Ferreting Out the Effects of Neonatal Hypoxia–Ischemia and Sex on Ferret Cortical Gyrification
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Hypoxic–Ischemic Injury Model
2.3. Behavioral Testing
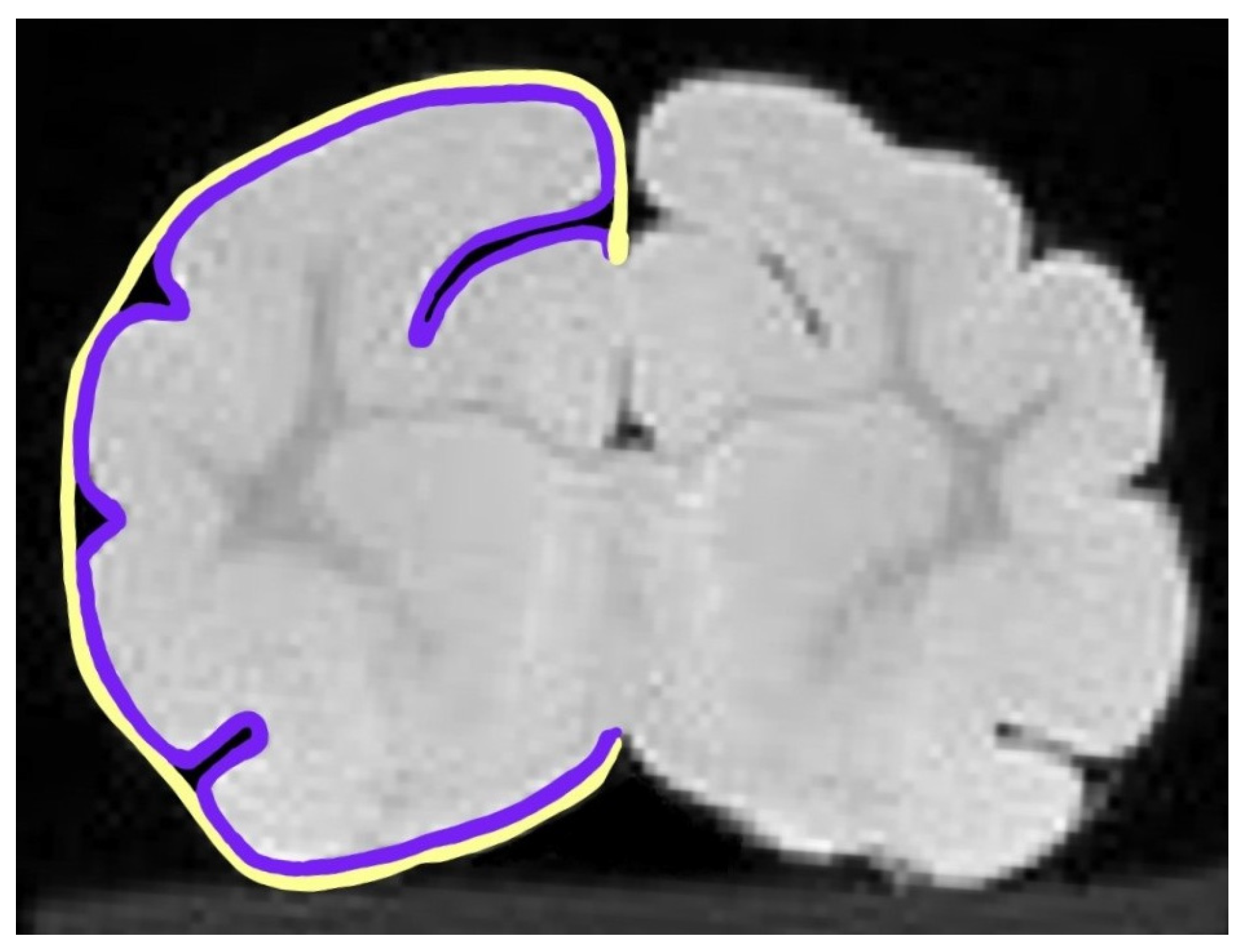
2.4. Gyrification Index
2.5. Statistical Analysis
3. Results
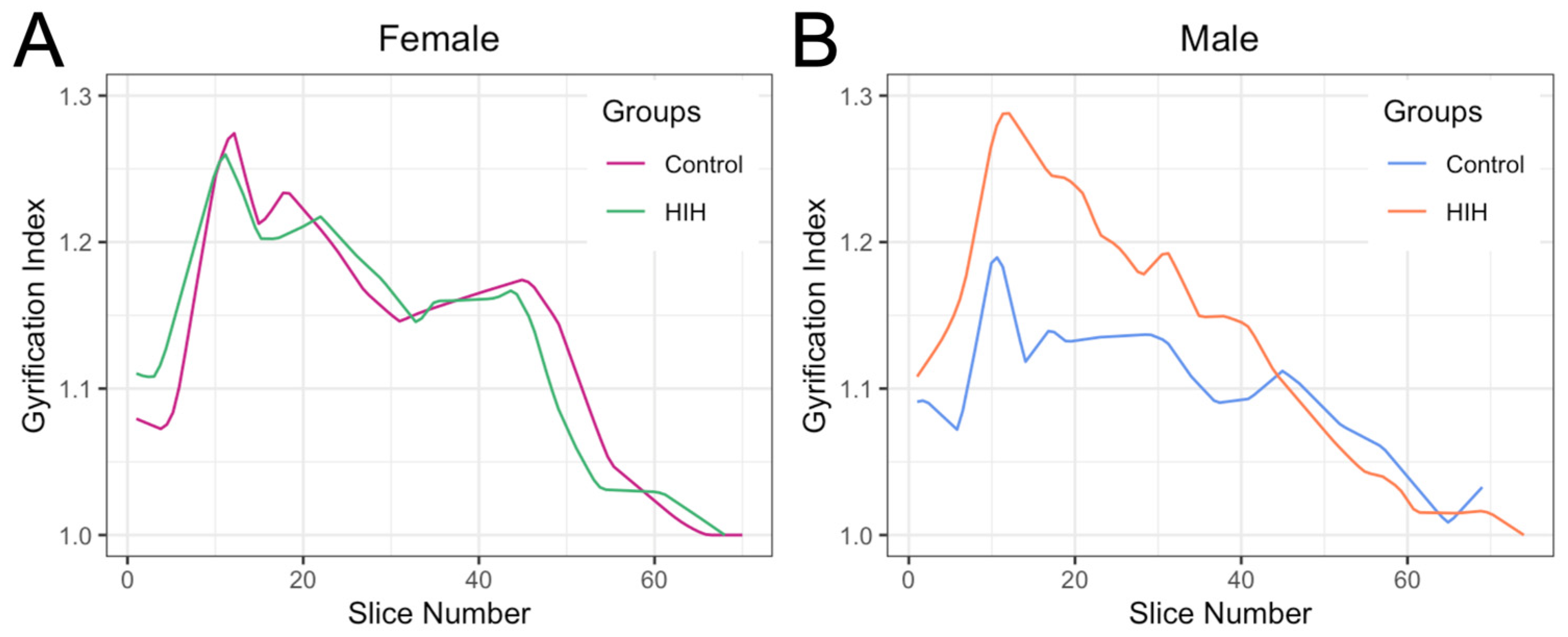
3.1. GI Trends Across the Cortex and Hemispheres
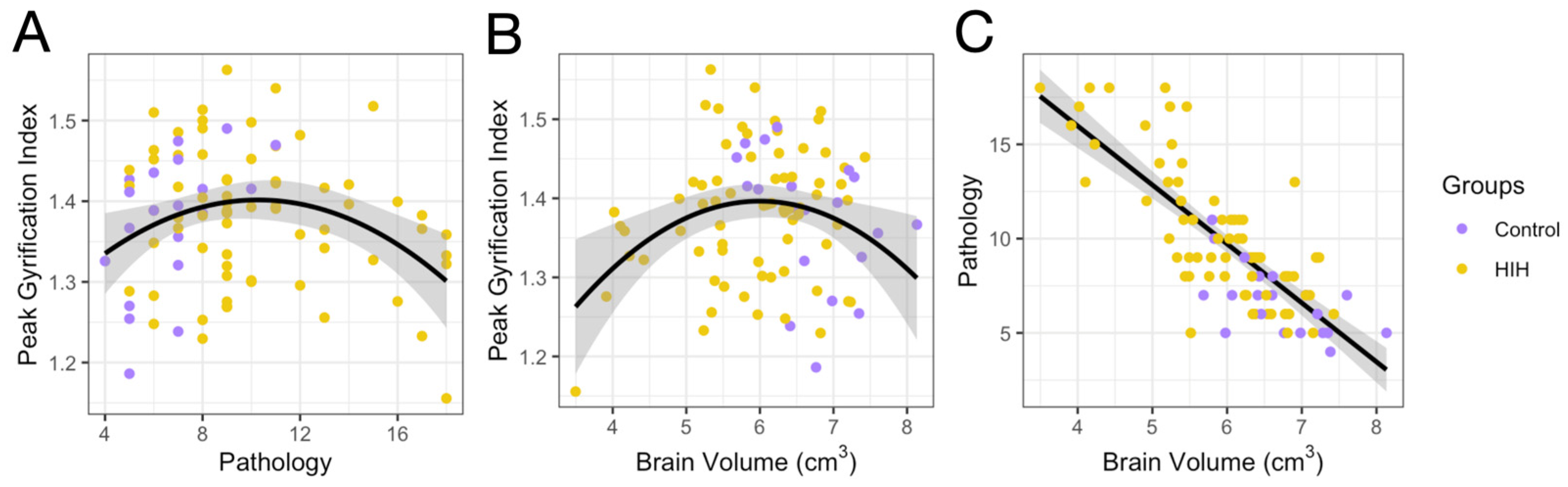
3.2. GI and Association with Injury Metrics
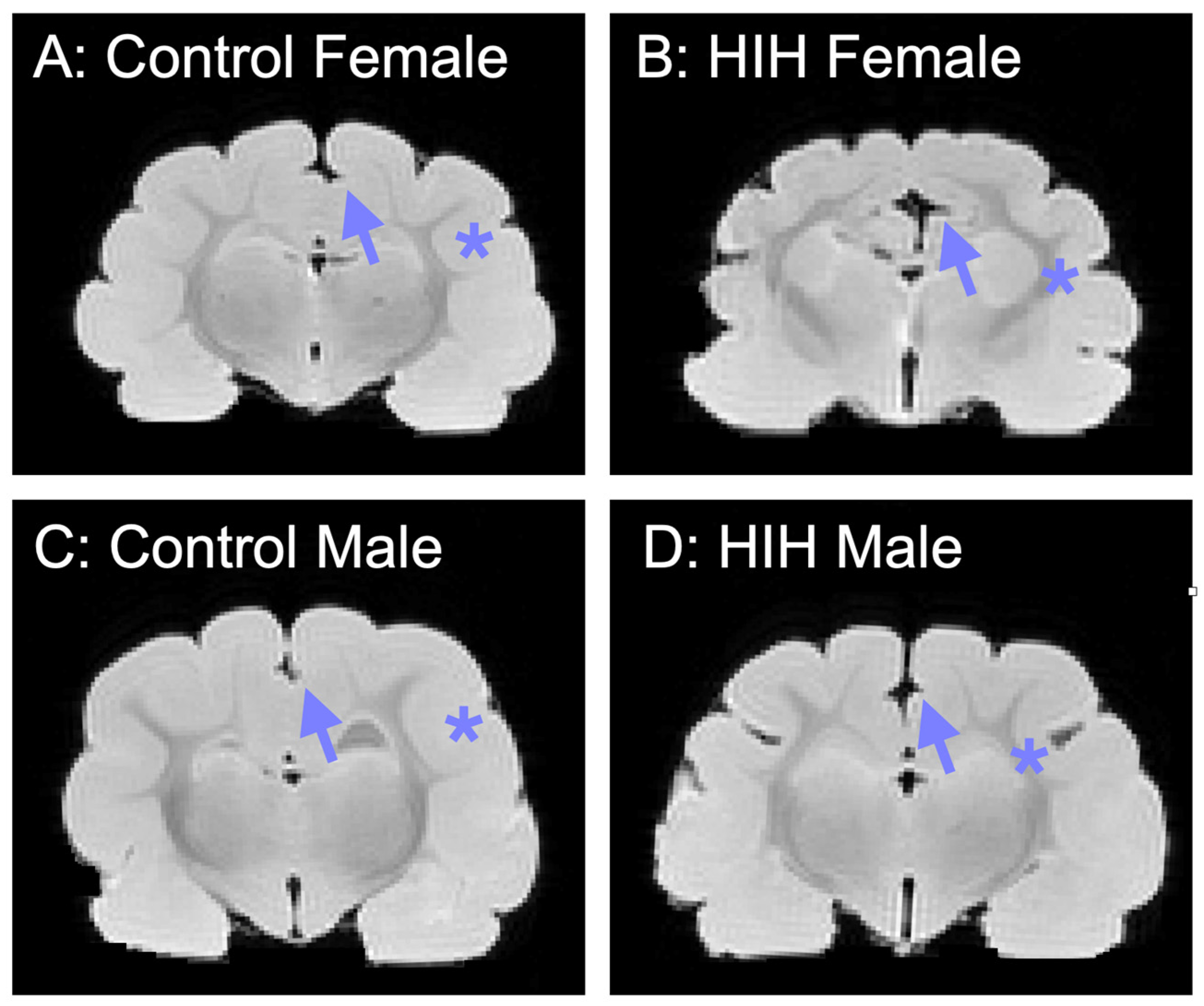
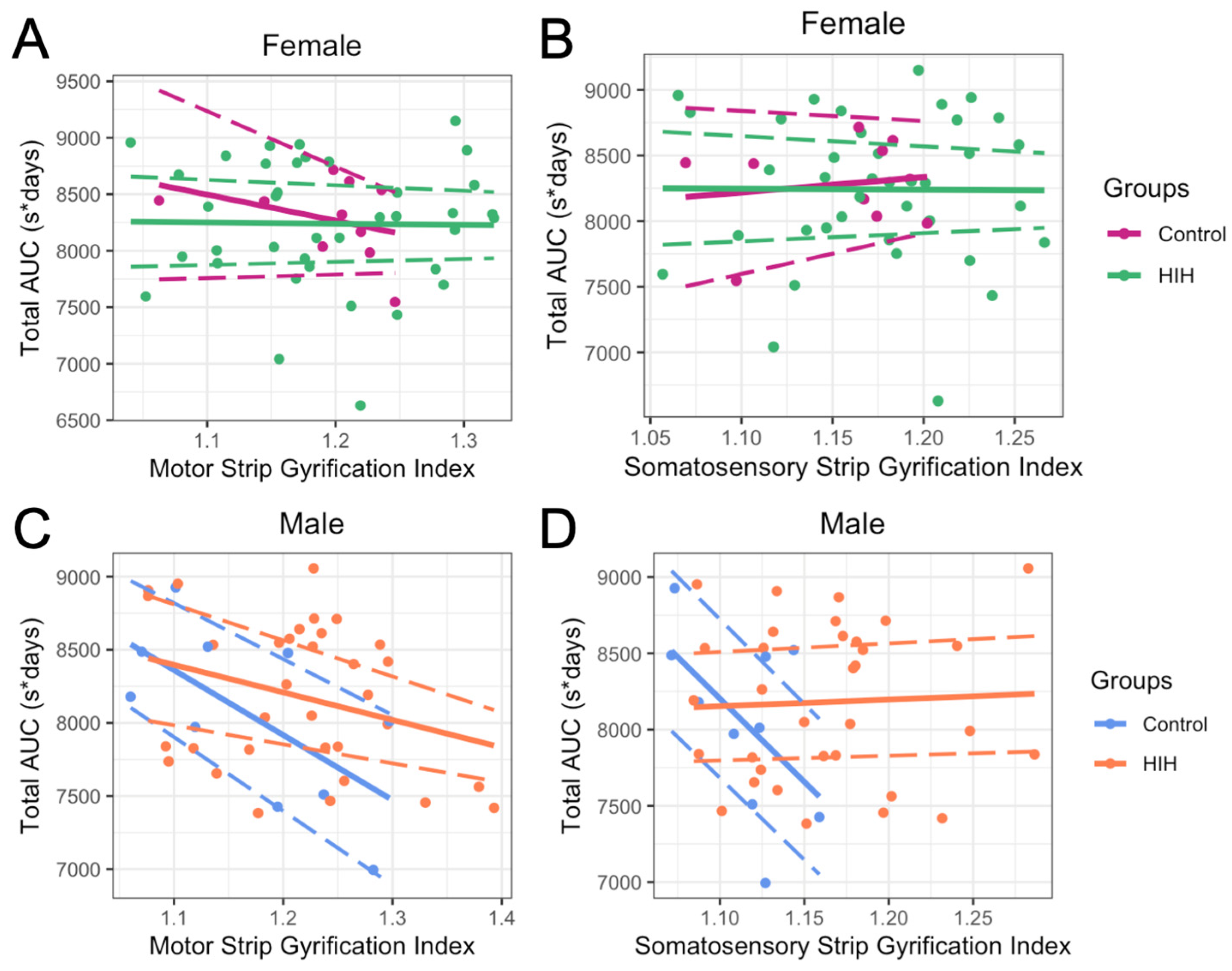
3.3. Effect of HIH on GI, Including Effect of Sex
3.4. Early Reflex Testing and GI
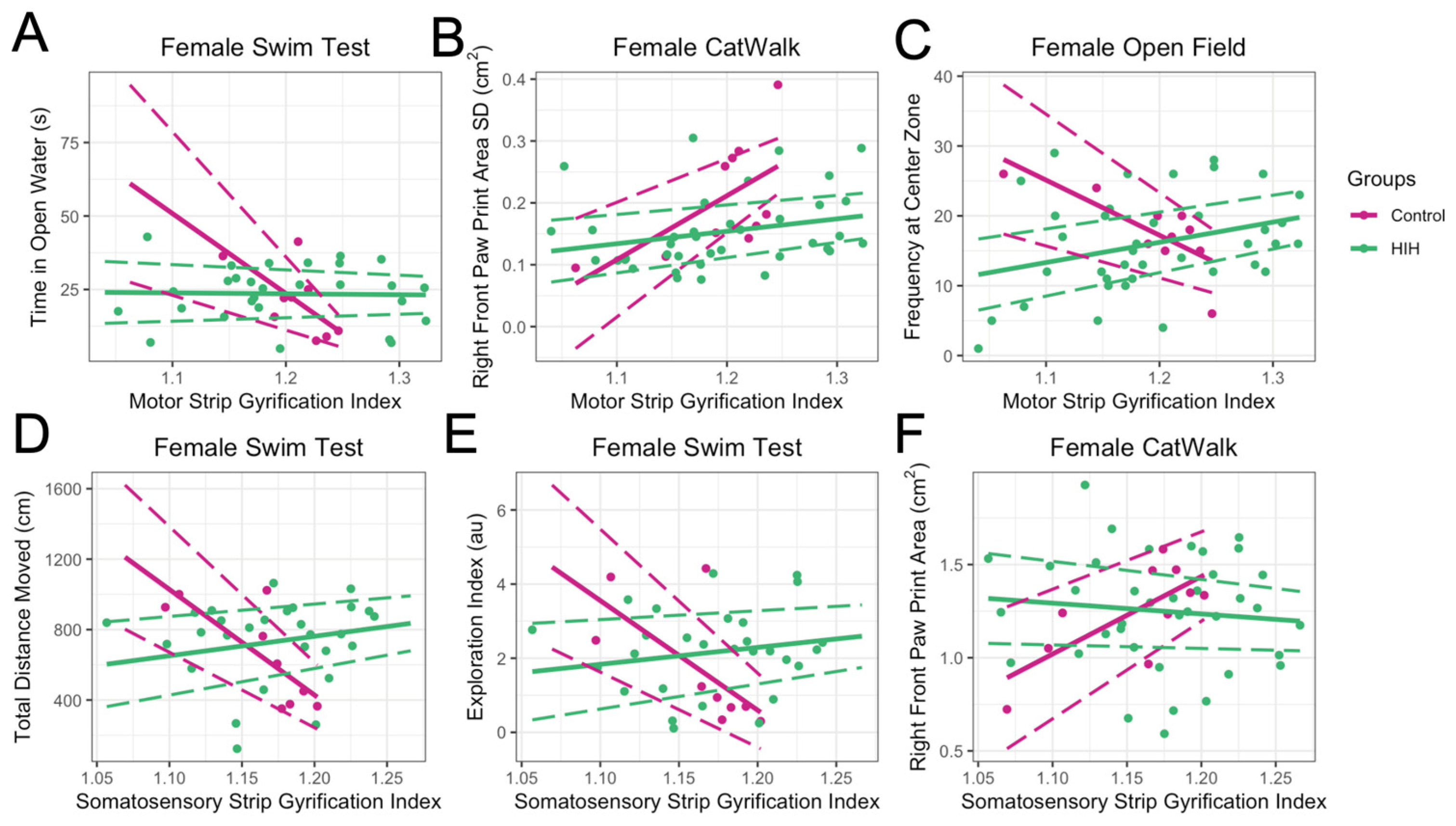
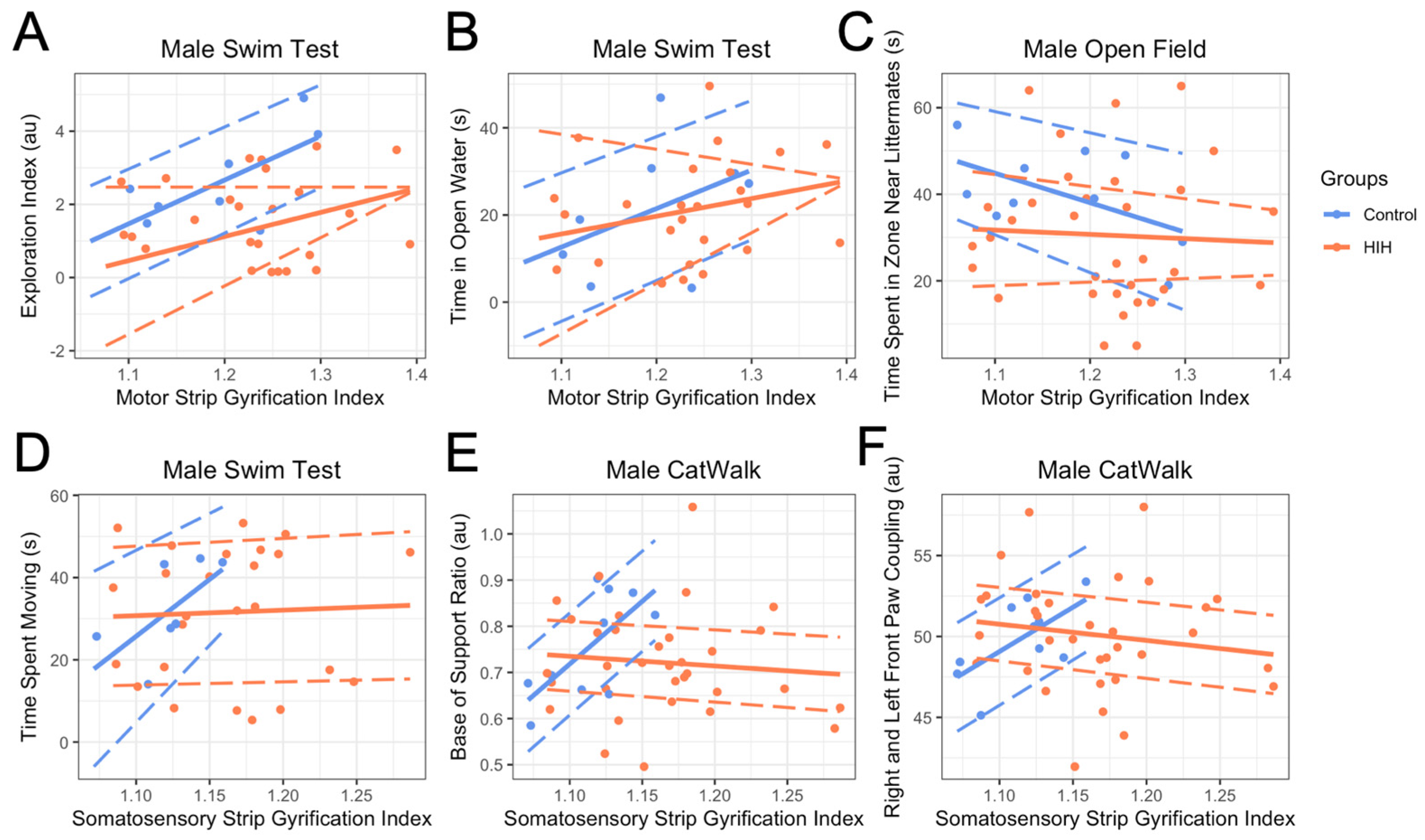
3.5. Late Behavioral Testing and GI
3.6. Effect of Treatment on GI Metrics and on Relationship Between GI and Behavior
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| AUC | Area under the curve |
| Epo | Erythropoietin |
| GI | Gyrification index |
| H&E | Hematoxylin and eosin |
| HI | Hypoxia–ischemia |
| HIH | Hypoxia–ischemia with hyperoxia |
| IACUC | Institutional Animal Care and Use Committee |
| IQ | Intelligence quotient |
| IQR | Interquartile range |
| LCA | Left carotid artery |
| LPS | Lipopolysaccharide |
| MRI | Magnetic resonance imaging |
| P | Postnatal day |
| p | Probability |
| PBS | Phosphate-buffered saline |
| RCA | Right carotid artery |
| TH | Therapeutic hypothermia |
References
- Yifan, S.; Cheng, C. Research Progress of Treatment for Neonatal Hypoxic Ischemic Encephalopathy. Chin. J. Appl. Clin. Pediatr. 2021, 24, 631–634. [Google Scholar]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Faix, R.G.; Laptook, A.R.; Shankaran, S.; Eggleston, B.; Chowdhury, D.; Heyne, R.J.; Das, A.; Pedroza, C.; Tyson, J.E.; Wusthoff, C.; et al. Whole-Body Hypothermia for Neonatal Encephalopathy in Preterm Infants 33 to 35 Weeks’ Gestation. JAMA Pediatr. 2025, 179, 396. [Google Scholar] [CrossRef]
- Lee, J.K.; Massaro, A.N.; Northington, F.J. The Search Continues: Neuroprotection for All Neonates with Hypoxic-Ischemic Encephalopathy. J. Thorac. Dis. 2017, 9, 3553–3556. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R.; Pappas, A.; McDonald, S.A.; Das, A.; Tyson, J.E.; Poindexter, B.B.; Schibler, K.; Bell, E.F.; Heyne, R.J.; et al. Effect of Depth and Duration of Cooling on Death or Disability at Age 18 Months Among Neonates With Hypoxic-Ischemic Encephalopathy. JAMA 2017, 318, 57. [Google Scholar] [CrossRef]
- Thayyil, S.; Pant, S.; Montaldo, P.; Shukla, D.; Oliveira, V.; Ivain, P.; Bassett, P.; Swamy, R.; Mendoza, J.; Moreno-Morales, M.; et al. Hypothermia for Moderate or Severe Neonatal Encephalopathy in Low-Income and Middle-Income Countries (HELIX): A Randomised Controlled Trial in India, Sri Lanka, and Bangladesh. Lancet Glob. Health 2021, 9, e1273–e1285. [Google Scholar] [CrossRef]
- Corry, K.A.; White, O.R.; Shearlock, A.E.; Moralejo, D.H.; Law, J.B.; Snyder, J.M.; Juul, S.E.; Wood, T.R. Evaluating Neuroprotective Effects of Uridine, Erythropoietin, and Therapeutic Hypothermia in a Ferret Model of Inflammation-Sensitized Hypoxic-Ischemic Encephalopathy. Int. J. Mol. Sci. 2021, 22, 9841. [Google Scholar] [CrossRef]
- Wood, T.; Moralejo, D.; Corry, K.; Snyder, J.M.; Traudt, C.; Curtis, C.; Nance, E.; Parikh, P.; Juul, S.E. A Ferret Model of Encephalopathy of Prematurity. Dev. Neurosci. 2018, 40, 475–489. [Google Scholar] [CrossRef]
- Wood, T.; Moralejo, D.; Corry, K.; Fisher, C.; Snyder, J.M.; Acuna, V.; Holden-Hunt, A.; Virk, S.; White, O.; Law, J.; et al. A Ferret Model of Inflammation-Sensitized Late Preterm Hypoxic-Ischemic Brain Injury. J. Vis. Exp. 2019, 153, e60131. [Google Scholar] [CrossRef]
- Gilardi, C.; Kalebic, N. The Ferret as a Model System for Neocortex Development and Evolution. Front. Cell Dev. Biol. 2021, 9, 661759. [Google Scholar] [CrossRef]
- Barnette, A.R.; Neil, J.J.; Kroenke, C.D.; Griffith, J.L.; Epstein, A.A.; Bayly, P.V.; Knutsen, A.K.; Inder, T.E. Characterization of Brain Development in the Ferret via MRI. Pediatr. Res. 2009, 66, 80–84. [Google Scholar] [CrossRef]
- Empie, K.; Rangarajan, V.; Juul, S.E. Is the Ferret a Suitable Species for Studying Perinatal Brain Injury? Int. J. Dev. Neurosci. 2015, 45, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Benders, M.; Cachia, A.; Lazeyras, F.; Ha-Vinh Leuchter, R.; Sizonenko, S.V.; Borradori-Tolsa, C.; Mangin, J.F.; Huppi, P.S. Mapping the Early Cortical Folding Process in the Preterm Newborn Brain. Cereb. Cortex 2008, 18, 1444–1454. [Google Scholar] [CrossRef]
- Engelhardt, E.; Inder, T.E.; Alexopoulos, D.; Dierker, D.L.; Hill, J.; Van Essen, D.; Neil, J.J. Regional Impairments of Cortical Folding in Premature Infants. Ann. Neurol. 2015, 77, 154–162. [Google Scholar] [CrossRef]
- Sawada, K.; Aoki, I. Biphasic Aspect of Sexually Dimorphic Ontogenetic Trajectory of Gyrification in the Ferret Cerebral Cortex. Neuroscience 2017, 364, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.E.; Illapani, V.S.P.; He, L.; Altaye, M.; Logan, J.W.; Parikh, N.A. Early Cortical Maturation Predicts Neurodevelopment in Very Preterm Infants. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 460–465. [Google Scholar] [CrossRef]
- Sawada, K.; Kamiya, S.; Aoki, I. Neonatal Valproic Acid Exposure Produces Altered Gyrification Related to Increased Parvalbumin-Immunopositive Neuron Density with Thickened Sulcal Floors. PLoS ONE 2021, 16, e0250262. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, D.; Sabir, H.; Falck, M.; Wood, T.; Maes, E.; Flatebø, T.; Puchades, M.; Thoresen, M. Hypothermia Does Not Reverse Cellular Responses Caused by Lipopolysaccharide in Neonatal Hypoxic-Ischaemic Brain Injury. Dev. Neurosci. 2015, 37, 390–397. [Google Scholar] [CrossRef]
- Falck, M.; Osredkar, D.; Wood, T.R.; Maes, E.; Flatebø, T.; Sabir, H.; Thoresen, M. Neonatal Systemic Inflammation Induces Inflammatory Reactions and Brain Apoptosis in a Pathogen-Specific Manner. Neonatology 2018, 113, 212–220. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hutchinson, E.B.; Schwerin, S.C.; Radomski, K.L.; Sadeghi, N.; Jenkins, J.; Komlosh, M.E.; Irfanoglu, M.O.; Juliano, S.L.; Pierpaoli, C. Population Based MRI and DTI Templates of the Adult Ferret Brain and Tools for Voxelwise Analysis. Neuroimage 2017, 152, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Williamson, B.D.; Okonek, T.; Wolock, C.J.; Spieker, A.J.; Wai, T.Y.H.; Hughes, J.P.; Emerson, S.S.; Willis, A.D. rigr: Regression, Inference, and General Data Analysis Tools in R. J. Open Source Softw. 2022, 7, 4847. [Google Scholar] [CrossRef]
- Vorland, C.J. Sex Difference Analyses under Scrutiny. eLife 2021, 10, 74135. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kline, J.E.; Illapani, V.S.P.; He, L.; Altaye, M.; Parikh, N.A. Retinopathy of Prematurity and Bronchopulmonary Dysplasia Are Independent Antecedents of Cortical Maturational Abnormalities in Very Preterm Infants. Sci. Rep. 2019, 9, 19679. [Google Scholar] [CrossRef]
- Hedderich, D.M.; Bäuml, J.G.; Berndt, M.T.; Menegaux, A.; Scheef, L.; Daamen, M.; Zimmer, C.; Bartmann, P.; Boecker, H.; Wolke, D.; et al. Aberrant Gyrification Contributes to the Link between Gestational Age and Adult IQ after Premature Birth. Brain 2019, 142, 1255–1269. [Google Scholar] [CrossRef]
- Kesler, S.R.; Vohr, B.; Schneider, K.C.; Katz, K.H.; Makuch, R.W.; Reiss, A.L.; Ment, L.R. Increased Temporal Lobe Gyrification in Preterm Children. Neuropsychologia 2006, 44, 445–453. [Google Scholar] [CrossRef]
- Papini, C.; Palaniyappan, L.; Kroll, J.; Froudist-Walsh, S.; Murray, R.M.; Nosarti, C. Altered Cortical Gyrification in Adults Who Were Born Very Preterm and Its Associations With Cognition and Mental Health. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 640–650. [Google Scholar] [CrossRef]
- Triplett, R.L.; Lean, R.E.; Parikh, A.; Miller, J.P.; Alexopoulos, D.; Kaplan, S.; Meyer, D.; Adamson, C.; Smyser, T.A.; Rogers, C.E.; et al. Association of Prenatal Exposure to Early-Life Adversity With Neonatal Brain Volumes at Birth. JAMA Netw. Open 2022, 5, e227045. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Diez-Sebastian, J.; Kapellou, O.; Gindner, D.; Allsop, J.M.; Rutherford, M.A.; Cowan, F.M. Predicting Motor Outcome and Death in Term Hypoxic-Ischemic Encephalopathy. Neurology 2011, 76, 2055–2061. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Shi, F.; Lyall, A.E.; Lin, W.; Gilmore, J.H.; Shen, D. Mapping Longitudinal Development of Local Cortical Gyrification in Infants from Birth to 2 Years of Age. J. Neurosci. 2014, 34, 4228–4238. [Google Scholar] [CrossRef]
- Kersbergen, K.J.; Leroy, F.; Išgum, I.; Groenendaal, F.; de Vries, L.S.; Claessens, N.H.P.; van Haastert, I.C.; Moeskops, P.; Fischer, C.; Mangin, J.-F.; et al. Relation between Clinical Risk Factors, Early Cortical Changes, and Neurodevelopmental Outcome in Preterm Infants. Neuroimage 2016, 142, 301–310. [Google Scholar] [CrossRef]
- Schaer, M.; Kochalka, J.; Padmanabhan, A.; Supekar, K.; Menon, V. Sex Differences in Cortical Volume and Gyrification in Autism. Mol. Autism 2015, 6, 42. [Google Scholar] [CrossRef]
- Mutlu, A.K.; Schneider, M.; Debbané, M.; Badoud, D.; Eliez, S.; Schaer, M. Sex Differences in Thickness, and Folding Developments throughout the Cortex. Neuroimage 2013, 82, 200–207. [Google Scholar] [CrossRef]
- Thompson, D.K.; Warfield, S.K.; Carlin, J.B.; Pavlovic, M.; Wang, H.X.; Bear, M.; Kean, M.J.; Doyle, L.W.; Egan, G.F.; Inder, T.E. Perinatal Risk Factors Altering Regional Brain Structure in the Preterm Infant. Brain 2007, 130, 667–677. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, G.-T.; Choi, J.W. Early Detection of Neonatal Hypoxic–Ischemic White Matter Injury. Neuroreport 2017, 28, 845–855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stockman, E.R.; Callaghan, R.S.; Gallagher, C.A.; Baum, M.J. Sexual Differentiation of Play Behavior in the Ferret. Behav. Neurosci. 1986, 100, 563–568. [Google Scholar] [CrossRef]
- Brougham, R.R.; Zail, C.M.; Mendoza, C.M.; Miller, J.R. Stress, Sex Differences, and Coping Strategies Among College Students. Curr. Psychol. 2009, 28, 85–97. [Google Scholar] [CrossRef]
- Foxworthy, W.A.; Meredith, M.A. An Examination of Somatosensory Area SIII in Ferret Cortex. Somat. Mot. Res. 2011, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rice, F.L.; Gomez, C.M.; Leclerc, S.S.; Dykes, R.W.; Moon, J.S.; Pourmoghadam, K. Cytoarchitecture of the Ferret Suprasylvian Gyrus Correlated with Areas Containing Multiunit Responses Elicited by Stimulation of the Face. Somat. Mot. Res. 1993, 10, 161–188. [Google Scholar] [CrossRef]
- McLaughlin, D.; Sonty, R.V.; Juliano, S.L. Organization of the Forepaw Representation in Ferret Somatosensory Cortex. Somat. Mot. Res. 1998, 15, 253–268. [Google Scholar] [CrossRef]
- Lempel, A.A.; Nielsen, K.J. Ferrets as a Model for Higher-Level Visual Motion Processing. Curr. Biol. 2019, 29, 179–191.e5. [Google Scholar] [CrossRef]
- Juul, S.E.; Pet, G.C. Erythropoietin and Neonatal Neuroprotection. Clin. Perinatol. 2015, 42, 469–481. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Crisostomo, A.; Zhou, K.Q.; Galinsky, R.; Dhillon, S.K.; Lear, C.A.; Bennet, L.; Gunn, A.J. Recombinant Erythropoietin Does Not Augment Hypothermic White Matter Protection after Global Cerebral Ischaemia in Near-Term Fetal Sheep. Brain Commun. 2021, 3, fcab172. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Comstock, B.A.; Heagerty, P.J.; Mayock, D.E.; Goodman, A.M.; Hauge, S.; Gonzalez, F.; Wu, Y.W. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial—Background, Aims, and Study Protocol. Neonatology 2018, 113, 331–338. [Google Scholar] [CrossRef]
- Snyder, J.M.; Wood, T.R.; Corry, K.; Moralejo, D.H.; Parikh, P.; Juul, S.E. Ontogeny of White Matter, Toll-like Receptor Expression, and Motor Skills in the Neonatal Ferret. Int. J. Dev. Neurosci. 2018, 70, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Reillo, I.; Borrell, V. Germinal Zones in the Developing Cerebral Cortex of Ferret: Ontogeny, Cell Cycle Kinetics, and Diversity of Progenitors. Cereb. Cortex 2012, 22, 2039–2054. [Google Scholar] [CrossRef]
- Wilson, S.; Pietsch, M.; Cordero-Grande, L.; Price, A.N.; Hutter, J.; Xiao, J.; McCabe, L.; Rutherford, M.A.; Hughes, E.J.; Counsell, S.J.; et al. Development of Human White Matter Pathways in Utero over the Second and Third Trimester. Proc. Natl. Acad. Sci. USA 2021, 118, e2023598118. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandon, O.C.; White, O.R.; Corry, K.A.; Stanescu, A.; Ariaye, A.; Moralejo, D.H.; Law, J.B.; Kolnik, S.E.; Juul, S.E.; Wood, T.R. Ferreting Out the Effects of Neonatal Hypoxia–Ischemia and Sex on Ferret Cortical Gyrification. Life 2025, 15, 1428. https://doi.org/10.3390/life15091428
Brandon OC, White OR, Corry KA, Stanescu A, Ariaye A, Moralejo DH, Law JB, Kolnik SE, Juul SE, Wood TR. Ferreting Out the Effects of Neonatal Hypoxia–Ischemia and Sex on Ferret Cortical Gyrification. Life. 2025; 15(9):1428. https://doi.org/10.3390/life15091428
Chicago/Turabian StyleBrandon, Olivia C., Olivia R. White, Kylie A. Corry, Andreea Stanescu, Arian Ariaye, Daniel H. Moralejo, Janessa B. Law, Sarah E. Kolnik, Sandra E. Juul, and Thomas R. Wood. 2025. "Ferreting Out the Effects of Neonatal Hypoxia–Ischemia and Sex on Ferret Cortical Gyrification" Life 15, no. 9: 1428. https://doi.org/10.3390/life15091428
APA StyleBrandon, O. C., White, O. R., Corry, K. A., Stanescu, A., Ariaye, A., Moralejo, D. H., Law, J. B., Kolnik, S. E., Juul, S. E., & Wood, T. R. (2025). Ferreting Out the Effects of Neonatal Hypoxia–Ischemia and Sex on Ferret Cortical Gyrification. Life, 15(9), 1428. https://doi.org/10.3390/life15091428







