Diabetic Kidney Disease: Evidence from Two Selected Cohorts of Patients from Low–Middle and High Income Countries
Abstract
1. Introduction
2. Methods
2.1. Integrated Multidisciplinary Outpatient Service
2.1.1. Physician Team
2.1.2. Visit Protocol
2.2. Integrated NCDs Clinic
2.2.1. Clinical Team
2.2.2. Visit Protocol
2.3. Variables Analyzed
2.4. Statistical Analysis
3. Results
3.1. Demographical Features and Complications
3.2. HbA1c over Time
3.3. Renal Function over Time
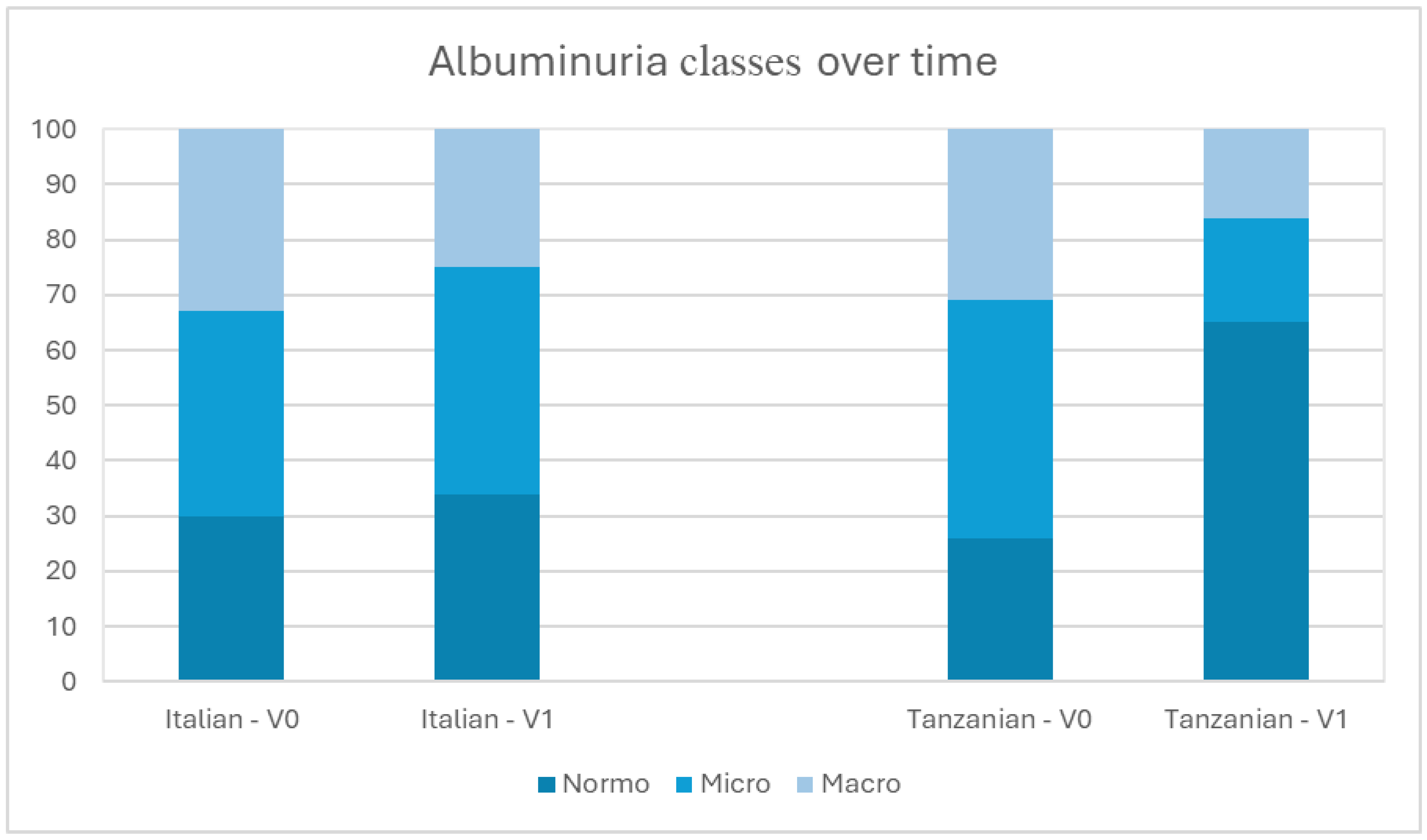
3.4. Albuminuria over Time
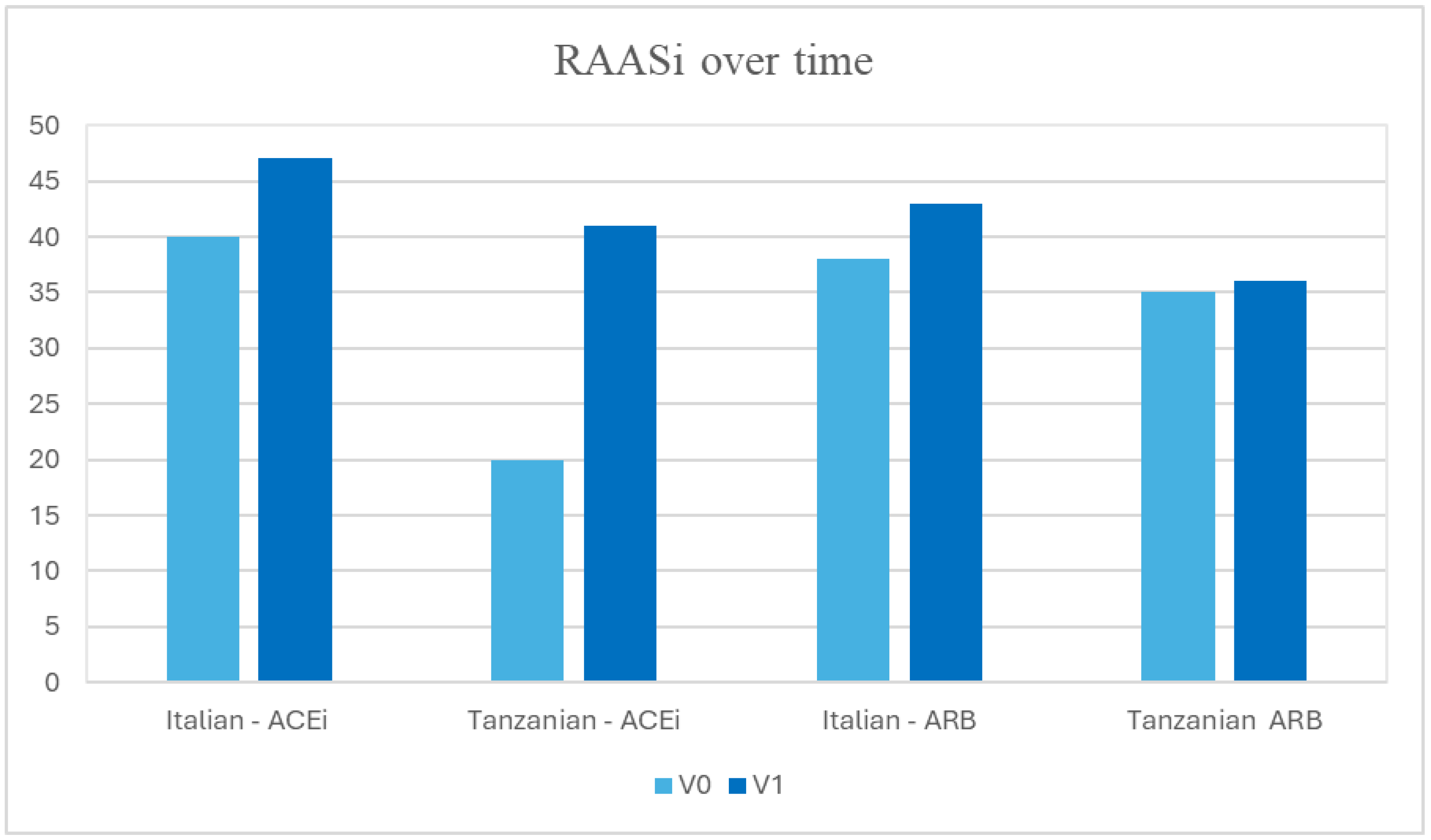
3.5. RAASi over Time
3.6. SGLT2i over Time
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Pugliese, G.; Penno, G.; Natali, A.; Barutta, F.; Di Paolo, S.; Reboldi, G.; Gesualdo, L.; De Nicola, L. Diabetic kidney disease: New clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1127–1150. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C.E. Microalbuminuria, blood pressure and diabetic renal disease: Origin and development of ideas. Diabetologia 1999, 42, 263–285. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Mahmud, H.; Korytkowski, M.T. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2018, 47, 81–96. [Google Scholar] [CrossRef]
- Chang, A.R.; Surapaneni, A.; Kirchner, H.L.; Young, A.; Kramer, H.J.; Carey, D.J.; Appel, L.J.; Grams, M.E. Metabolically Healthy Obesity and Risk of Kidney Function Decline. Obesity 2018, 26, 762–768. [Google Scholar] [CrossRef]
- Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [CrossRef]
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Bukhman, G.; Mocumbi, A.O.; Horton, R. Reframing NCDs and injuries for the poorest billion: A Lancet Commission. Lancet 2015, 386, 1221–1222. [Google Scholar] [CrossRef]
- World Bank. Monitoring Global Poverty; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Peck, R.; Mghamba, J.; Vanobberghen, F.; Kavishe, B.; Rugarabamu, V.; Smeeth, L.; Hayes, R.; Grosskurth, H.; Kapiga, S. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: A cross-sectional survey. Lancet. Glob. Health 2014, 2, e285–e292. [Google Scholar] [CrossRef]
- Maruyama, H.; Franks, J.; Laki, D.; Msumi, O.; Makyao, N.; Rwabiyago, O.E.; Rabkin, M.; Kagashe, M.J.; El-Sadr, W.M. Bringing HIV services to key populations and their communities in Tanzania: From pilot to scale. J. Int. AIDS Soc. 2021, 24 (Suppl. S3), e25718. [Google Scholar] [CrossRef] [PubMed]
- Jayapaul, M.K.; Messersmith, R.; Bennett-Jones, D.N.; Mead, P.A.; Large, D.M. The joint diabetic-renal clinic in clinical practice: 10 years of data from a District General Hospital. QJM 2006, 99, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Asmelash, D.; Asmelash, Y. The Burden of Undiagnosed Diabetes Mellitus in Adult African Population: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 4134937. [Google Scholar] [CrossRef]
- Ponce, D.; Balbi, A. Acute kidney injury: Risk factors and management challenges in developing countries. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 193–200. [Google Scholar] [CrossRef]
- Halle, M.P.E.; Chipekam, N.M.; Beyiha, G.; Fouda, H.; Coulibaly, A.; Hentchoya, R.; Kaze, F.F.; Luma, N.H.; Ashuntantang, G. Incidence, characteristics and prognosis of acute kidney injury in Cameroon: A prospective study at the Douala General Hospital. Ren. Fail. 2018, 40, 30–37. [Google Scholar] [CrossRef]
- Masewu, A.; Makulo, J.-R.; Lepira, F.; Amisi, E.B.; Sumaili, E.K.; Bukabau, J.; Mokoli, V.; Longo, A.; Nlandu, Y.; Engole, Y.; et al. Acute kidney injury is a powerful independent predictor of mortality in critically ill patients: A multicenter prospective cohort study from Kinshasa, the Democratic Republic of Congo. BMC Nephrol. 2016, 17, 118. [Google Scholar] [CrossRef]
- Dlamini, T.A.L.; Heering, P.J.; Chivese, T.; Rayner, B. A prospective study of the demographics, management and outcome of patients with acute kidney injury in Cape Town, South Africa. PLoS ONE 2017, 12, e0177460. [Google Scholar] [CrossRef]
- Kohli, H.S.; Bhat, A.; Jairam, A.; Aravindan, A.N.; Sud, K.; Jha, V.; Gupta, K.L.; Sakhuja, V. Predictors of mortality in acute renal failure in a developing country: A prospective study. Ren. Fail. 2007, 29, 463–469. [Google Scholar] [CrossRef]
- Safari, S.; Hashemi, B.; Forouzanfar, M.M.; Shahhoseini, M.; Heidari, M. Epidemiology and Outcome of Patients with Acute Kidney Injury in Emergency Department; a Cross-Sectional Study. Emergency 2018, 6, e30. [Google Scholar]
- Lutale, J.J.K.; Thordarson, H.; Abbas, Z.G.; Vetvik, K. Microalbuminuria among Type 1 and Type 2 diabetic patients of African origin in Dar Es Salaam, Tanzania. BMC Nephrol. 2007, 8, 2. [Google Scholar] [CrossRef]
- Janmohamed, M.N.; Kalluvya, S.E.; Mueller, A.; Kabangila, R.; Smart, L.R.; Downs, J.A.; Peck, R.N. Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrol. 2013, 14, 183. [Google Scholar] [CrossRef]
- Patel, M.; Shilliday, I.R.; McKay, G.A. A combined diabetes renal clinic improves risk factor management and progression of renal disease in a district general hospital. J. Eval. Clin. Pract. 2009, 15, 832–835. [Google Scholar] [CrossRef]
- Joss, N.; Paterson, K.R.; Deighan, C.J.; Simpson, K.; Boulton-Jones, J.M. Diabetic nephropathy: How effective is treatment in clinical practice? QJM 2002, 95, 41–49. [Google Scholar] [CrossRef]
- Slade, H.; Williams, S.M.; Manning, P.J.; Walker, R.J. High-risk diabetic nephropathy patients: The outcome of evidence-based clinical practice in an outpatient clinic. Diabetes Res. Clin. Pract. 2011, 92, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef] [PubMed]
- Liew, B.S.; Perry, C.; Boulton-Jones, J.M.; Simpson, K.; Paterson, K. Diabetic nephropathy: An observational study on patients attending a joint diabetes renal clinic. QJM 1997, 90, 353–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, C.; Beaulieu, M.; Djurdjev, O.; Er, L.; Taylor, P.; Ignaszewski, A.; Burnett, S.; Levin, A. Towards rational approaches of health care utilization in complex patients: An exploratory randomized trial comparing a novel combined clinic to multiple specialty clinics in patients with renal disease-cardiovascular disease-diabetes. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27 (Suppl. S3), iii104–iii110. [Google Scholar] [CrossRef]
- Zimbudzi, E.; Lo, C.; Ranasinha, S.; Earnest, A.; Teede, H.; Usherwood, T.; Polkinghorne, K.R.; Fulcher, G.; Gallagher, M.; Jan, S.; et al. A co-designed integrated kidney and diabetes model of care improves mortality, glycaemic control and self-care. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2022, 37, 1472–1481. [Google Scholar] [CrossRef]
- Zimbudzi, E.; Lo, C.; Ranasinha, S.; Teede, H.; Usherwood, T.; Polkinghorne, K.R.; Fulcher, G.; Gallagher, M.; Jan, S.; Cass, A.; et al. Health-related quality of life among patients with comorbid diabetes and kidney disease attending a codesigned integrated model of care: A longitudinal study. BMJ Open Diabetes Res. Care 2020, 8, e000842. [Google Scholar] [CrossRef]
- Adedokun, S.T.; Yaya, S. Factors influencing mothers’ health care seeking behaviour for their children: Evidence from 31 countries in sub-Saharan Africa. BMC Health Serv. Res. 2020, 20, 842. [Google Scholar] [CrossRef]
- Balcha, S.A.; Phillips, D.I.W.; Trimble, E.R. Type 1 Diabetes in a Resource-Poor Setting: Malnutrition Related, Malnutrition Modified, or Just Diabetes? Curr. Diab. Rep. 2018, 18, 47. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Yu, R.; Craig, J.C.; Polkinghorne, K.R.; Atkins, R.C.; Chadban, S.J. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 58, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Nadkarni, G.N.; Grams, M.E.; Sang, Y.; Ballew, S.H.; Coresh, J.; Matsushita, K.; Surapaneni, A.; Brunskill, N.; Chadban, S.J.; et al. Conversion of Urine Protein-Creatinine Ratio or Urine Dipstick Protein to Urine Albumin-Creatinine Ratio for Use in Chronic Kidney Disease Screening and Prognosis: An Individual Participant-Based Meta-analysis. Ann. Intern. Med. 2020, 173, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, A.; Zidi, I.; Zidi, N.; Mnif, W.; Zinelabidine, H.T. Nephropathy following type 2 diabetes mellitus in Tunisian population. West Indian Med. J. 2012, 61, 881–889. [Google Scholar] [CrossRef][Green Version]
- Alebiosu, C.O.; Ayodele, O.E. The increasing prevalence of diabetic nephropathy as a cause of end stage renal disease in Nigeria. Trop. Doct. 2006, 36, 218–219. [Google Scholar] [CrossRef]
- Mafundikwa, A.; Ndhlovu, C.E.; Gomo, Z. The prevalence of diabetic nephropathy in adult patients with insulin dependent diabetes mellitus attending Parirenyatwa Diabetic Clinic, Harare. Cent. Afr. J. Med. 2007, 53, 1–6. [Google Scholar] [CrossRef]
- Reutens, A.T.; Atkins, R.C. Epidemiology of diabetic nephropathy. Contrib. Nephrol. 2011, 170, 1–7. [Google Scholar] [CrossRef]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Smide, B. Outcome of foot examinations in Tanzanian and Swedish diabetic patients, a comparative study. J. Clin. Nurs. 2009, 18, 391–398. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J. V Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
| Tosamaganga (N = 139) | Bologna (N = 235) | Total (N = 374) | p-Value | |
|---|---|---|---|---|
| Sex, n (%) | <0.001 | |||
| F | 93 (66.9%) | 60 (25.5%) | 153 (40.9%) | |
| M | 46 (33.1%) | 175 (74.5%) | 221 (59.1%) | |
| Age, years | 56.65 (13.93) | 67.66 (12.21) | 63.56 (13.92) | <0.001 |
| Smoke, n (%) | <0.001 | |||
| 9 (6.5%) | 33 (14.1%) | 42 (11.3%) | |
| 0 (0.0%) | 103 (44.0%) | 103 (27.6%) | |
| Retinopathy, n (%) | 54 (38.8%) | 61 (26.5%) | 115 (31.2%) | 0.015 |
| Diabetic foot, n (%) | 7 (5.0%) | 20 (8.7%) | 27 (7.3%) | 0.296 |
| HF, n (%) | 10 (7.2%) | 13 (8.8%) | 23 (8.0%) | 0.668 |
| MACE, n (%) | 1 (0.7%) | 70 (30.3%) | 71 (19.2%) | <0.001 |
| Stroke, n (%) | 10 (7.2%) | 18 (12.0%) | 28 (9.7%) | |
| BMI, kg/m2 (SD) | 26.39 (5.58) | 30.18 (5.80) | 28.75 (6.00) | <0.001 |
| Hba1c, mmol/mol (SD) | 83.71 (32.03) | 56.92 (13.79) | 65.96 (25.12) | <0.001 |
| Total Cholesterol, mg/dL (SD) | 159.18 (57.53) | 168.12 (44.91) | 164.58 (50.40) | 0.028 |
| sCr, mg/dL (SD) | 1.38 (0.98) | 1.49 (0.52) | 1.45 (0.73) | <0.001 |
| eGFR, mL/min/1.73m2 (SD) | 70.13 (31.93) | 52.31 (23.37) | 58.97 (28.20) | <0.001 |
| Albuminuria class, n (%) | 0.617 | |||
| 36 (26.5%) | 54 (29.7%) | 90 (28.3%) | |
| 58 (42.6%) | 68 (37.4%) | 126 (39.6%) | |
| 42 (30.9%) | 60 (33.0%) | 102 (32.1%) | |
| ACEi, n (%) | 16 (20.0%) | 94 (40.0%) | 110 (34.9%) | 0.001 |
| ARB, n (%) | 28 (35.0%) | 89 (37.9%) | 117 (37.1%) | 0.689 |
| CCB, n (%) | 36 (45.0%) | 69 (42.6%) | 105 (43.4%) | 0.783 |
| Diuretics, n (%) | 24 (30%) | 69 (43%) | 93 (38.5%) | 0.045 |
| Metoformin, n (%) | 112 (80.6%) | 114 (48.5%) | 226 (60.4%) | <0.001 |
| Sulfonylureas, n (%) | 75 (54.0%) | 31 (13.2%) | 106 (28.3%) | <0.001 |
| Insulin, n (%) | 9 (6.5%) | 102 (43.4%) | 111 (29.7%) | <0.001 |
| SGLT2i, n (%) | - | 42 (18.0%) | 42 (11.2%) | - |
| GLP1-RA, n (%) | - | 30 (12.7%) | 30 (8.0%) | - |
|
Tosamaganga
(N = 139) |
Bologna
(N = 235) |
Total
(N = 374) | p-Value | |
|---|---|---|---|---|
| BMI kg/m2 (SD) | 27.35 (5.88) | 29.64 (5.00) | 28.86 (5.41) | 0.015 |
| Hba1c, mmol/mol (SD) | 59.07 (28.80) | 55.77 (12.18) | 56.65 (18.12) | 0.53’ |
| Total Cholesterol, mg/dL (SD) | 155.71 (52.65) | 170.00 (47.09) | 166.73 (48.61) | 0.061 |
| sCr, mg/dL (SD) | 1.20 (0.92) | 1.52 (0.55) | 1.43 (0.69) | <0.001 |
| eGFR, mL/min/1.73m2 (SD) | 79.38 (34.17) | 49.90 (21.37) | 58.80 (29.17) | <0.001 |
| Albuminuria class, n (%) | 0.617 | |||
| 65.0% | 34.0% | 28.3% | |
| 19.0% | 41.0% | 39.6% | |
| 16.0% | 25.0% | 32.1% | |
| ACEi, (%) | 41.0% | 47.0% | 45.0% | 0.001 |
| ARB, (%) | 36.0% | 43.0% | 41.9% | 0.689 |
| SLT2i, n (%) | - | 112 (48%) | 112 (30%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattiotti, M.; Righini, M.; Vetrano, D.; Ribichini, D.; Vicennati, V.; Aiello, V.; Notaro, E.; Belardi, P.; Bazzanini, N.; Mutalemwa, K.; et al. Diabetic Kidney Disease: Evidence from Two Selected Cohorts of Patients from Low–Middle and High Income Countries. Life 2025, 15, 1429. https://doi.org/10.3390/life15091429
Mattiotti M, Righini M, Vetrano D, Ribichini D, Vicennati V, Aiello V, Notaro E, Belardi P, Bazzanini N, Mutalemwa K, et al. Diabetic Kidney Disease: Evidence from Two Selected Cohorts of Patients from Low–Middle and High Income Countries. Life. 2025; 15(9):1429. https://doi.org/10.3390/life15091429
Chicago/Turabian StyleMattiotti, Maria, Matteo Righini, Daniele Vetrano, Danilo Ribichini, Valentina Vicennati, Valeria Aiello, Ermanno Notaro, Paolo Belardi, Noemi Bazzanini, Katunzi Mutalemwa, and et al. 2025. "Diabetic Kidney Disease: Evidence from Two Selected Cohorts of Patients from Low–Middle and High Income Countries" Life 15, no. 9: 1429. https://doi.org/10.3390/life15091429
APA StyleMattiotti, M., Righini, M., Vetrano, D., Ribichini, D., Vicennati, V., Aiello, V., Notaro, E., Belardi, P., Bazzanini, N., Mutalemwa, K., Ndile, E., Itambu, R., Pagotto, U., Azzimonti, G., Cianciolo, G., Capelli, I., & La Manna, G. (2025). Diabetic Kidney Disease: Evidence from Two Selected Cohorts of Patients from Low–Middle and High Income Countries. Life, 15(9), 1429. https://doi.org/10.3390/life15091429








