Deciphering the Complex Intertwining Between Cytopenia and Transfusion Needs After CAR-T-Cell Therapy for B-Cell Malignancies
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Transfusion Requirements
3.2. Predictive Factors of Post-CAR-T-Cell Transfusion Needs
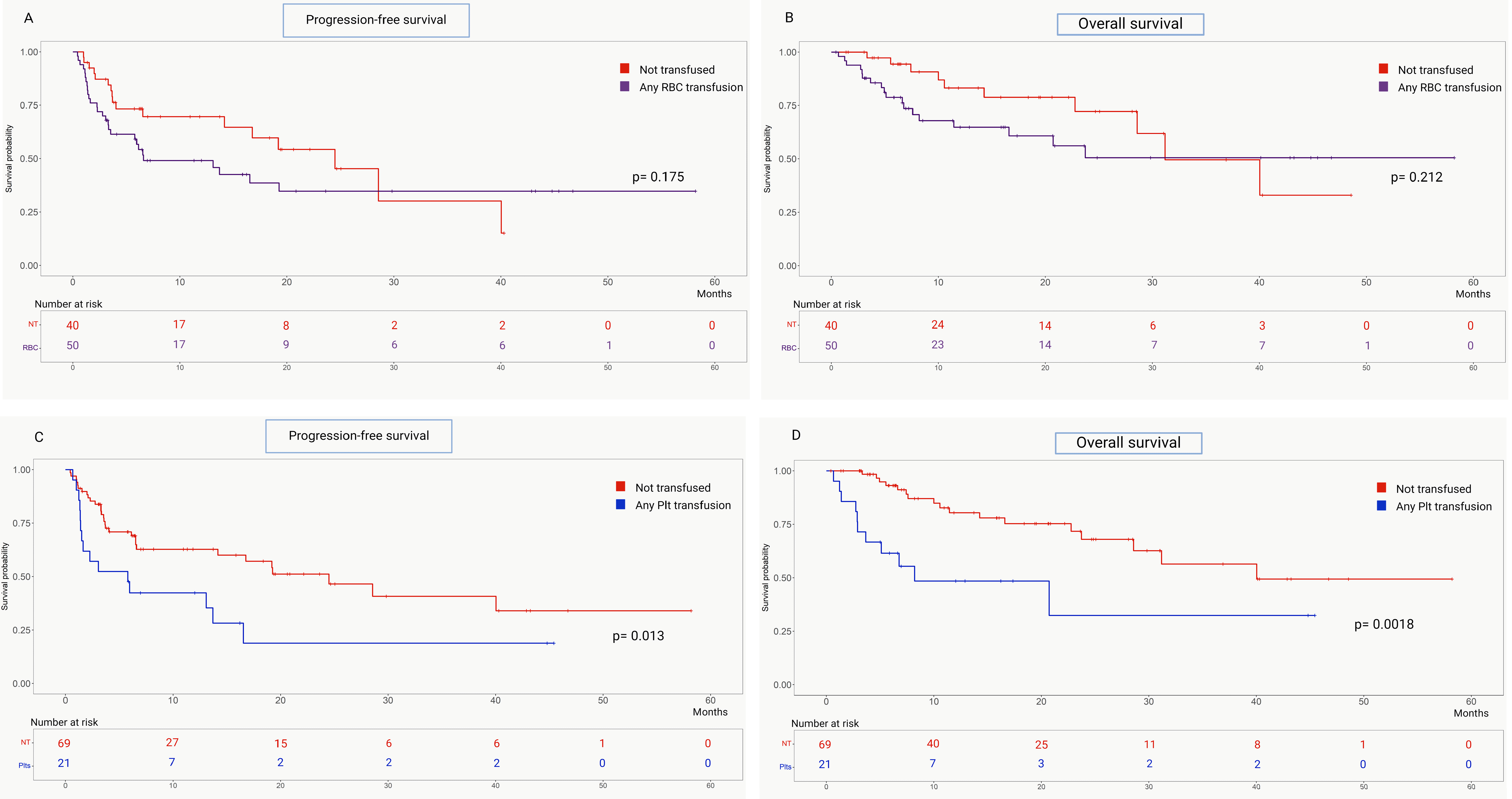
3.3. Correlation Between Transfusion Support and Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18FDG PET-CT | 18-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography |
| aOR | Adjusted Odds Ratio |
| ASTCT | American Society for Transplantation and Cellular Therapy |
| CAR-T | Chimeric Antigen Receptor T Cell |
| CAR-HEMATOTOX | Chimeric Antigen Receptor Hematotoxicity |
| CI | Confidence Interval |
| CRS | Cytokine Release Syndrome |
| CR | Complete Response |
| ddPCR | Digital Droplet Polymerase Chain Reaction |
| EBMT | European Society for Blood and Marrow Transplantation |
| EHA | European Hematology Association |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| EPO | Erythropoietin |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| HR | Hazard Ratio |
| ICAHT | Immune-Effector-Cell-Associated Hematotoxicity |
| ICANS | Immune-Effector-Cell-Associated Neurotoxicity Syndrome |
| IQR | Interquartile Range |
| MRD | Minimal Residual Disease |
| MCL | Mantle Cell Lymphoma |
| PLT | Platelet |
| PR | Partial Response |
| PFS | Progression-Free Survival |
| RBC | Red Blood Cell |
| R/R | Relapsed/Refractory |
| TRIM | Transfusion-related immunomodulation |
| TRFS | Transfusion and Relapse-Free Survival |
| TPO-RAs | Thrombopoietin Receptor Agonists |
References
- Sharma, N.; Reagan, P.M.; Liesveld, J.L. Cytopenia after CAR-T cell therapy—A brief review of a complex problem. Cancers 2022, 14, 1501. [Google Scholar] [CrossRef]
- Rejeski, K.; Jain, M.D.; Shah, N.N.; Perales, M.A.; Subklewe, M. Immune effector cell-associated haematotoxicity after CAR T-cell therapy: From mechanism to management. Lancet Haematol. 2024, 11, e459–e470. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Gu, T.; Liu, L.; Huang, Y.; Han, Y.; Qian, P.; Huang, H. Hematologic cytopenia post CAR T cell therapy: Etiology, potential mechanisms and perspective. Cancer Lett. 2022, 550, 215920. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, K.; Perez, A.; Sesques, P.; Hoster, E.; Berger, C.; Jentzsch, L.; Mougiakakos, D.; Frölich, L.; Ackermann, J.; Bücklein, V.; et al. CAR-HEMATOTOX: A model for CAR T-cell–related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood 2021, 138, 2499–2513. [Google Scholar] [CrossRef]
- Rejeski, K.; Perez, A.; Iacoboni, G.; Penack, O.; Bücklein, V.; Jentzsch, L.; Mougiakakos, D.; Johnson, G.; Arciola, B.; Carpio, C.; et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 2022, 10, e004475. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, K.; Subklewe, M.; Aljurf, M.; Bachy, E.; Balduzzi, A.C.; Barba, P.; Bruno, B.; Benjamin, R.; Carrabba, M.G.; Chabannon, C.; et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood 2023, 142, 865–877. [Google Scholar] [CrossRef]
- Rejeski, K.; Wang, Y.; Hansen, D.K.; Iacoboni, G.; Bachy, E.; Bansal, R.; Penack, O.; Müller, F.; Bethge, W.; Munoz, J.; et al. Applying the EHA/EBMT grading for ICAHT after CAR-T: Comparative incidence and association with infections and mortality. Blood Adv. 2024, 8, 1857–1868. [Google Scholar] [CrossRef]
- Vic, S.; Thibert, J.-B.; Bachy, E.; Cartron, G.; Gastinne, T.; Morschhauser, F.; Le Bras, F.; Bouabdallah, K.; Despas, F.; Bay, J.-O.; et al. Transfusion needs after CAR T-cell therapy for large B-cell lymphoma: Predictive factors and outcome (a DESCAR-T study). Blood Adv. 2024, 8, 1573–1585. [Google Scholar] [CrossRef]
- Galli, E.; Viscovo, M.; Fosso, F.; Pansini, I.; Di Cesare, G.; Iacovelli, C.; Maiolo, E.; Sorà, F.; Hohaus, S.; Sica, S.; et al. Unlocking predictive power: Quantitative assessment of CAR-T expansion with digital droplet polymerase chain reaction (ddPCR). Int. J. Mol. Sci. 2024, 25, 2673. [Google Scholar] [CrossRef]
- Thiele, J.; Kvasnicka, H.M.; Facchetti, F.; Franco, V.; van der Walt, J.; Orazi, A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 2005, 90, 1128–1132. [Google Scholar]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.; Roddie, C.; Bader, P.; Basak, G.; Bonig, H.; Bonini, C.; Chabannon, C.; Ciceri, F.; Corbacioglu, S.; Ellard, R.; et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2022, 33, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Wandt, H.; Schaefer-Eckart, K.; Wendelin, K.; Pilz, B.; Wilhelm, M.; Thalheimer, M.; Mahlknecht, U.; Ho, A.; Schaich, M.; Kramer, M.; et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: An open-label, multicentre, randomised study. Lancet 2012, 380, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Foukaneli, T.; Kerr, P.; Bolton-Maggs, P.H.; Cardigan, R.; Coles, A.; Gennery, A.; Jane, D.; Kumararatne, D.; Manson, A.; New, H.V.; et al. Guidelines on the use of irradiated blood components. Br. J. Haematol. 2020, 191, 704–724. [Google Scholar] [CrossRef]
- Gökbuget, N.; Boissel, N.; Chiaretti, S.; Dombret, H.; Doubek, M.; Fielding, A.K.; Foà, R.; Giebel, S.; Hoelzer, D.; Hunault, M.; et al. Diagnosis, prognostic factors and assessment of ALL in adults: 2023 ELN recommendations from a European expert panel. Blood 2024, 143, 1891–1902. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR T cell therapy: What we know so far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef]
- Goubran, H.; Sheridan, D.; Radosevic, J.; Burnouf, T.; Seghatchian, J. Transfusion-related immunomodulation and cancer. Transfus. Apher. Sci. 2017, 56, 336–340. [Google Scholar] [CrossRef]
- Etheridge, J.; Shah, P.; Stanworth, S.J.; Harrison, E.; Gillies, M.; Walsh, T.S.; Shah, A. Association between peri-operative red blood cell transfusion and cancer recurrence in patients undergoing major cancer surgery: An umbrella review. Anaesthesia 2025, 80 (Suppl. S2), 65–74. [Google Scholar] [CrossRef]
- Marco-Ayala, J.; Cantó, P.A.; Suarez, M.; Lamas, B.; Santiago, M.; Gómez, I.; Arnao, M.; Sanz, J.; Montava, A.; Sanz, M.Á.; et al. Single-unit transfusion policy in autologous hematopoietic stem cell transplantation: Less is not worse. Transfus. Med. Rev. 2024, 38, 150859. [Google Scholar] [CrossRef]
- Murphy, W.G. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef]
- Buecklein, V.L.; Rejeski, K.; Perez, A.; Iacoboni, G.; Jurinovic, V.; Kharboutli, S.; Blumenberg, V.; Ackermann, J.; Frölich, L.; Johnson, G.; et al. Impact of sex on clinical outcomes after CD19 CAR T-cell therapy for large B-cell lymphoma: Response and survival are significantly superior in female compared to male patients. Blood 2023, 142 (Suppl. S1), 3787. [Google Scholar] [CrossRef]
- Jain, T.; Knezevic, A.; Pennisi, M.; Chen, Y.; Ruiz, J.D.; Purdon, T.J.; Devlin, S.M.; Smith, M.; Shah, G.L.; Halton, E.; et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020, 4, 3776–3787. [Google Scholar] [CrossRef]
- Juluri, K.R.; Wu, Q.V.; Voutsinas, J.M.; Hou, J.; Hirayama, A.V.; Mullane, E.; Miles, N.; Maloney, D.G.; Turtle, C.J.; Bar, M.; et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2022, 6, 2055–2068. [Google Scholar] [CrossRef]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.A.; Spitalnik, S.L. Transfusion-related immunomodulation: A reappraisal. Curr. Opin. Hematol. 2017, 24, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Alhomoud, M.; Rejeski, K. Navigating the prognostic role of transfusions after CAR-T. Blood Adv. 2024, 8, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Albendea, M.Á.C.; Canonico, P.L.; Cartron, G.; Deiters, B.; Jommi, C.; Marks, R.; Rioufol, C.; Cia, J.M.S.; Santoro, A.; Wagner-Drouet, E.M. Comparative analysis of CAR T-cell therapy access for DLBCL patients: Associated challenges and solutions in the four largest EU countries. Front. Med. 2023, 10, 1128295. [Google Scholar] [CrossRef]
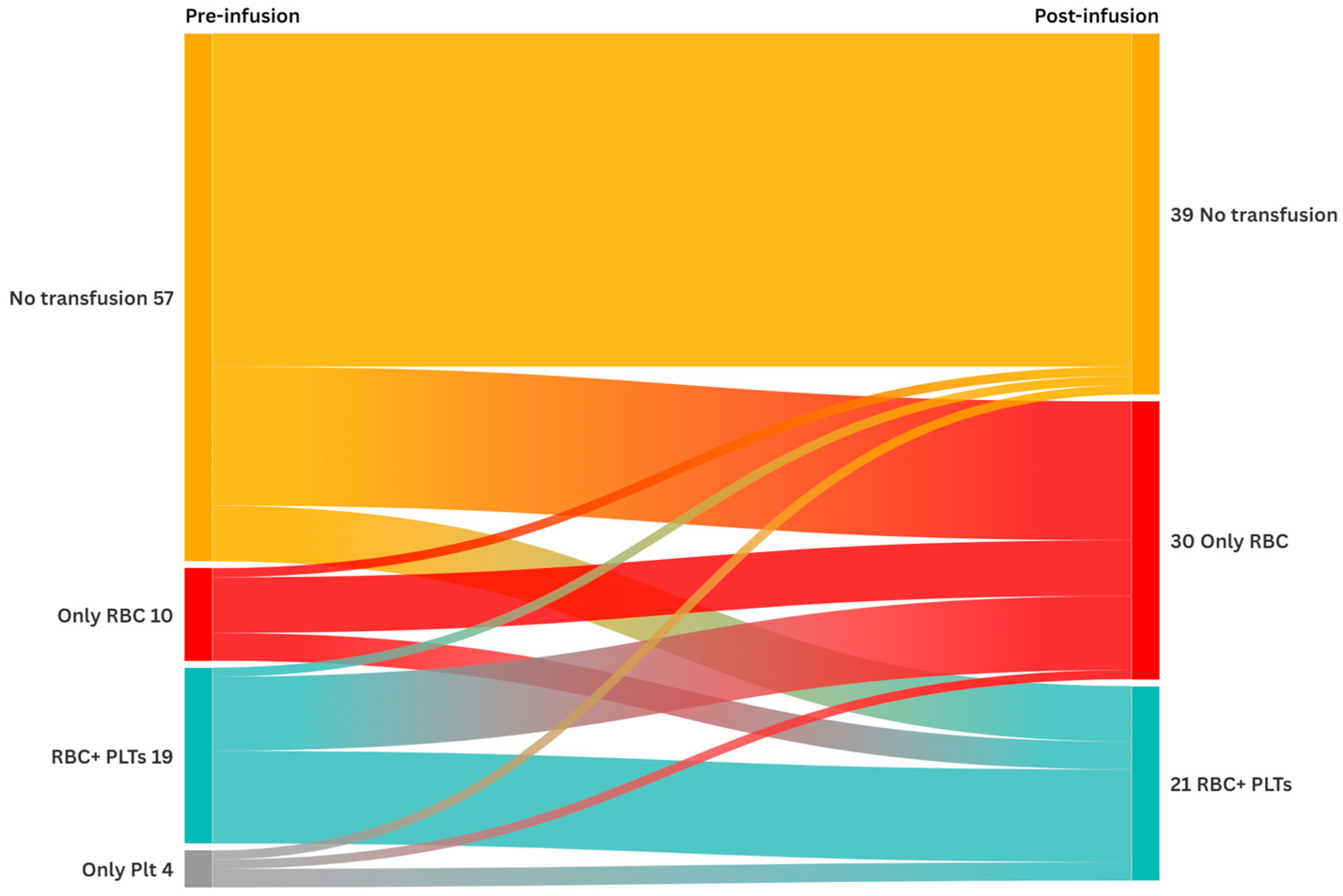
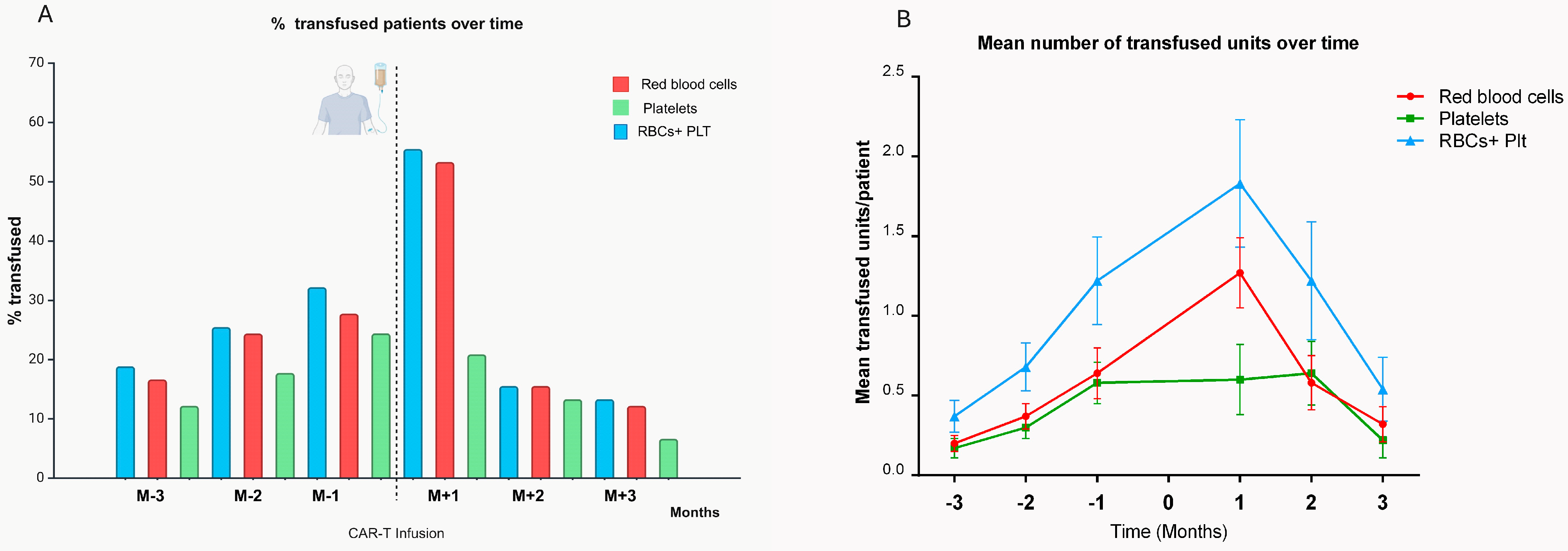
| Entire Cohort (n = 90) | At Least One Transfusion (n = 50) | No Transfusion (n = 40) | p-Value | |
|---|---|---|---|---|
| Disease Histology (n, %) | ||||
| DLBCL | 72 (80.0) | 39 (78.0) | 33 (82.5) | |
| MCL | 11 (12.2) | 8 (16.0) | 3 (7.5) | 0.388 |
| B-ALL | 7 (7.8) | 3 (6.0) | 4 (10.0) | |
| Sex (M/F, %) | 40/50 | 20/30 (40.0) | 30/10 (75.0) | 0.001 |
| Age at infusion (Years) | 57 (47.0–65.0) | 56.0 (46.5–63.2) | 57.8 (51.0–65.0) | 0.572 |
| ECOG PS at CAR-T infusion (Score) | 1 (0–1) | 1 (1–2) | 1 (0–1) | 0.038 |
| Prior lines (number) | 2 (2–3) | 2 (2–4) | 2 (2–3) | 0.383 |
| Prior lines > 3 (n, %) | 17 (18.9) | 14 (28.0) | 3 (7.5) | 0.013 |
| Bridging therapy (n, %) | ||||
| None | 6 (6.7) | 4 (8.0) | 2 (5.0) | |
| ASCT | 9 (10.0) | 8 (16.0) | 1 (2.5) | 0.078 |
| Chemoimmunotherapy | 33 (36.7) | 20 (40.0) | 9 (32.5) | |
| Local radiotherapy | 26 (28.9) | 10 (20.0) | 11 (40.0) | |
| Others * | 16 (17.7) | 8 (16.0) | 6 (20.0) | |
| Partial/Complete response to bridging therapy (n, %) | 38 (42.2) | 15 (30.0) | 23 (57.5) | 0.010 |
| RBCs transfused within 3 months before CAR-T-cell therapy (units) | 0 (0–2) | 1 (0–2) | 0 (0–0) | <0.001 |
| Platelets transfused within 3 months before CAR-T-cell therapy (units) | 0 (0–1) | 0 (0–1) | 0 (0–0) | <0.001 |
| CAR-HEMATOTOX score high risk (≥2) (n, %) | 54 (60.0) | 40 (80.0) | 14 (35.0) | <0.001 |
| BM status at the infusion (n, %) | ||||
| Reduced cellularity | 48 (53.3) | 36 (72.0) | 12 (30.0) | <0.001 |
| Disease infiltration | 12 (13.3) | 8 (16.0) | 4 (10.0) | 0.405 |
| CAR-T product infused (n, %) | ||||
| Axi-cel | 49 (54.4) | 26 (52.0) | 23 (57.5) | |
| Tisa-cel | 24 (26.7) | 14 (28.0) | 10 (25.0) | 0.872 |
| Brexu-cel | 17 (18.9) | 10 (20.0) | 7 (17.5) | |
| CRS incidence (n, %) | 81 (90.0) | 48 (96.0) | 33 (82.5) | 0.033 |
| CRS severity (n, %) | ||||
| CRS grade 1 | 27 (30.0) | 13 (26.0) | 14 (35.0) | |
| CRS grade 2 | 41 (45.5) | 23 (46.0) | 18 (45.0) | 0.008 |
| CRS grade 3–4 | 13 (14.5) | 12 (24.0) | 1 (2.5) | |
| Tocilizumab use (n, %) | 61 (67.8) | 36 (72.0) | 25 (62.5) | 0.337 |
| Tocilizumab (doses) | 1 (0–3) | 1.5 (0–3.0) | 1 (0–2) | 0.128 |
| ICANS incidence (n, %) | 30 (33.3) | 19 (38.0) | 11 (27.5) | 0.294 |
| ICANS maximum grade (n, %) | 0 (0–2) | 0 (0–2) | 1 (0–1) | 0.257 |
| ICANS ≥ 2 (n, %) | 11 (12.2) | 8 (16.0) | 3 (7.5) | 0.333 |
| Serum IL-6 peak (pg/mL) | 183.0 (34.0–1190.7) | 257.9 (61.6–1679.5) | 102.3 (24.7–677.2) | 0.054 |
| Serum IL-2R peak (UI/L) | 4124.0 (2105.2–6153.2) | 4323.5 (2227–6935) | 3188.0 (1964–5578) | 0.137 |
| ● CAR-T expansion (ddPCR assay, copies/microg gDNA) n = 70 | ||||
| ● Day 7 | 8683 (1616–20,831) | 7147 (1101–21,532) | 10618 (3391–20,375) | 0.218 |
| ● Day 14 | 8395 (2543–17,962) | 8520 (2500–19,850) | 8270 (2789–14,800) | 0.776 |
| ● Day 30 | 2387 (1644–13,191) | 717.5 (200–4392) | 749 (205–4451) | 0.879 |
| Neutropenia ≥ G3 (n, %) | 54 (60.0) | 36 (72) | 18 (45) | 0.084 |
| Neutropenia phenotype (n, %) | ||||
| Quick recovery | 47 (52.2) | 22 (44.0) | 25 (62.5) | |
| Intermittent recovery | 16 (17.8) | 9 (18.0) | 7 (17.5) | 0.001 |
| Aplastic | 20 (22.2) | 18 (36.0) | 2 (5.0) | |
| Early ICAHT > 2 (n, %) | 26 (28.8) | 22 (44.0) | 4 (10.0) | 0.001 |
| Late ICAHT > 2 (n = 86) (n, %) | 25 (27.7) | 21 (42.0) | 4 (10.0) | 0.005 |
| 3-month ORR (n = 86) ** (n, %) | 60 (69.7) | 29 (58.8) | 31 (77.5) | 0.073 |
| Early RBCs | Early Platelets | Late RBCs | Late Platelets | |
|---|---|---|---|---|
| Transfusion support before CAR-T-cell therapy | 8.7 (2.1–36.9) p = 0.002 | 8.3 (1.7–41.3) p = 0.009 | 7.6 (1.4–41) p = 0.019 | 8.1 (1.5–46.1) p = 0.017 |
| High (≥2) CAR-HEMATOTOX score | 5.1 (1.4–18.7) p = 0.013 | N.S. | N.S. | N.S. |
| Female sex | 6.3 (1.7–23.3) p = 0.005 | N.S. | N.S. | N.S. |
| ECOG PS ≥ 2 | N.S. | 4.9 (1.1–22.5) p = 0.041 | N.S. | N.S. |
| No response to bridging therapy | 4.4 (1.2–16.0) p = 0.026 | N.S. | N.S. | N.S. |
| Severe (grade > 2) ICANS | N.S. | 6.3 (1.1–38.6) p = 0.042 | N.S. | N.S. |
| Severe (>2) early ICAHT | N.S. | N.S. | N.S. | 4.9 (1.1–22.2) p = 0.036 |
| Median PFS (Months, 95% CI) | Median OS (Months, 95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Transfused | Not Transfused | HR | p-Value | Transfused | Not Transfused | HR | p-Value | |
| Any RBCs | 6.6 (3.5–19.3) | 24.5 (14.2–28.6) | 1.51 (0.84–1.71) | 0.175 | 17.4 (12.0–23.6) | 31.2 (28.6–40.0) | 1.62 (0.77–3.41) | 0.212 |
| Any Platelets | 5.8 (1.5–13.1) | 24.5 (14.2–40.0) | 2.14 (1.11–4.50) | 0.013 | 8.2 (3.7–20.7) | 40.0 (28.6-NR) | 3.12 (1.17–8.33) | 0.001 |
| Early RBCs | 6.6 (3.3–19.3) | 24.5 (14.2–28.6) | 1.50 (0.84–2.69) | 0.177 | 17.4 (16.6–23.7) | 31.2 (28.6–40.0) | 1.51 (0.72–3.17) | 0.282 |
| Early Platelets | 5.8 (1.4–13.1) | 24.5 (6.6–40.0) | 2.19 (1.05–5.04) | 0.016 | 20.7 (2.9–20.7) | NR (28.6-NR) | 2.73 (1.11–8.29) | 0.011 |
| Late RBCs | 6.6 (1.6–13.1) | 19.2 (6.5–28.6) | 1.15 (0.54–2.47) | 0.706 | 7.6 (6.8–8.2) | 40.0 (23.7-NR) | 1.58 (0.59–4.19) | 0.288 |
| Late Platelets | 6.0 (1.4–13.1) | 16.8 (6.5–28.6) | 1.36 (0.58–3.19) | 0.421 | 7.1 (3.7–8.2) | 40.0 (23.7-NR) | 2.25 (0.74–6.78) | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, C.; Galli, E.; Chiusolo, P.; Ladiana, R.; Valentini, C.G.; Viscovo, M.; Sorà, F.; Sica, S.; Teofili, L. Deciphering the Complex Intertwining Between Cytopenia and Transfusion Needs After CAR-T-Cell Therapy for B-Cell Malignancies. Life 2025, 15, 1419. https://doi.org/10.3390/life15091419
Pellegrino C, Galli E, Chiusolo P, Ladiana R, Valentini CG, Viscovo M, Sorà F, Sica S, Teofili L. Deciphering the Complex Intertwining Between Cytopenia and Transfusion Needs After CAR-T-Cell Therapy for B-Cell Malignancies. Life. 2025; 15(9):1419. https://doi.org/10.3390/life15091419
Chicago/Turabian StylePellegrino, Claudio, Eugenio Galli, Patrizia Chiusolo, Rossella Ladiana, Caterina Giovanna Valentini, Marcello Viscovo, Federica Sorà, Simona Sica, and Luciana Teofili. 2025. "Deciphering the Complex Intertwining Between Cytopenia and Transfusion Needs After CAR-T-Cell Therapy for B-Cell Malignancies" Life 15, no. 9: 1419. https://doi.org/10.3390/life15091419
APA StylePellegrino, C., Galli, E., Chiusolo, P., Ladiana, R., Valentini, C. G., Viscovo, M., Sorà, F., Sica, S., & Teofili, L. (2025). Deciphering the Complex Intertwining Between Cytopenia and Transfusion Needs After CAR-T-Cell Therapy for B-Cell Malignancies. Life, 15(9), 1419. https://doi.org/10.3390/life15091419






