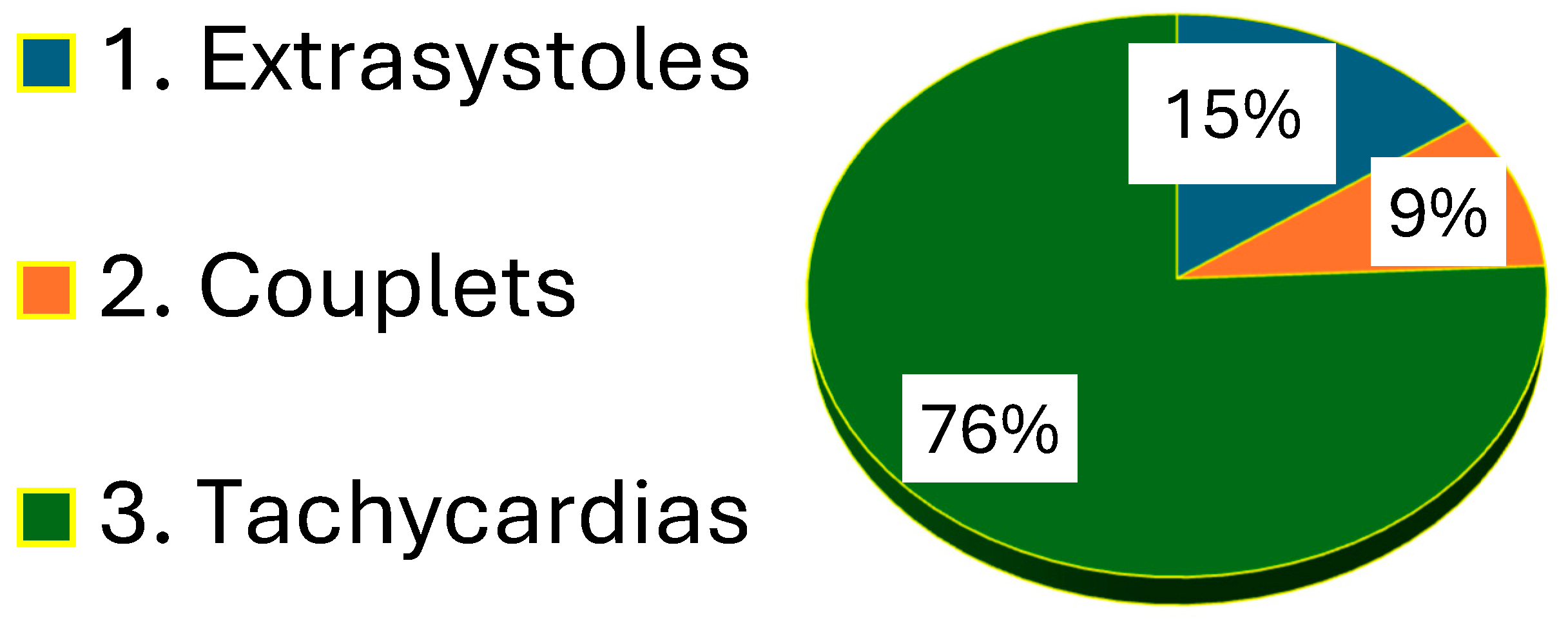
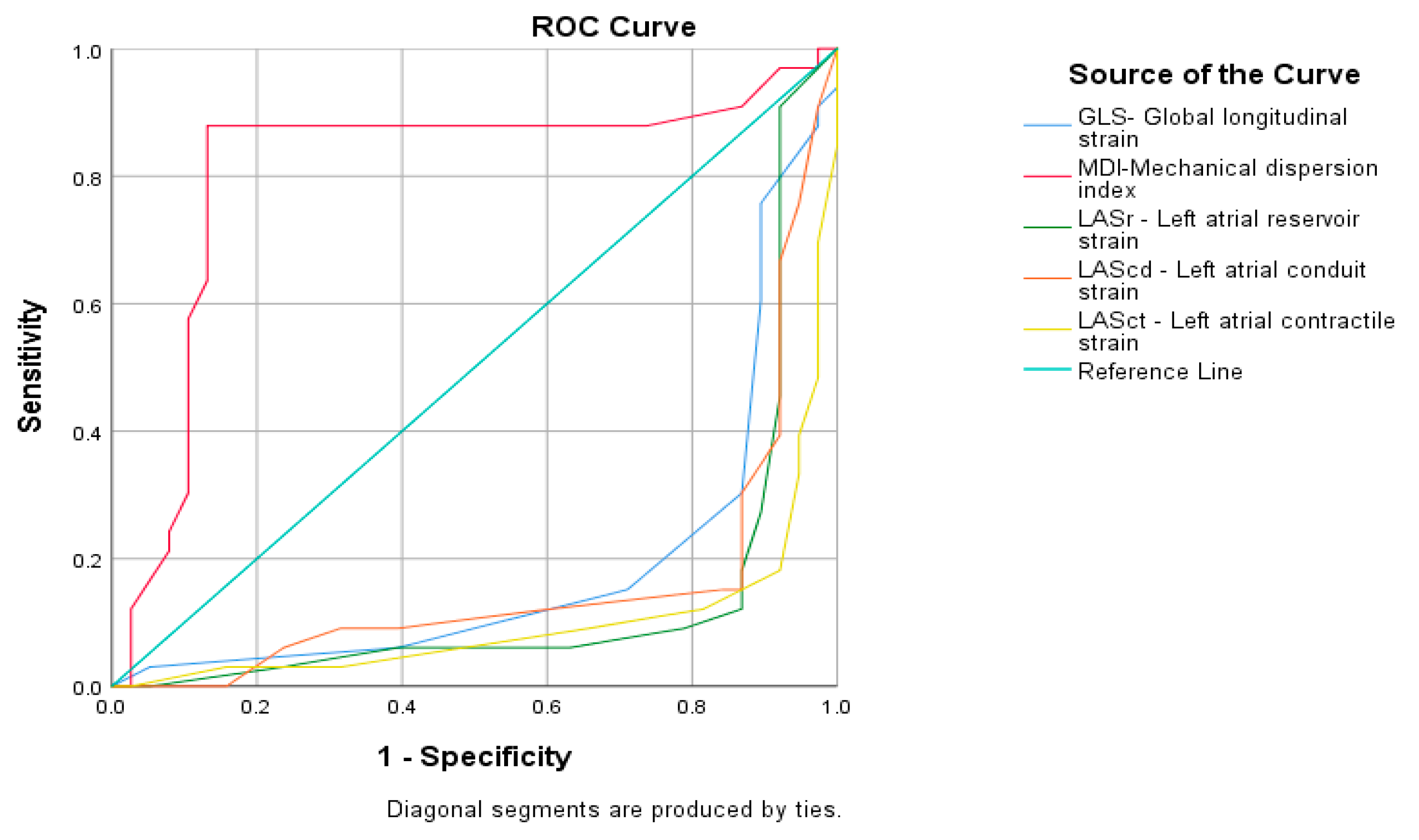
3. Results
4. Discussion
- Speckle tracking echocardiography in metabolic syndrome can be used as proarrhythmogenic screening in newly diagnosed asymptomatic metabolic syndrome.
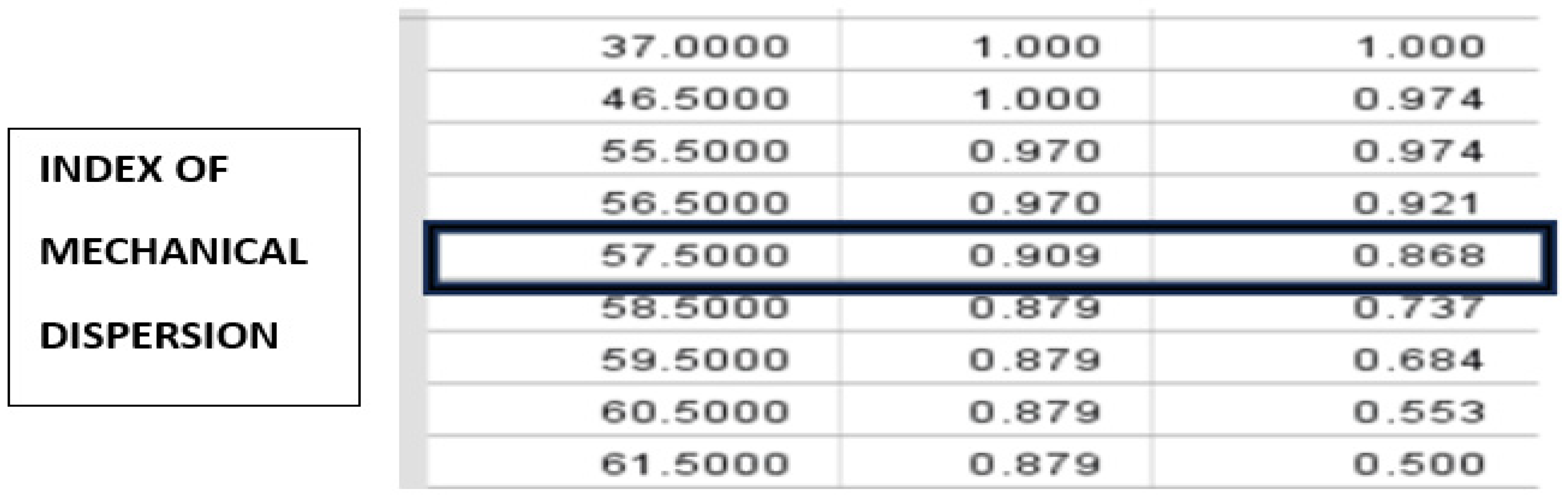
- Mechanical dispersion index with duration over 57.50 msec may be useful in the clinical prognostic assessment of proarrhythmogenicity in newly diagnosed metabolic syndrome. Their determination during routine echocardiography should not be underestimated.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MetS | Metabolic syndrome |
| GLS | Global longitudinal strain |
| MDI | Mechanical dispersion index |
| LASr | Left atrial reservoir strain |
| LAScd | Left atrial conduit strain |
| LASct | Left atrial contractile strain |
References
- Pigeot, I.; Ahrens, W. Epidemiology of metabolic syndrome. Pflügers Arch.-Eur. J. Physiol. 2025, 477, 669–680. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Available online: https://idf.org/media/uploads/2023/05/attachments-30.pdf (accessed on 1 January 2020).
- Patel, K.H.K.; Li, X.; Xu, X.; Sun, L.; Ardissino, M.; Punjabi, P.P.; Purkayastha, S.; Peters, N.S.; Ware, J.S.; Ng, F.S. Increasing Adiposity Is Associated With QTc Interval Prolongation and Increased Ventricular Arrhythmic Risk in the Context of Metabolic Dysfunction: Results From the UK Biobank. Front. Cardiovasc. Med. 2022, 9, 939156. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Russo, A.D.; Casella, M.; Pelargonio, G.; Di Biase, L.; Natale, A. Left ventricular ejection fraction for the risk stratification of sudden cardiac death: Friend or foe? Intern. Med. J. 2011, 41, 55–60. [Google Scholar] [CrossRef]
- Karlsen, S.; Dahlslett, T.; Grenne, B.; Sjøli, B.; Smiseth, O.; Edvardsen, T.; Brunvand, H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc. Ultrasound 2019, 17, 18. [Google Scholar] [CrossRef]
- Papadopoulos, C.H.; Oikonomidis, D.; Lazaris, E.; Nihoyannopoulos, P. Echocardiography and cardiac arrhythmias. Hellenic J. Cardiol. 2018, 59, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Verdugo-Marchese, M.; Coiro, S.; Selton-Suty, C.; Kobayashi, M.; Bozec, E.; Lamiral, Z.; Venner, C.; Zannad, F.; Rossignol, P.; Girerd, N.; et al. Left ventricular myocardial deformation pattern, mechanical dispersion, and their relation with electrocardiogram markers in the large population-based STANISLAS cohort: Insights into electromechanical coupling. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1237–1245. [Google Scholar] [CrossRef]
- Bjerregaard, C.L.; Skaarup, K.G.; Lassen, M.C.H.; Biering-Sørensen, T.; Olsen, F.J. Strain Imaging and Ventricular Arrhythmia. Diagnostics 2023, 13, 1778. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Kurosawa, K.; Hristova, K.; Popescu, B.A.; Vinereanu, D.; Yuda, S.; Marwick, T.H. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc. Imaging 2015, 8, 489–492. [Google Scholar] [CrossRef]
- DeVore, A.D.; McNulty, S.; Alenezi, F.; Ersboll, M.; Vader, J.M.; Oh, J.K.; Lin, G.; Redfield, M.M.; Lewis, G.; Semigran, M.J.; et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: Insights from the RELAX trial. Eur. J. Heart Fail. 2017, 19, 893–900. [Google Scholar] [CrossRef]
- Munk, K.; Andersen, N.H.; Nielsen, S.S.; Bibby, B.M.; BøTker, H.E.; Nielsen, T.T.; Poulsen, S.H. Global longitudinal strain by speckle tracking for infarct size estimation. Eur. J. Echocardiogr. 2011, 12, 156–165. [Google Scholar] [CrossRef]
- Stecker, E.C.; Vickers, C.; Waltz, J.; Socoteanu, C.; John, B.T.; Mariani, R.; McAnulty, J.H.; Gunson, K.; Jui, J.; Chugh, S.S. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon Sudden Unexpected Death Study. J. Am. Coll. Cardiol. 2006, 47, 1161–1166. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Subačius, H.; Patel, T.; Cunnane, R.; Kadish, A.H. Kadish Sudden cardiac death risk stratification in patients with nonischemic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 1879–1889. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Patel, R.B.; Michel, A.; Shah, S.J.; Senni, M.; Gheorghiade, M.; Butler, J. Mode of death in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2017, 69, 556–569. [Google Scholar] [CrossRef]
- Mornoş, C.; Muntean, D.; Mornoş, A.; Crişan, S.; Petrescu, L.; Ionac, A.; Sosdean, R.; Cozma, D. Risk stratification in patients with heart failure: The value of considering both global longitudinal left ventricular strain and mechanical dispersion. Can. J. Physiol. Pharmacol. 2017, 95, 1360–1368. [Google Scholar] [CrossRef]
- Drabik, I.; Mazurek, A.; Czyz, L.; Skubera, M.; Kwiecien, E.; Sikorska, M.; Kulaga, A.; Mikunda, A.; Szot, W.; Kostkiewicz, M.; et al. Relationship between left ventricular global longitudinal strain, infarct size and left ventricular function in patients with acute myocardial infarction in a stem cell therapy study. Eur. Heart J. 2021, 42 (Suppl. S1), ehab724.0277. [Google Scholar] [CrossRef]
- Ersbøll, M.; Valeur, N.; Mogensen, U.M.; Andersen, M.J.; Møller, J.E.; Velazquez, E.J.; Hassager, C.; Søgaard, P.; Køber, L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J. Am. Coll. Cardiol. 2013, 61, 2365–2373. [Google Scholar] [CrossRef]
- Ersbøll, M.; Valeur, N.; Andersen, M.J.; Mogensen, U.M.; Vinther, M.; Svendsen, J.H.; Møller, J.E.; Kisslo, J.; Velazquez, E.J.; Hassager, C.; et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; Langet, H.; Saloux, E.; Manrique, A.; Boileau, L.; Slimani, A.; Allain, P.; Roy, C.; de Meester, C.; Pasquet, A.; et al. Head-to-Head Comparison of Global and Regional Two-Dimensional Speckle Tracking Strain Versus Cardiac Magnetic Resonance Tagging in a Multicenter Validation Study. Circ. Cardiovasc. Imaging 2017, 10, e006530. [Google Scholar] [CrossRef] [PubMed]
- Cañon-Montañez, W.; Santos, A.B.; Nunes, L.A.; Pires, J.C.; Freire, C.M.; Ribeiro, A.L.; Mill, J.G.; Bessel, M.; Duncan, B.B.; Schmidt, M.I.; et al. Central Obesity is the Key Component in the Association of Metabolic Syndrome With Left Ventricular Global Longitudinal Strain Impairment. Rev. Espanola Cardiol. (Engl. Ed.) 2018, 71, 524–530. [Google Scholar] [CrossRef]
- Shen, M.-T.; Li, Y.; Shi, K.; Wang, J.; Jiang, L.; Jiang, Y.; Gao, Y.; Yu, S.-Q.; Li, X.-M.; Yan, W.-F.; et al. The adverse effect of metabolic syndrome on left ventricular global strains and myocardial energetic efficiency in non-ischemic dilated cardiomyopathy patients: A cardiac magnetic resonance study. Cardiovasc. Diabetol. 2025, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, E.N.; Kvisvik, B.; O Pervez, M.; Lyngbakken, M.N.; Berge, T.; Enger, S.; Orstad, E.B.; Smith, P.; Omland, T.; Tveit, A.; et al. Left ventricular mechanical dispersion in a general population: Data from the Akershus Cardiac Examination 1950 study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zanella, H.; Haugaa, K.; Boccalini, F.; Secco, E.; Edvardsen, T.; Badano, L.P.; Muraru, D. Physiological Determinants of Left Ventricular Mechanical Dispersion: A 2-Dimensional Speckle Tracking Echocardiographic Study in Healthy Volunteers. JACC Cardiovasc. Imaging 2018, 11, 650–651. [Google Scholar] [CrossRef]
- Matsuzoe, H.; Tanaka, H.; Matsumoto, K.; Toki, H.; Shimoura, H.; Ooka, J.; Sano, H.; Sawa, T.; Motoji, Y.; Mochizuki, Y.; et al. Leftventricular dyssynergy and dispersion as determinant factors of fatal ventricular arrhythmias in patients with mildly reduced ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 334–342. [Google Scholar] [CrossRef]
- van der Bijl, P.; Khidir, M.J.; Leung, M.; Yilmaz, D.; Mertens, B.; Marsan, N.A.; Delgado, V.; Bax, J.J. Reduced left ventricular mechanical dispersion at 6 months follow-up after cardiac resynchronization therapy is associated with superior long-term outcome. Heart Rhythm 2018, 15, 1683–1689. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Edvardsen, T.; Leren, T.P.; Gran, J.M.; Smiseth, O.A.; Amlie, J.P. Left ventricular mechanical dispersion by tissue Doppler imaging: A novel approach for identifying high-risk individuals with long QT syndrome. Eur. Heart J. 2009, 30, 330–337. [Google Scholar] [CrossRef]
- Lie, Ø.H.; Rootwelt-Norberg, C.; Dejgaard, L.A.; Leren, I.S.; Stokke, M.K.; Edvardsen, T.; Haugaa, K.H. Prediction of Life-Threatening Ventricular Arrhythmia in Patients with Arrhythmogenic Cardiomyopathy: A Primary Prevention Cohort Study. JACC Cardiovasc. Imaging 2018, 11, 1377–1386. [Google Scholar] [CrossRef]
- van Grootel, R.W.; van Den Bosch, A.E.; Baggen, V.J.; Menting, M.E.; Baart, S.J.; Cuypers, J.A.; Witsenburg, M.; Roos-Hesselink, J.W. The Prognostic Value of Myocardial Deformation in Adult Patients with Corrected Tetralogy of Fallot. J. Am. Soc. Echocardiogr. 2019, 32, 866–875.e2. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Nerlekar, N.; Haugaa, K.H.; Edvardsen, T.; Marwick, T.H. Prediction of Ventricular Arrhythmias With Left Ventricular Mechanical Dispersion: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging. 2020, 13 Pt 2, 562–572. [Google Scholar] [CrossRef]
- Aga, Y.S.; Kroon, D.; Snelder, S.M.; Biter, L.U.; Laat, L.E.d.G.-D.; Zijlstra, F.; Brugts, J.J.; van Dalen, B.M. Decreased left atrial function in obesity patients without known cardiovascular disease. Int. J. Cardiovasc. Imaging 2023, 39, 471–479. [Google Scholar] [CrossRef]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: Evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J.; et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ. Cardiovasc. Imaging. 2016, 9, 10. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Morris, D.A.; Cardim, N.; Cikes, M.; Delgado, V.; Donal, E.; Flachskampf, F.A.; Galderisi, M.; Gerber, B.L.; Gimelli, A.; et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2022, 23, e34–e61. [Google Scholar] [CrossRef]
- Frydas, A.; Morris, D.A.; Belyavskiy, E.; Radhakrishnan, A.; Kropf, M.; Tadic, M.; Roessig, L.; Lam, C.S.; Shah, S.J.; Solomon, S.D.; et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail. 2020, 7, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left atrial strain in left ventricular diastolic dysfunction: Have we fnally found the missing piece of the puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef]
- Mălăescu, G.-G.; Mirea, O.; Capotă, R.; Petrescu, A.M.; Duchenne, J.; Voigt, J.-U. Left atrial strain determinants during the cardiac phases. JACC Cardiovasc. Imaging 2022, 15, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Potter, E.L.; Ramkumar, S.; Kawakami, H.; Yang, H.; Wright, L.; Negishi, T.; Marwick, T.H. Association of asymptomatic diastolic dysfunction assessed by left atrial strain with incident heart failure. JACC Cardiovasc. Imaging 2020, 13, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Aung, S.M.; Guler, A.; Guler, Y.; Huraibat, A.; Karabay, C.Y.; Akdemir, I. Left atrial strain in heart failure with preserved ejection fraction. Herz 2017, 42, 194–199. [Google Scholar] [CrossRef]
- Singh, A.; Medvedofsky, D.; Mediratta, A.; Balaney, B.; Kruse, E.; Ciszek, B.; Shah, A.P.; Blair, J.E.; Maffessanti, F.; Addetia, K.; et al. Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular flling pressures. Int. J. Cardiovasc. Imaging 2019, 35, 23–32. [Google Scholar] [CrossRef]
- Fan, J.-L.; Su, B.; Zhao, X.; Zhou, B.-Y.; Ma, C.-S.; Wang, H.-P.; Hu, S.-D.; Zhou, Y.-F.; Ju, Y.-J.; Wang, M.-H. Correlation of left atrial strain with left ventricular end-diastolic pressure in patients with normal left ventricular ejection fraction. Int. J. Cardiovasc. Imaging 2020, 36, 1659–1666. [Google Scholar] [CrossRef]
- Vaishnav, J.; Chasler, J.E.; Lee, Y.J.; Ndumele, C.E.; Hu, J.R.; Schulman, S.P.; Russell, S.D.; Sharma, K. Highest obesity category associated with largest decrease in N-terminal pro-B-type natriuretic peptide in patients hospitalized with heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2020, 9, e015738. [Google Scholar] [CrossRef]
- Lavie, C.J.; Alpert, M.A.; Arena, R.; Mehra, M.R.; Milani, R.V.; Ventura, H.O. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure. JACC Heart Fail. 2013, 1, 93–102. [Google Scholar] [CrossRef]
- Plasek, J.; Skopelidou, V.S.V.; Strakos, J.S.J.; Drienikova, D.D.D.; Rachela, M.R.M.; Pudich, J.P.J.; Vrtal, J.V.J.; Homza, M.H.M.; Vaclavik, J.V.J. Left atrial strain parameters are able to predict presence of atrial fibrillation. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.355. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Ness-Abramof, R.; Apovian, C.M. Waist circumference measurement in clinical practice. Nutr. Clin. Pract. 2008, 23, 397–404. [Google Scholar] [CrossRef]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Pathan, F.; D’Elia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Cuspidi, C.; Majstorovic, A.; Pencic, B.; Backovic, S.; Ivanovic, B.; Scepanovic, R.; Martinov, J.; Kocijancic, V.; Celic, V. Does the metabolic syndrome impact left-ventricular mechanics? A two-dimensional speckle tracking study. J. Hypertens. 2014, 32, 1870–1878. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Smedsrud, M.K.; Steen, T.; Kongsgaard, E.; Loennechen, J.P.; Skjaerpe, T.; Voigt, J.-U.; Willems, R.; Smith, G.; Smiseth, O.A.; et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhythmia. JACC Cardiovasc. Imaging 2010, 3, 247–256. [Google Scholar] [CrossRef]
- Hasselberg, N.E.; Haugaa, K.H.; Bernard, A.; Ribe, M.P.; Kongsgaard, E.; Donal, E.; Edvardsen, T. Left ventricular markers of mortality and ventricular arrhythmias in heart failure patients with cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 343–350. [Google Scholar] [CrossRef]
- Banasik, G.; Segiet, O.; Elwart, M.; Szulik, M.; Lenarczyk, R.; Kalarus, Z.; Kukulski, T. LV mechanical dispersion as a predictor of ventricular arrhythmia in patients with advanced systolic heart failure: A pilot study. Herz 2016, 41, 599–604. [Google Scholar] [CrossRef]
- Segura-Rodríguez, D.; Bermúdez-Jiménez, F.J.; González-Camacho, L.; Escobar, E.M.; García-Orta, R.; Alcalá-López, J.E.; Pavés, A.B.; Oyonarte-Ramírez, J.M.; López-Fernández, S.; Álvarez, M.; et al. Layer-Specific Global Longitudinal Strain Predicts Arrhythmic Risk in Arrhythmogenic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 748003. [Google Scholar] [CrossRef] [PubMed]
- Harapoz, M.; Zada, M.; Matthews, J.; Kumar, S.; Thomas, L. Echocardiographic predictors of ventricular arrhythmias in patients with non-ischemic cardiomyopathy. Int. J. Cardiol. Heart Vasc. 2022, 39, 100962. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Pouleur, A.-C.; Knappe, D.; Al-Ahmad, A.; Gibinski, M.; Wang, P.J.; McNitt, S.; Merkely, B.; Goldenberg, I.; Solomon, S.D.; et al. Dyssynchrony and the risk of ventricular arrhythmias. JACC Cardiovasc. Imaging 2013, 6, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.S. Left Atrial Strain Insights in Atrial Fibrillation and the Interplay with Metabolic Syndrome. Adv. Ther. 2024, 41, 1685–1697. [Google Scholar] [CrossRef]
| Low Arrhythmogenicity (n = 38) | High Arrhythmogenicity (n = 33) | |
|---|---|---|
| Male/Female, n (%) | 20/18 (52.6%/47.4%) | 23/10 (69.7%/30.3%) |
| Age, year | 45.47 ± 1.11 | 45.97 ± 1.32 |
| BMI (kg/m2) | 38.71 ± 1.54 | 40.85 ± 1.22 |
| Waist circumference (sm) | 108.10 ± 19.52 | 129.43 ± 20.35 |
| Waist-hip ratio | 1.07 ± 0.015 | 1.09 ± 0.012 |
| Fat percentage (%) | 39.26 ± 1.32 | 38.92 ± 1.31 |
| Internal fat level | 16.89 ± 0.57 | 18.15 ± 0.50 |
| Arterial hypertension (%) | 34.83± 31.47 | 28.25 ± 29.08 |
| Diabetes Mellitus (%) | 35.12± 25.33 | 33.99 ± 29.55 |
| Total cholesterol (mmol/L) | 5.76 ± 1.34 | 5.8 ± 1.09 |
| Triglycerides (mmol/L) | 2.75 ± 1.9 | 2.0 ± 0.95 |
| HDL-cholesterol (mmol/L) | 1.2 ± 0.24 | 1.27 ± 0.27 |
| LDL-cholesterol (mmol/L) | 3.38 ± 1.23 | 3.7 ± 0.86 |
| Non-HDL-cholesterol | 4.15 ± 1.23 | 4.55 ± 0.86 |
| Apolipoprotein-A1 (g/L) | 1.54 ± 0.40 | 1.52 ± 0.34 |
| Apolipoprotein-B (g/L) | 1.33 ± 0.39 | 1.32 ± 0.53 |
| Apolipoprotein- B/A1 ratio | 0.92 ± 0.38 | 0.97 ± 0.72 |
| TG/HDL ratio | 2.47 ± 2.03 | 1.68 ± 1.02 |
| TG/GL ratio | 0.47 ± 0.34 | 0.35 ± 0.16 |
| LDL/HDL ratio | 2.843 ± 0.15 | 3.06 ± 0.16 |
| LDL/ApoB ratio | 2.7± 0.17 | 3.21 ± 0.21 |
| TG/HDL ratio | 2.47 ± 2.03 | 1.6 ± 1.02 |
| Glucose (mmol/L) | 5.9 ± 1.47 | 5.7 ± 1.34 |
| НОМА index | 5.17 ± 4.27 | 6,5 ± 7.3 |
| Glycated hemoglobin (%) | 5.6 ± 0.76 | 5.7 ± 0.66 |
| Immunoreactive insulin (μU/mL) | 18.66 ± 12.47 | 23.81 ± 19.55 |
| Adiponectin (µg/mL) | 8.1 ± 3.8 | 7.9 ± 3.31 |
| Leptin (ng/mL) | 64.7 ± 51.12 | 74.8 ± 57.9 |
| Adiponectin/Leptin | 1.7 ± 1.42 | 0.20 ± 0.04 |
| hsCRP (mg/dL) | 9.8 ± 3.3 | 8.9 ± 4.0 |
| Visfatin (ng/mL) | 5.97 ± 3 | 5.62 ± 2.7 |
| Interleukin-6 (pg/mL) | 15.9 ± 10.16 | 58.08 ± 77.75 |
| TNF- α (pg/mL) | 6.5 ± 7.08 | 26.96 ± 30.59 |
| Left ventricular endodiastolic size (sm) | 4.867 (0.148–0.179) | 4.217 (0.136–0.227) |
| E/A | 0.68 (0.56–0.89) | 0.64 (0.50–0.87) |
| E/e‘ | 17.5 (13.75–18) | 18.7 (15–19) |
| Septal velocity e‘ (m/s) | 6.5 (5.3–7.25) | 4.5 (4.9–7.5) |
| V max tricuspid insufficiency (m/s) | 2.0 (1.75–2.5) | 2.05(1.6–2.6) |
| Left atrial volume indexed for height2 | 19.5 (17.6–22.5) | 21.7 (18.5–22.5) |
| GLS (%) | 21.9 ± 1.3 | 20.1 ± 1.3 |
| MDI (ms) | 64 ± 10.5 | 79.8 ± 10.5 |
| LASr (%) | 36.31 ± 5.7 | 25.3 ± 5.2 |
| LAScd (%) | 20.3 ± 3.4 | 14.5 ± 3.7 |
| LAct (%) | 15.7 ± 1.9 | 11.2 ± 2.6 |
| Group | Low Arrhythmogenicity (n = 38) | High Arrhythmogenicity (n = 33) | p-Value |
| Echo-marker | Median (25th–75th) | Median (25th–75th) | |
| Left ventricular endodiastolic size (sm) | 4.867 (0.148–0.179) | 4.217 (0.136–0.227) | 0.44 |
| E/A | 0.68 (0.56–0.89) | 0.64 (0.50–0.87) | 0.854 |
| E/e′ | 17.5 (13.75–18) | 18.7 (15–19) | 0.045 |
| Septal velocity e′ | 6.5 (5.3–7.25) | 4.5 (4.9–7.5) | 0.55 |
| V max tricuspid insufficiency | 2.0 (1.75–2.5) | 2.05 (1.6–2.6) | 0.712 |
| Left atrial volume indexed for h2 | 19.5 (17.6–22.5) | 21.7 (18.5–22.5) | 0.913 |
| Group | Low Arrhythmogenicity (n = 38) | High Arrhythmogenicity (n = 33) | p-Value |
| Marker | mean ± SEM | mean ± SEM | |
| GLS | 21.90±0.23 | 20.17 ± 0.23 | <0.0001 |
| MDI | 64 ± 1.72 | 79 ± 1.83 | <0.0001 |
| LASr | 36.32 ± 0.93 | 25.39 ± 0.91 | <0.0001 |
| LAScd | 20.38 ± 0.56 | 14.52 ± 0.66 | <0.0001 |
| LASct | 15.74 ± 0.31 | 11.21 ± 0.46 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitov, S.; Kitova, M.-F.; Nonchev, B.; Tokmakova, M.; Kitova, L. Proarrhythmogenic Echocardiographic Markers in Metabolic Syndrome: A Cross-Sectional Study. Life 2025, 15, 1443. https://doi.org/10.3390/life15091443
Kitov S, Kitova M-F, Nonchev B, Tokmakova M, Kitova L. Proarrhythmogenic Echocardiographic Markers in Metabolic Syndrome: A Cross-Sectional Study. Life. 2025; 15(9):1443. https://doi.org/10.3390/life15091443
Chicago/Turabian StyleKitov, Spas, Maria-Florance Kitova, Boyan Nonchev, Mariya Tokmakova, and Lyudmila Kitova. 2025. "Proarrhythmogenic Echocardiographic Markers in Metabolic Syndrome: A Cross-Sectional Study" Life 15, no. 9: 1443. https://doi.org/10.3390/life15091443
APA StyleKitov, S., Kitova, M.-F., Nonchev, B., Tokmakova, M., & Kitova, L. (2025). Proarrhythmogenic Echocardiographic Markers in Metabolic Syndrome: A Cross-Sectional Study. Life, 15(9), 1443. https://doi.org/10.3390/life15091443






