Microbiota and Gut Inflammatory Markers (Zonulin and Fecal Calprotectin) Exhibit Age-Dependent Variation in Patients with Ulcerative Colitis
Abstract
1. Introduction
2. Aim
3. Material and Methods
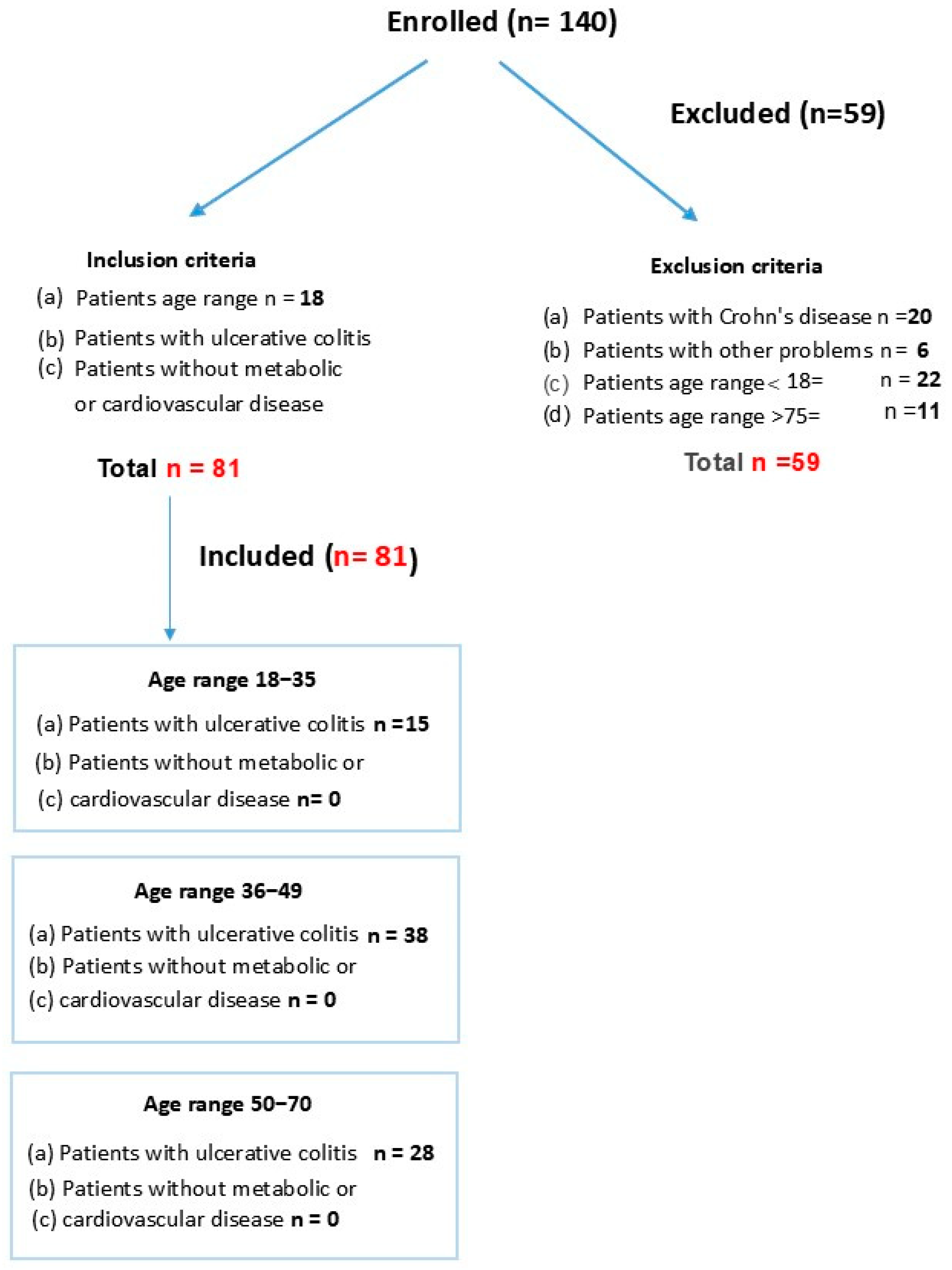
3.1. Patient Study Groups
3.2. Inclusion Criteria
3.3. Exclusion Criteria
3.4. Study Groups
3.5. Measurement of PCR
3.6. Measurement of Calprotectin
3.7. Measurement of Zonulin
3.8. Measurement of Microbiota
3.9. Measurement of Vitamin D
4. Results
4.1. Zonulin
4.2. Calprotectin
4.3. Vitamin D
4.4. Total Species Carriers of LPS
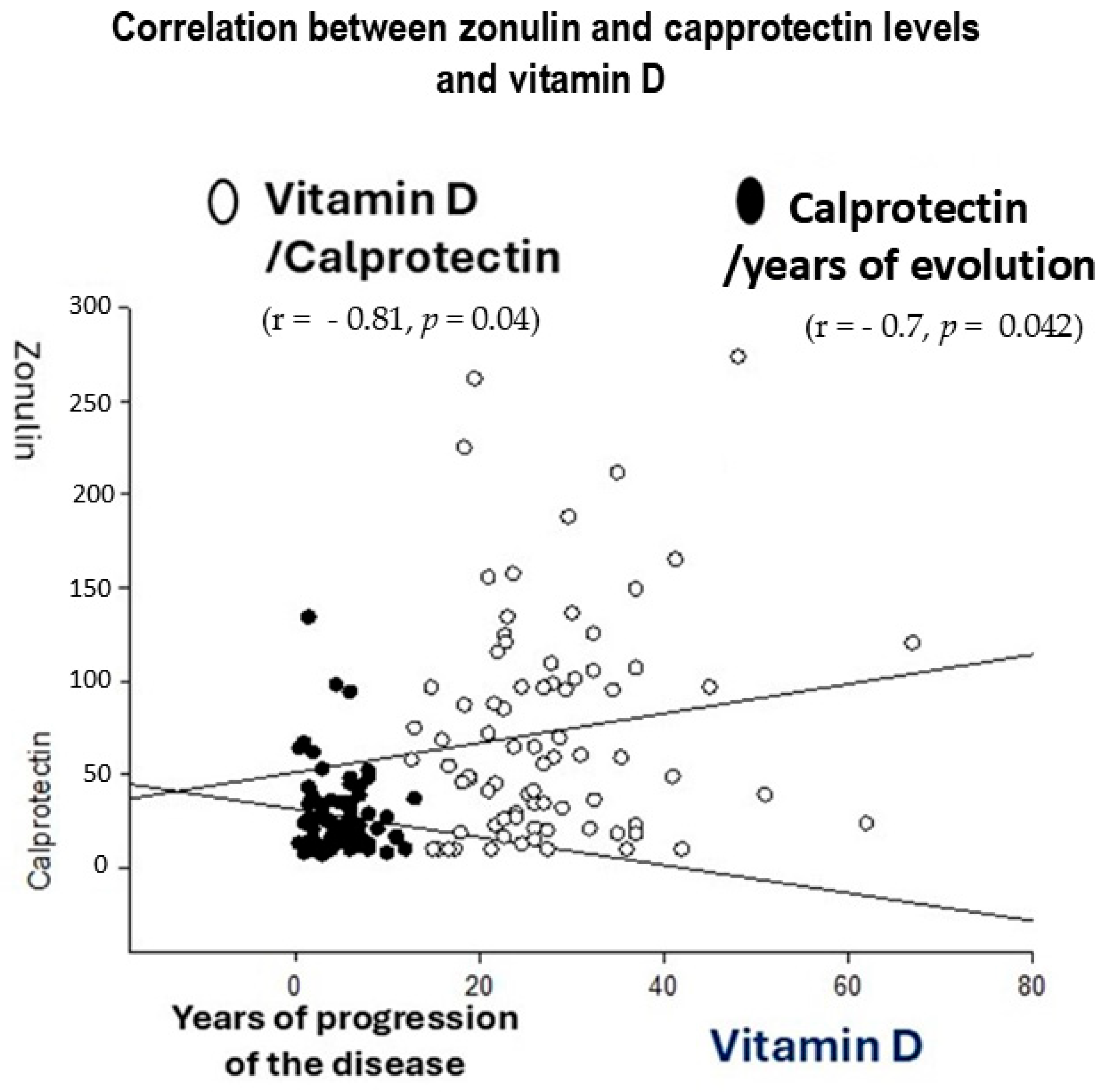
4.5. R Spearman Correlations Between Inflammatory Markers (Zonulin and Calprotectin) and Years of Ulcerative Colitis Evolution
4.6. r Spearman Correlations Between Inflammatory Markers (Zonulin and Calprotectin) and LPS Microbiota Strains Producers in Patients with Ulcerative Colitis by Age
4.7. Numbers of UCF/g of Enteroccocus spp., E. Coli biovare, and Candida albicans
4.8. Analysis of Microbiota Strains by PCR
4.9. r Spearman Correlations Between LPS Producers and Rest of Markers
4.10. Other Parameters (PCR/Ig G/A/M)
4.10.1. Leucocytes (Number of Leycoytes/mm3)
4.10.2. Statistical Analysis
5. Discussion
6. Limitations and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón, P.; Núñez, P.; Nos, P.; Quera, R. Personalised therapy in inflammatory bowel disease. Gastroenterol. Hepatol. 2024, 47, 763–770. [Google Scholar] [CrossRef]
- Aliu, A.; Bosch, D.H.; Keszthelyi, D.; Rezazadeh Ardabili, A.; Colombel, J.F.; Sawyer, R.; Törnblom, H.; Hart, A.; Jonkers, D.M.; Pierik, M.J.; et al. Review article: A practical approach to persistent gastrointestinal symptoms in inflammatory bowel disease in remission. Aliment. Pharmacol. Ther. 2024, 59, 1470–1488. [Google Scholar] [CrossRef]
- Szymanska, E.; Wierzbicka, A.; Dadalski, M.; Kierkus, J. Fecal Zonulin as a Noninvasive Biomarker of Intestinal Permeability in Pediatric Patients with Inflammatory Bowel Diseases-Correlation with Disease Activity and Fecal Calprotectin. J. Clin. Med. 2021, 10, 3905. [Google Scholar] [CrossRef]
- Duricova, D.; Burisch, J.; Jess, T.; Gower-Rousseau, C.; Lakatos, P.L.; ECCO-EpiCom. Age-related differences in presentation and course of inflammatory bowel disease: An update on the population-based literature. J. Crohn’s Colitis 2014, 8, 1351–1361. [Google Scholar] [CrossRef]
- Freeman, H.J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 2014, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Chen, G.Y.; Núñez, G. Host-pathobiont interactions in Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 22, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, A.; Chan, J.J.; De Wolfe, T.J.; Yang, H.; Vallance, B.A. Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care. Gastroenterology 2024, 66, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients with Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Hecht, A.L.; Harling, L.C.; Friedman, E.S.; Tanes, C.; Lee, J.; Firrman, J.; Hao, F.; Tu, V.; Liu, L.; Patterson, A.D.; et al. Dietary carbohydrates regulate intestinal colonization and dissemination of Klebsiella pneumoniae. J. Clin. Investig. 2024, 134, e174726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Wang, Z.; Guan, Z.; Miao, J.; Li, W.; Yu, P.; Molina Jimenez, C. UCFNNet: Ulcerative colitis evaluation based on fine-grained lesion learner and noise suppression gating. Comput. Methods Programs Biomed. 2024, 247, 108080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Dou, P.; Pan, Y.S. Associations between eicosapentaenoic acid and docosahexaenoic acid consumption and inflammatory bowel disease in adults: The National Health and Nutrition Examination Survey (NHANES) 2009–2010. Asia Pac. J. Clin. Nutr. 2024, 33, 562–568. [Google Scholar]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef] [PubMed]
- Giampazolias, E.; Pereira da Costa, M.; Lam, K.C.; Lim, K.H.J.; Cardoso, A.; Piot, C.; Chakravarty, P.; Blasche, S.; Patel, S.; Biram, A.; et al. Vitamin D regulates microbiome-dependent cancer immunity. Science 2024, 384, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Jouët, P.; Altman, C.; Bruley, D.E.S.; Varannes, S.; Juhel, C.; Henri, F. Probiotics plus vitamin D in irritable bowel syndrome: A prospective multicentric non-interventional study. Minerva Gastroenterol. 2024, 70, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Jin, M.L.; Yuan, M.X.; Liu, M.X.; Zang, M.G. Compound Xuejie Preparation Improved Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Mitigating Inflammation-Induced Damage and Downregulating TNF-α Expression. Int. J. Pharmacol. 2024, 20, 652–659. [Google Scholar] [CrossRef]
- Gubatan, J.; Mitsuhashi, S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2017, 15, 240–246.e1. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levinson, T.; Wasserman, A. C-Reactive Protein Velocity (CRPv) as a New Biomarker for the Early Detection of Acute Infection/Inflammation. Int. J. Mol. Sci. 2022, 23, 8100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashton, J.J.; Beattie, R.M. Inflammatory bowel disease: Recent developments. Arch. Dis. Child. 2024, 109, 370–376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Swaminathan, A.; Borichevsky, G.M.; Frampton, C.M.; Day, A.S.; Hampton, M.B.; Kettle, A.J.; Gearry, R.B. Comparison of Fecal Calprotectin and Myeloperoxidase in Predicting Outcomes in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2024, 31, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G11–G17. [Google Scholar] [CrossRef]
- Schwiertz, A.; Spiegel, J.; Dillmann, U.; Grundmann, D.; Bürmann, J.; Faßbender, K.; Schäfer, K.H.; Unger, M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Park. Relat. Disord. 2018, 50, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malíčková, K.; Francová, I.; Lukáš, M.; Kolář, M.; Králíková, E.; Bortlík, M.; Ďuricová, D.; Štěpánková, L.; Zvolská, K.; Pánková, A.; et al. Fecal zonulin is elevated in Crohn’s disease and in cigarette smokers. Pract. Lab. Med. 2017, 9, 39–44. [Google Scholar] [CrossRef]
- Xu, M.; Li, F.; Zhang, X.; Chen, B.; Geng, Y.; Ouyang, P.; Chen, D.; Li, L.; Huang, X. Microbiome analysis reveals the intestinal microbiota characteristics and potential impact of Procambarus clarkii. Appl. Microbiol. Biotechnol. 2024, 108, 77. [Google Scholar] [CrossRef]
- Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; Kato, J. Fecal Immunochemical Test and Fecal Calprotectin Results Show Different Profiles in Disease Monitoring for Ulcerative Colitis. Gut Liver. 2018, 12, 142–148. [Google Scholar]
- Li, F.; Ma, J.; Geng, S.; Wang, J.; Liu, J.; Zhang, J.; Sheng, X. Fecal calprotectin concentrations in healthy children aged 1–18 months. PLoS ONE. 2015, 10, e0119574. [Google Scholar] [CrossRef]
- Penckofer, S.; Ridosh, M.; Adams, W.; Grzesiak, M.; Woo, J.; Byrn, M.; Kouba, J.; Sheean, P.; Kordish, C.; Durazo-Arvizu, R.; et al. Vitamin D Supplementation for the Treatment of Depressive Symptoms in Women with Type 2 Diabetes: A Randomized Clinical Trial. J. Diabetes Res. 2022, 2022, 4090807. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Marangos, M.; Assimakopoulos, S.F.; Mouzaki, A.; Thomopoulos, K.; Triantos, C. Vitamin D and Microbiome: Molecular Interaction in Inflammatory Bowel Disease Pathogenesis. Am. J. Pathol. 2023, 193, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Kapel, N.; Ouni, H.; Benahmed, N.A.; Barbot-Trystram, L. Fecal Calprotectin for the Diagnosis and Management of Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2023, 14, e00617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and UC: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Tibble, J.A.; Sigthorsson, G.; Foster, R.; Forgacs, I.; Bjarnason, I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 2002, 123, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Poullis, A.; Foster, R.; Shetty, A.; Fagerhol, M.K.; Mendall, M.A. Bowel inflammation as measured by fecal calprotectin: A link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Safroneeva, E.; Romero, Y.; Armstrong, D.; Schmidt, C.; Trummler, M.; Pittet, V.; Vavricka, S.R. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm. Bowel Dis. 2013, 19, 332–341. [Google Scholar] [CrossRef]
- Kopylov, U.; Yung, D.E.; Engel, T.; Avni, T.; Battat, R.; Ben-Horin, S.; Plevris, J.N.; Eliakim, R.; Koulaouzidis, A. Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1137–1144. [Google Scholar] [CrossRef]
- Canani, R.B.; Terrin, G.; Rapacciuolo, L.; Miele, E.; Siani, M.C.; Puzone, C.; Cosenza, L.; Staiano, A.; Troncone, R. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig. Liver Dis. 2008, 40, 547–553. [Google Scholar] [CrossRef]
- Chang, M.H.; Chou, J.W.; Chen, S.M.; Tsai, M.C.; Sun, Y.S.; Lin, C.C.; Lin, C.P. Faecal calprotectin as a novel biomarker for differentiating between inflammatory bowel disease and irritable bowel syndrome. Mol. Med. Rep. 2014, 10, 522–526. [Google Scholar] [CrossRef]
- Yeaman, F.; Nguyen, A.; Abasszade, J.; Gupta, S.; Bell, S.; Moore, G. Assessing vitamin D as a biomarker in inflammatory bowel disease. JGH Open 2023, 7, 953–958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Muñoz, P.; Beltrán, B.; Sáez-González, E.; Alba, A.; Nos, P.; Iborra, M. Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in UC Patients. Nutrients 2019, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, L.; Hao, Y.; Xu, Y.; Yang, Q.; Dai, Z.; Yang, Y.; Wu, Z.; Ji, Y. Contemporary Perspectives on the Role of Vitamin D in Enhancing Gut Health and Its Implications for Preventing and Managing Intestinal Diseases. Nutrients 2024, 16, 2352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gubatan, J.; Mitsuhashi, S.; Longhi, M.S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 2018, 103, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.Y.; Chiang Chiau, J.S.; Cheng, M.L.; Chan, W.T.; Jiang, C.B.; Chang, S.W.; Liu, C.Y.; Chang, C.W.; Lee, H.C. Effects of Vitamin D-Deficient Diet on Intestinal Epithelial Integrity and Zonulin Expression in a C57BL/6 Mouse Model. Front. Med. 2021, 8, 649818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.; Ferreira, S.R. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Yoon, H.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.Y.; Lee, D.H.; Kim, J.S. Tu1715—The Effect of Vitamin D Administration on Inflammatory Marker in Patients with Inflammatory Bowel Disease. Gastroenterology 2018, 154, S-998. [Google Scholar] [CrossRef]
- Jørgensen, S.P.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef]
- Abreu, M.T.; Kantorovich, V.; Vasiliauskas, E.A.; Gruntmanis, U.; Matuk, R.; Daigle, K.; Chen, S.; Zehnder, D.; Lin, Y.C.; Yang, H.; et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut 2004, 53, 1129–1136. [Google Scholar] [CrossRef]
- Jones, K.S.; Assar, S.; Harnpanich, D.; Bouillon, R.; Lambrechts, D.; Prentice, A.; Schoenmakers, I. 25(OH)D-2 Half-Life Is Shorter Than 25(OH)D-3 Half-Life and Is Influenced by DBP Concentration and Genotype. J. Clin. Endocrinol. Metab. 2014, 99, 3373–3381. [Google Scholar] [CrossRef]
- Cusato, J.; Bertani, L.; Antonucci, M.; Tomasello, C.; Caviglia, G.P.; Dibitetto, S.; Massano, A.; Mangia, M.; Mula, J.; Ceccarelli, L.; et al. Vitamin D-Related Genetics as Predictive Biomarker of Clinical Remission in Adalimumab-Treated Patients Affected by Crohn’s Disease: A Pilot Study. Pharmaceuticals 2021, 14, 1230. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, H.; Zhang, Z.; Gao, J.; Li, J.; Li, X. Vitamin D3 alleviates inflammation in ulcerative colitis by activating the VDR-NLRP6 signaling pathway. Front. Immunol. 2023, 14, 1135930. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Milestone, A.N.; Walters, J.R.; Hart, A.L.; Ghosh, S. Vitamin D and gastrointestinal diseases: Inflammatory bowel disease and colorectal cancer. Therap. Adv. Gastroenterol. 2011, 4, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.G. Vitamin D Receptor Influences Intestinal Barriers in Health and Disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Raftery, T.; Martineau, A.R.; Greiller, C.L.; Ghosh, S.; McNamara, D.; Bennett, K.; Meddings, J.; O’Sullivan, M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United Eur. Gastroenterol. J. 2015, 3, 294–302. [Google Scholar] [CrossRef]
- Sninsky, J.A.; Sansgiry, S.; Taylor, T.; Perrin, M.; Kanwal, F.; Hou, J.K. The Real-World Impact of Vitamin D Supplementation on Inflammatory Bowel Disease Clinical Outcomes. Clin. Gastroenterol. Hepatol. 2025, 22, S1542-356500624-X. [Google Scholar] [CrossRef]
- Ullah, H. Gut-vitamin D interplay: Key to mitigating immunosenescence and promoting healthy ageing. Immun. Ageing 2025, 22, 20. [Google Scholar] [CrossRef]
- Hamza, F.N.; Daher, S.; Fakhoury, H.M.A.; Grant, W.B.; Kvietys, P.R.; Al-Kattan, K. Immunomodulatory Properties of Vitamin D in the Intestinal and Respiratory Systems. Nutrients 2023, 15, 1696. [Google Scholar] [CrossRef]
- Schaffler, H.; Herlemann, D.P.R.; Klinitzke, P.; Berlin, P.; Kreikemeyer, B.; Jaster, R.; Lamprecht, G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 2018, 19, 225–234. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin d Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 362. [Google Scholar] [CrossRef]
- Dell’Anna, G.; Fanizzi, F.; Zilli, A.; Furfaro, F.; Solitano, V.; Parigi, T.L.; Ciliberto, A.; Fanizza, J.; Mandarino, F.V.; Fuccio, L.; et al. The Role of Vitamin D in Inflammatory Bowel Diseases: From Deficiency to Targeted Therapeutics and Precise Nutrition Strategies. Nutrients 2025, 17, 2167. [Google Scholar] [CrossRef] [PubMed]
- Caviezel, D.; Maissen, S.; Niess, J.H.; Kiss, C.; Hruz, P. High Prevalence of Vitamin D Deficiency among Patients with Inflammatory Bowel Disease. Inflamm. Intest. Dis. 2018, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Frigstad, S.O.; Høivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.; Huppertz-Hauss, G.; Hovde, Ø.; Torp, R. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef]
- Mo, C.; Bajgai, J.; Rahman, M.H.; Choe, S.; Abdul-Nasir, S.; Ma, H.; He, W.; Pham, T.T.; Zhang, H.; Hoon, G.S.; et al. A pilot clinical trial to explore the effects of UV exposure on vitamin D synthesis and inflammatory responses in vitamin D-Deficient adults. Sci. Rep. 2025, 15, 22557. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Ferrandiz, L.; Salvador, P.; Ortiz-Masia, D.; Macias-Ceja, D.C.; Orden, S.; Esplugues, J.V.; Calatayud, S.; Hinojosa, J.; Barrachina, M.D.; Hernandez, C. A Single Nucleotide Polymorphism in the Vitamin D Receptor Gene Is Associated with Decreased Levels of the Protein and a Penetrating Pattern in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 1462–1470. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm. Bowel Dis. 2019, 25, 97–110. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef]
- Froicu, M.; Weaver, V.; Wynn, T.A.; McDowell, M.A.; Welsh, J.E.; Cantorna, M.T. A Crucial Role for the Vitamin D Receptor in Experimental Inflammatory Bowel Diseases. Mol. Endocrinol. 2003, 17, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.L.; Targan, S.R.; Elson, C.O., III. Microbiota activation and regulation of innate and adaptive immunity. Immunol. Rev. 2014, 260, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Bartolacci, A.; Stocchi, F.; Stocchi, V.; Zeppa, S.D. The Crucial Role of Vitamin D in Regulating Gut Microbiota in Inflammatory Bowel Disease. Recent. Progress. Nutr. 2025, 5, 1–8. [Google Scholar] [CrossRef]
- Ahmadi, A.; Yousefimashouf, R.; Mohammadi, A.; Nikkhoo, B.; Shokoohizadeh, L.; Khan Mirzaei, M.; Alikhani, M.Y.; Sheikhesmaili, F.; Khodaei, H. Investigating the expression of anti/pro-inflammatory cytokines in the pathogenesis and treatment of ulcerative colitis and its association with serum level of vitamin D. Sci. Rep. 2025, 15, 7569. [Google Scholar] [CrossRef]
- Nitzan, O.; Elias, M.; Chazan, B.; Raz, R.; Saliba, W. Clostridium difficile and inflammatory bowel disease: Role in pathogenesis and implications in treatment. World J. Gastroenterol. 2013, 19, 7577–7585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagishetty, V.; Misharin, A.V.; Liu, N.Q.; Lisse, T.S.; Chun, R.F.; Ouyang, Y.; McLachlan, S.M.; Adams, J.S.; Hewison, M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology 2010, 151, 2423–2432. [Google Scholar] [CrossRef]
- Hamza, K.H.; Dunér, E.; Ulmert, I.; Arias, A.; Sorobetea, D.; Lahl, K. Minor alterations in the intestinal microbiota composition upon Rotavirus infection do not affect susceptibility to DSS colitis. Sci. Rep. 2021, 11, 13485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macfarlane, S.; Furrie, E.; Cummings, J.H.; Macfarlane, G.T. Chemotaxonomic analysis of bacterial populations colonizing the rectal mucosa in patients with ulcerative colitis. Clin. Infect. Dis. 2004, 38, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.E.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.-H.; Yang, H.; Qian, J.M.; Li, J.N. VSL#3 Can Prevent Ulcerative Colitis-Associated Carcinogenesis in Mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Dutta, U. Role of fungus in inflammatory bowel disease: The butterfly effect? Indian. J. Gastroenterol. 2024, 43, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Kotsiliti, E. Fungal strain-dependent inflammation in IBD. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, Y.; Zheng, M.; Zhou, M.; Weng, C.; Fan, Y.; Lv, B. Association of Recreational Physical Activity with Gut Microbiota Diversity and Composition, and Symptom Burden in Crohn’s Disease: A Cross-Sectional Study. J. Inflamm. Res. 2025, 18, 10295–10310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zwolinska-Wcislo, M.; Brzozowski, T.; Budak, A.; Kwiecien, S.; Sliwowski, Z.; Drozdowicz, D.; Trojanowska, D.; Rudnicka-Sosin, L.; Mach, T.; Konturek, S.J.; et al. Effect of Candida colonization on human ulcerative colitis and the healing of inflammatory changes of the colon in the experimental model of colitis ulcerosa. J. Physiol. Pharmacol. 2009, 60, 107–118. [Google Scholar] [PubMed]
- Xie, H.; Yu, S.; Tang, M.; Xun, Y.; Shen, Q.; Wu, G. Gut microbiota dysbiosis in inflammatory bowel disease: Interaction with intestinal barriers and microbiota-targeted treatment options. Front. Cell Infect. Microbiol. 2025, 15, 1608025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The Role of Probiotic Lactic Acid Bacteria and Bifidobacteria in the Prevention and Treatment of Inflammatory Bowel Disease and Other Related Diseases: A Systematic Review of Randomized Human Clinical Trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE. 2016, 11, e0146162. [Google Scholar] [CrossRef]
- D’Amico, F.; Nancey, S.; Danese, S.; Peyrin-Biroulet, L. A Practical Guide for Faecal Calprotectin Measurement: Myths and Realities. J. Crohns Colitis. 2021, 15, 152–161. [Google Scholar] [CrossRef] [PubMed]


| LPS (36–49) | Calprotectin (36–49) | Clostridium (36–49) |  | |
| Zonulin (36–49) | r = −0.69 p = 0.001 | r = −0.9 p = 0.04 | r = 0.75 p = 0.00005 | |
| LPS (50–70) | C. albicans (50–70) | |||
| Zonulin (50–70) | r = 0.41 p = 0.047 | r = −0.58 p = 0.02 | ||
| LPS (36–49) | E. Coli biovare (36–49) | Clostridium spp. (36–49) | Enterococcus spp. (36–49) | |
| Calprotectin (36–49) | r = 0.79 p = 0.0012 | r = 0.74 p = 0.0000001 | r = 0.75 p = 0.0005 | r = 0.48 p = 0.018 |
| LPS (36–49) | Calprotectin (36–49) | E. Coli biovare (36–49) | ||
| Vitamin D (36–49) | r = 0.68 p = 0.003 | r = 0.5 p = 0.014 | r = 0.74 p = 0.0038 | |
| LPS (50–70) | Leucocytes (50–70) | |||
| Vitamin D (50–70) | r = −0.6 p = 0.0059 | r = 0.41 p = 0.047 | ||
| Bifidubacterium (36–49) | Rummicocus (36–49) | |||
| LPS (36–49) | r = 0.73 p = 0.00000002 | r = 0.68 p = 0.029 | ||
| Bacteroides | ||||
| 18–35 | 68,173,333,800 ± 6,656,031,308 | H = 2.38; p = 0.3 | ||
| 36–49 | 1,911,106,842 ± 363,491,140 | (MW: bact 36–49 vs. bact 50–70), p = 0.12, n.s | ||
| 50–70 | 11,108,000,000 ± 4,633,849,803 | (MW: 18–35 vs. (36–49) group, p = 0.1; n.s | ||
| Lactobacillus plantarum | ||||
| 18–35 | 7187 ± 5812 | H = 2.56, p = 0.27, n.s | ||
| 36–49 | 8351.3 ± 45,412.4 | |||
| 50–70 | 9564.8 ± 4370 | |||
| Clostridium botulinum | ||||
| 18–35 | 10,000 ± 0 | |||
| 36–39 | 33,913 ± 17,328 | H = 2.33, p = 0.312, n.s | ||
| 50–70 | 53,125 ± 32,583 | |||
| E. coli | ||||
| 18–35 | 31,350,000 ± 15,640,119 | H = 0.6, p = 0.73, n.s | ||
| 36–39 | 5,633,243.2 ± 1,444,353 | |||
| 50–70 | 7,346,538.4 ± 2,341,705.6 | |||
| Faecalibacterium prausnitzii | ||||
| 18–35 | 1,961,578,947 ± 573,939,022 | H = 0.224, p = 0.89; n.s | ||
| 36–39 | 1,657,222,777 ± 340,905,218 | |||
| 50–70 | 2,085,937,037 ± 537,806,296 | |||
| E. Coli biovare | ||||
| 18–35 | 1,961,578,947 ± 573,939,022 | H = 4.071, p = 0.131; n.s | ||
| 36–39 | 1,657,222,777 ± 340,905,218 * | 18–35 vs. 36–49 (p = 0.049, MW) | ||
| 50–70 | 2,085,937,037 ± 537,806,296 | |||
| Enterococcus spp. | ||||
| 18–35 | 2,514,000 ± 810,135 | H = 3.77, p = 0.15; n.s | ||
| 36–39 | 1,753,076 ± 606,510 * | |||
| 50–70 | 1,450,400 ± 518,619 | |||
| Akkermansia muciniphila | ||||
| 18–35 | 9,182,333 ± 2,828,963 | H = 1.47, p = 0.47; n.s | ||
| 36–39 | 139,963,162 ± 41,909,305 | |||
| 50–70 | 124,314,160 ± 47,206,763 | |||
| Ruminococcus bromii | ||||
| 18–35 | 128,137,333 ± 66,003,115 | |||
| 36–39 | 183,045,238 ± 43,022,266 | H = 0.928, p = 0.62; n.s | ||
| 50–70 | 280,800,000 ± 114,649,573 | |||
| PCR | ||||
| 18–35 | 0.51 ± 102.6 | H = 1.92; p = 0.38, n.s | ||
| 36–49 | 0.29 ± 0.039 | (MW: bact 36–49 vs. bact 50–70) | ||
| 50–70 | 0.39 ± 0.044 | |||
| Leucocytes (number/mm3) | pH | |||
| 18–35 | 7.04 ± 0.073 | 6.53 + 0.22 | H = 1.48; p = 0.47, n.s (leucocytes) | |
| 36–49 | 1191.63 ± 43.3 | 6.51 + 0.12 | F (2, 64) = 0.48; p = 0.62; n.s (pH) | |
| 50–70 | 1252.61 ± 59.4 | 6.71 + 0.1 | ||
| IgA | Ig G | Ig M | ||
| 18–35 | 104 ± 8 | 1120.3 ± 0.07 | 190.8 ± 12.49 | Ig A [F (2, 64) = 0.4; p = 0.6; n.s] |
| 36–49 | 109 ± 11 | 1191.6 ± 43 | 196.02 ± 12.8 | Ig G (H = 1.48, p = 0.4, n.s) |
| 50–70 | 111 ± 13 | 1252.61 ± 59 | 194.58 ± 10 | Ig M (H = 0.218, p = 0.89, n.s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, J.J.; Bastande Rey, N.; Fernández-García, R. Microbiota and Gut Inflammatory Markers (Zonulin and Fecal Calprotectin) Exhibit Age-Dependent Variation in Patients with Ulcerative Colitis. Life 2025, 15, 1412. https://doi.org/10.3390/life15091412
Merino JJ, Bastande Rey N, Fernández-García R. Microbiota and Gut Inflammatory Markers (Zonulin and Fecal Calprotectin) Exhibit Age-Dependent Variation in Patients with Ulcerative Colitis. Life. 2025; 15(9):1412. https://doi.org/10.3390/life15091412
Chicago/Turabian StyleMerino, José Joaquín, Nuría Bastande Rey, and Rubén Fernández-García. 2025. "Microbiota and Gut Inflammatory Markers (Zonulin and Fecal Calprotectin) Exhibit Age-Dependent Variation in Patients with Ulcerative Colitis" Life 15, no. 9: 1412. https://doi.org/10.3390/life15091412
APA StyleMerino, J. J., Bastande Rey, N., & Fernández-García, R. (2025). Microbiota and Gut Inflammatory Markers (Zonulin and Fecal Calprotectin) Exhibit Age-Dependent Variation in Patients with Ulcerative Colitis. Life, 15(9), 1412. https://doi.org/10.3390/life15091412






