The Effect of Physical Exercise on Non-Oncological Musculoskeletal Chronic Pain and Its Associated Biomarkers: Systematic Review on Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Selection and Data Extraction
2.3. Data Synthesis
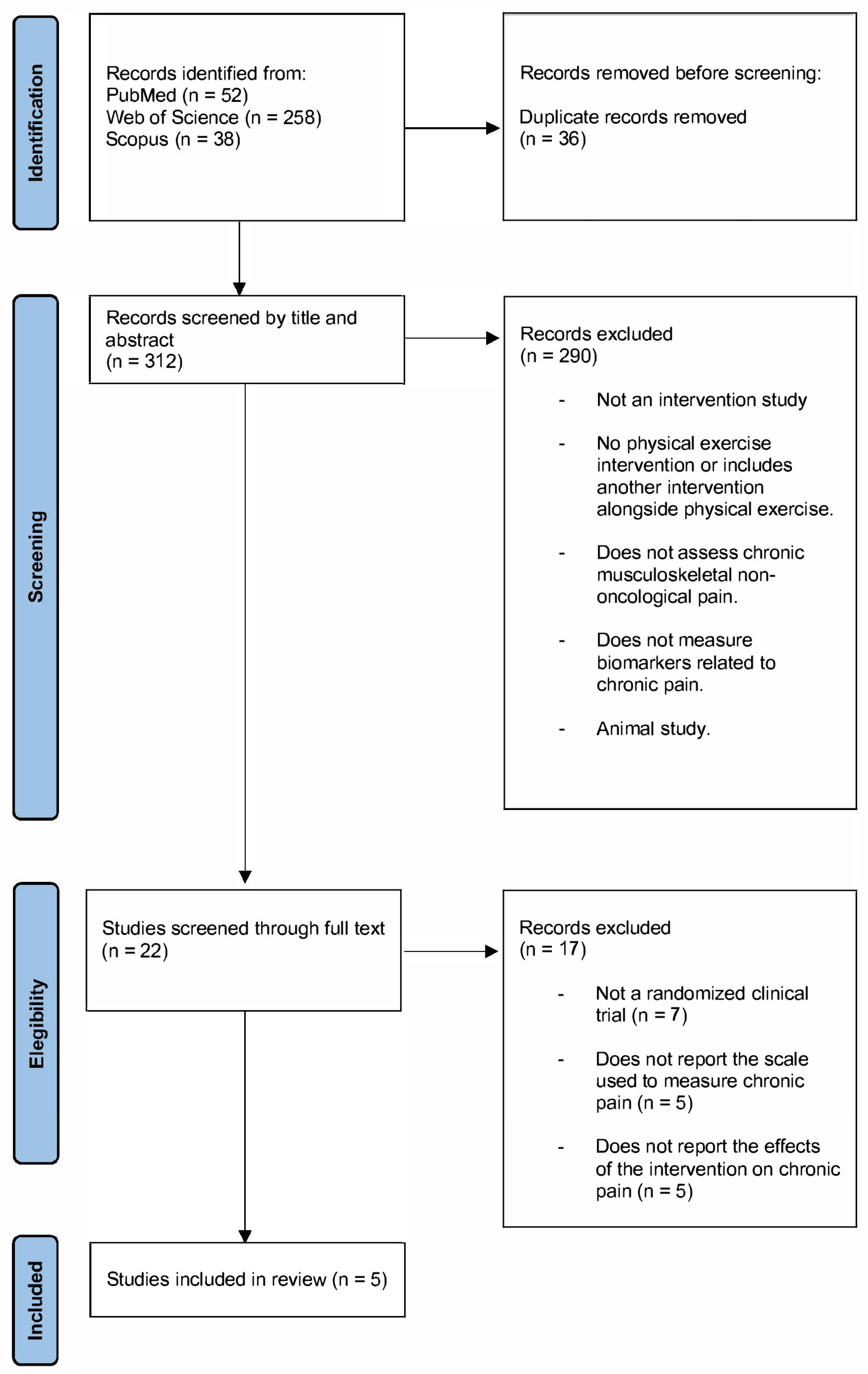
3. Results
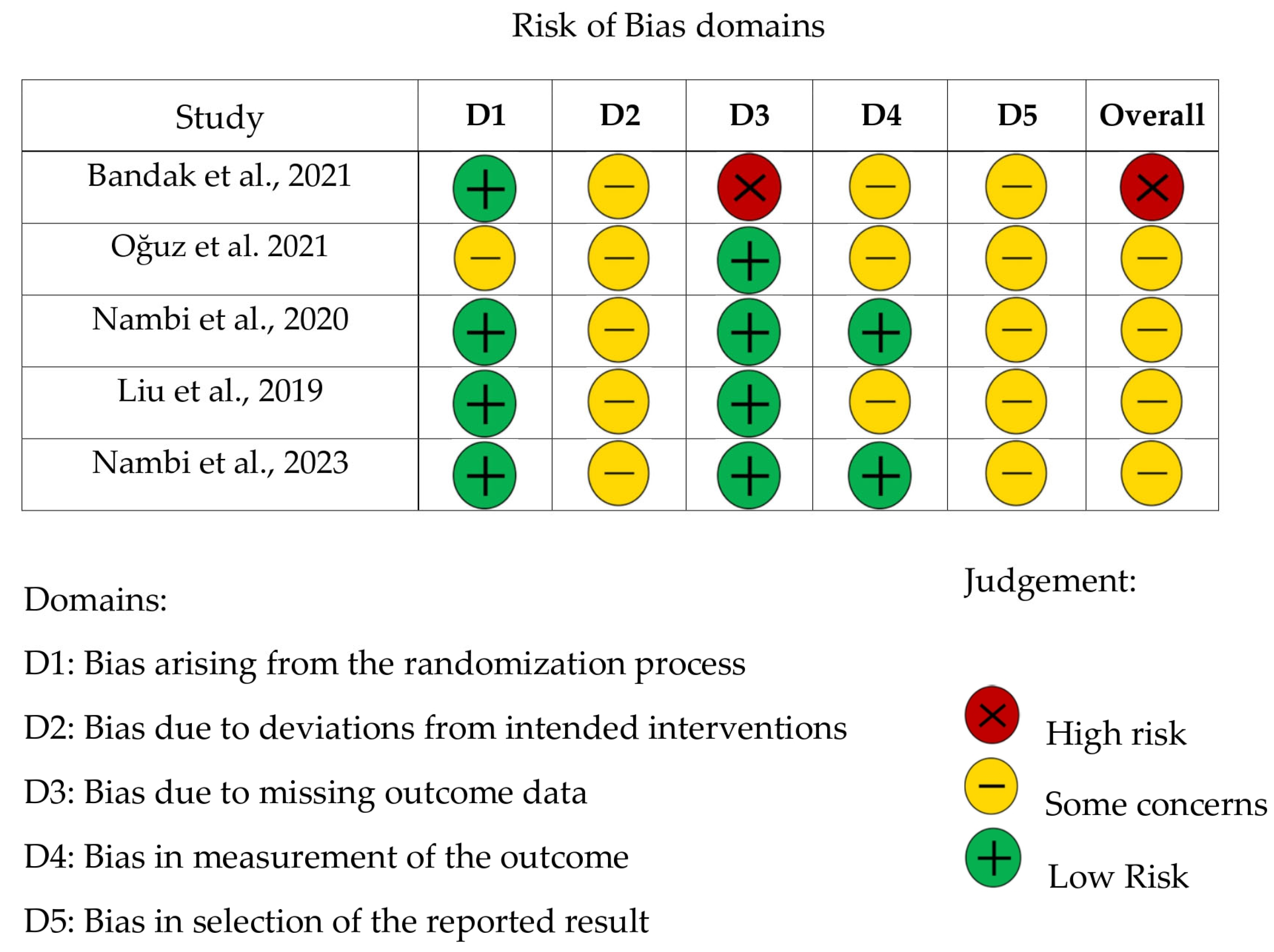
3.1. Risk of Bias Assessment
3.2. Characteristics of the Studies
- -
- Imaging-based biomarkers: magnetic resonance imaging with and without contrast, and functional brain magnetic resonance imaging.
- -
- Inflammatory and immune response regulation biomarkers: IL-2, IL-4, IL-6, IL-10, IFN-γ, PD-1, TIM-3, CRP, TNF-α.
- -
- Neurotrophic biomarkers: BDNF.
- -
- Cartilage metabolism-related biomarkers: COMP, MMP-1, MMP-3.
- -
- Bone morphogenetic proteins: BMP-2, BMP-4, BMP-6, BMP-7.
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | Brain-Derived Neurotrophic Factor |
| BMP | Bone Morphogenic Protein |
| COMP | Cartilage Oligomeric Matrix Protein |
| CRP | C-reactive Protein |
| IASP | International Association for the Study of Pain |
| IL | Interleukin |
| INF-γ | Interferon γ |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| MMP | Matrix Metalloproteinase |
| MRI | Magnetic Resonance Imaging |
| NICE | National Institute for Health and Care Excellence |
| NIH | National Institutes of Health |
| PET | Positron Emission Tomography |
| PD-1 | Programmed Death 1 |
| TIM-3 | T-Cell Ig- And Mucin-Domain-Containing Molecule-3 |
| TNF-α | Tumor Necrosis Factor A |
| VAS | Visual Analogue Scale |
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J. Chronic Pain: Where the Body Meets the Brain. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 167–183. [Google Scholar] [PubMed]
- Van Hecke, O.; Torrance, N.; Smith, B.H. Chronic Pain Epidemiology and Its Clinical Relevance. Br. J. Anaesth. 2013, 111, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.; Moore, R.; Clarke, C.; Martin, D.; Colvin, L.; Smith, B. Physical Activity and Exercise for Chronic Pain in Adults: An Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 1, CD011279. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Eldabe, S.; Obara, I.; Panwar, C.; Brookes, M.; Gulve, A.; Klukinov, M.; Hegarty, D.; Johnson, M.I. Biomarkers for Chronic Pain: Significance and Summary of Recent Advances. Pain Res. Manag. 2022, 2022, 1940906. [Google Scholar] [CrossRef]
- Gunn, J.; Hill, M.M.; Cotten, B.M.; Mittal, D.; Aouizerat, B.E.; Finley, P.J. An Analysis of Biomarkers in Patients with Chronic Pain: An Observational Study. Pain Physician 2020, 23, E41–E49. [Google Scholar] [CrossRef]
- Levitt, J.; Saab, C.Y. What Does a Pain ‘Biomarker’ Mean and Can a Machine Be Taught to Measure Pain? Neurosci. Lett. 2019, 702, 40–43. [Google Scholar] [CrossRef]
- De Assis, E.B.; De Carvalho, C.D.; Martins, C.; Diniz, L.R.L.; de Oliveira, L.R.C.; Silva, L.R.; Macêdo, M.C.S.; Leite, H.R. Beta-Endorphin as a Biomarker in the Treatment of Chronic Pain with Non-Invasive Brain Stimulation: A Systematic Scoping Review. J. Pain Res. 2021, 14, 2191–2200. [Google Scholar] [CrossRef]
- Pinto, E.M.; Neves, J.R.; Laranjeira, M.; Almeida, L.; Silva, R.; Gonçalves, J.; Fernandes, R.; Sousa, R. The Importance of Inflammatory Biomarkers in Non-Specific Acute and Chronic Low Back Pain: A Systematic Review. Eur. Spine J. 2023, 32, 3230–3244. [Google Scholar] [CrossRef]
- Kashanian, A.; Tsolaki, E.; Caruso, J.; Kasper, E.M. Imaging as a Pain Biomarker. Neurosurg. Clin. N. Am. 2022, 33, 345–350. [Google Scholar] [CrossRef]
- Rockholt, M.M.; Kenefati, G.; Doan, L.V.; Llinas, R.H.; Agrawal, A.; Zhou, Y. In Search of a Composite Biomarker for Chronic Pain by Way of EEG and Machine Learning: Where Do We Currently Stand? Front. Neurosci. 2023, 17, 1186418. [Google Scholar] [CrossRef] [PubMed]
- Vagaska, E.; Litavcova, A.; Srotova, I.; Kralovicova, L.; Kocmalova, M.; Madarasova-Geckova, A.; van Dijk, J.P.; Reijneveld, S.A.; Rosenberger, J. Do Lumbar Magnetic Resonance Imaging Changes Predict Neuropathic Pain in Patients with Chronic Non-Specific Low Back Pain? Medicine 2019, 98, e15377. [Google Scholar] [CrossRef] [PubMed]
- D’Agnelli, S.; Gerra, M.C.; Bignami, E.; Arendt-Nielsen, L. Exosomes as a New Pain Biomarker Opportunity. Mol. Pain 2020, 16, 1744806920957800. [Google Scholar] [CrossRef]
- Aguiar, G.C.; do Nascimento, M.R.; de Miranda, A.S.; Rocha, N.P.; Teixeira, A.L.; Scalzo, P.L. Effects of an Exercise Therapy Protocol on Inflammatory Markers, Perception of Pain, and Physical Performance in Individuals with Knee Osteoarthritis. Rheumatol. Int. 2015, 35, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Uzunel, E.; Kronhed, A.C.G.; Alin, C.K.; Ahlgren, C.; Salminen, H. The Effect of Group Training or Spinal Orthosis on Quality of Life and Potential Plasma Markers of Pain in Older Women with Osteoporosis: A Randomized Controlled Trial. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100297. [Google Scholar] [CrossRef]
- Kawi, J.; Lukkahatai, N.; Inouye, J.; Hinds, P.; Saligan, L. Effects of Exercise on Select Biomarkers and Associated Outcomes in Chronic Pain Conditions: Systematic Review. Biol. Res. Nurs. 2016, 18, 147–159. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bandak, E.; Boesen, M.; Bliddal, H.; Brix, M.; Henriksen, M. The Effect of Exercise Therapy on Inflammatory Activity Assessed by MRI in Knee Osteoarthritis: Secondary Outcomes from a Randomized Controlled Trial. Knee 2021, 28, 256–265. [Google Scholar] [CrossRef]
- Oğuz, R.; Belviranlı, M.; Okudan, N. Effects of Exercise Training Alone and in Combination with Kinesio Taping on Pain, Functionality, and Biomarkers Related to Cartilage Metabolism in Knee Osteoarthritis. Cartilage 2021, 13 (Suppl. 1), 1791S–1800S. [Google Scholar] [CrossRef]
- Nambi, G.; Abdelbasset, W.K.; Elsayed, S.H.; Elnegamy, T.E.; Alqahtani, B.A.; Ibrahim, A.A.; Verma, A.; Venugopal, A.; Aldhafian, O.R.; Kakaraparthi, V.N. Comparative Effects of Virtual Reality Training and Sensory Motor Training on Bone Morphogenic Proteins and Inflammatory Biomarkers in Post-Traumatic Osteoarthritis. Sci. Rep. 2020, 10, 72587. [Google Scholar] [CrossRef]
- Nambi, G.; Alghadier, M.; Kashoo, F.Z.; Alqahtani, B.A.; Aldhafian, O.R.; Alnahdi, A.H.; Alsubaie, S.F.; Alghamdi, M.S.; Abdelbasset, W.K. Effects of Virtual Reality Exercises versus Isokinetic Exercises in Comparison with Conventional Exercises on the Imaging Findings and Inflammatory Biomarker Changes in Soccer Players with Non-Specific Low Back Pain: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Chen, X.; Hu, K.; Tu, Y.; Lin, M.; Kong, J. Modulatory Effects of Different Exercise Modalities on the Functional Connectivity of the Periaqueductal Grey and Ventral Tegmental Area in Patients with Knee Osteoarthritis: A Randomised Multimodal Magnetic Resonance Imaging Study. Br. J. Anaesth. 2019, 123, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Mobasheri, A.; Bay-Jensen, A.C.; van Spil, W.E.; Larkin, J.; Levesque, M.C. Osteoarthritis Year in Review 2016: Biomarkers (Biochemical Markers). Osteoarthr. Cartil. 2017, 25, 199–208. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Driban, J.B.; Henrotin, Y.; Hunter, D.J.; Jiang, G.L.; Skou, S.T.; Wang, S.X.; Schnitzer, T.J. OARSI Clinical Trials Recommendations: Design, Conduct, and Reporting of Clinical Trials for Knee Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 747–760. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory Biomarkers of Low Back Pain and Disc Degeneration: A Review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Reckziegel, D.; Vachon-Presseau, E.; Petre, B.; Schnitzer, T.J.; Baliki, M.N.; Apkarian, A.V. Deconstructing Biomarkers for Chronic Pain: Context- and Hypothesis-Dependent Biomarker Types in Relation to Chronic Pain. Pain 2019, 160 (Suppl. 1), S37–S45. [Google Scholar] [CrossRef]
- Thudium, C.S.; Löfvall, H.; Karsdal, M.A.; Bay-Jensen, A.C.; Bihlet, A.R. Protein Biomarkers Associated with Pain Mechanisms in Osteoarthritis. J. Proteom. 2019, 190, 55–66. [Google Scholar] [CrossRef]
- Overstreet, D.S.; Strath, L.J.; Jordan, M.; Quinn, T.L.; LaVigne, T.; DeVon, H.A.; Goodin, B.R. A Brief Overview: Sex Differences in Prevalent Chronic Musculoskeletal Conditions. Int. J. Environ. Res. Public Health 2023, 20, 4521. [Google Scholar] [CrossRef]
- Wolf, J.M.; Cannada, L.; Van Heest, A.E.; O’Connor, M.I.; Ladd, A.; Goldfarb, C.A.; Gelberman, R.H. Male and Female Differences in Musculoskeletal Disease. J. Am. Acad. Orthop. Surg. 2015, 23, 339–347. [Google Scholar] [CrossRef]
- Pedulla, R.; Glugosh, J.; Jeyaseelan, N.; Nicolson, P.J.A.; Sharma, S.; Hall, A.; Costa, M.L. Associations of Gender Role and Pain in Musculoskeletal Disorders: A Mixed-Methods Systematic Review. J. Pain 2024, 25, 104644. [Google Scholar] [CrossRef]
- Alonso-Sal, A.; Alonso-Perez, J.L.; Sosa-Reina, M.D.; Silva, A.; Cuesta-Vargas, A.I. Effectiveness of Physical Activity in the Management of Nonspecific Low Back Pain: A Systematic Review. Medicina 2024, 60, 2065. [Google Scholar] [CrossRef]
| Authors (Year) | Sample | Objective | Intervention | Biomarkers | Studied Variable | Results |
|---|---|---|---|---|---|---|
| Bandak et al., (2021) [19] | N = 60; intervention group = 31 (27 women, 4 men; age = 65.9); control group = 29 (21 women, 8 men; age = 61.3). | To determine the effect of physical exercise on inflammatory activity and the relationship between biomarkers of structural changes and reported pain in individuals with knee osteoarthritis. | - Intervention group: Functional exercise sessions (duration not reported), 3 times a week for 12 weeks. - Control group: No intervention. | Knee MRI with and without contrast, IL-6, IL-10 | Pain reported using the KOOS pain subscale | Statistically significant reduction in perceived pain in the intervention group compared to the control group (p = 0.0075). No statistically significant differences between groups in MRI changes with contrast (p = 0.052) and without contrast (p = 0.122), IL-6 (p = 0.672), and IL-10 (p = 0.871). |
| Liu et al., (2019) [23] | N = 108; Tai Chi group = 28 (22 women, 6 men; age = 40–70); Baduanjin group = 29 (24 women, 5 men; age = 40–68); static bicycle group = 27 (23 women, 4 men; age = 40–70); control group = 24 (14 women, 10 men; age = 40–70) | To determine the effect of different physical exercise modalities on pain, changes in brain regions associated with the opioid and reward/motivation system, and immune and inflammatory biomarkers in patients with knee osteoarthritis. | - Tai Chi group: 1 h sessions, 5 times a week for 12 weeks. - Baduanjin group: 1 h sessions, 5 times a week for 12 weeks. - Static bicycle group: 1 h sessions, 5 times a week for 12 weeks. - Control group: No intervention. | Functional brain MRI, BDNF, IFN-γ, PD-1, TIM-3 | Pain reported using the KOOS pain subscale | Compared to the control group: - Statistically significant reduction in perceived pain (Tai Chi, p < 0.01; Baduanjin, p < 0.01; static bicycle, p = 0.05). - Significant reductions in PD-1 concentrations in the Baduanjin (p = 0.05) and static bicycle groups (p < 0.01), and in IFN-γ levels across all intervention groups (p < 0.01). - Significant reduction in resting-state functional connectivity between the right periaqueductal gray area and medial orbitofrontal cortex (no p-value reported). - Significant increase in gray matter volume in the medial orbitofrontal cortex in all intervention groups (Tai Chi, p = 0.003; Baduanjin, p = 0.048; static bicycle, p = 0.03). |
| Oğuz et al., (2021) [20] | N = 22; physical exercise group = 11 (11 women; age = 51 ± 3.69); physical exercise + kinesio-taping group = 11 (11 women; age = 48.18 ± 7.56) | To investigate the effects of physical exercise alone or in combination with kinesio-taping on pain, functionality, and biomarkers related to cartilage metabolism in patients with knee osteoarthritis. | - Physical exercise group: Strengthening, balance/stability, and stretching exercises for 60 min, 3 times a week for 6 weeks. - Physical exercise + kinesio-taping group: same exercise protocol. | COMP, MMP-1, MMP-3 | Pain reported using the VAS scale | Statistically significant reduction in perceived pain in both groups (p < 0.05), with no statistically significant differences between groups (p > 0.05). No statistically significant changes in COMP, MMP-1, or MMP-3 concentrations in the physical exercise group, with no significant differences between groups (p > 0.05). |
| Nambi et al., (2020) [21] | N = 60; virtual reality training group = 20, age = 22.8 ± 1.3; sensori-motor training group = 20, age = 22.6 ± 1.4; control group = 20, age = 21.9 ± 1.3. Sex not reported. | To compare the effects of virtual reality training and sensori-motor training on bone morphogenetic proteins and inflammatory biomarkers in individuals with post-traumatic knee osteoarthritis. | - Virtual reality training group: 2 sessions of 20 min, 5 days a week for 4 weeks. - Sensori-motor training group: 3 exercises, 5 repetitions per set, 3 sets with 3 min of rest between sets, 5 days a week for 4 weeks. - Control group: conventional knee exercises, 10–15 repetitions per set, 3 sets with 1 min of rest between sets, 5 days a week for 4 weeks. | BMP-2; BMP-4; BMP-6; BMP-7; CRP; TNF-α, IL-2; IL-4; IL-6 | Pain reported using the VAS scale | Significant reduction in pain intensity in the virtual reality training group compared to the control group (p < 0.001). No statistically significant variations between groups in bone morphogenetic protein concentrations BMP-2 (p = 0.946), BMP-4 (p = 0.967), BMP-6 (p = 0.930), and BMP-7 (p = 0.924). Significant reduction in inflammatory biomarkers such as CRP (p = 0.001), TNF-α (p = 0.001), IL-2 (p = 0.015), IL-4 (p = 0.001), and IL-6 (p = 0.001) in the virtual reality training group compared to the other groups. |
| Nambi et al., (2023) [22] | N = 60; virtual reality training group = 20, age = 23.2 ± 1.6; isokinetic training group = 20, age 22.9 ± 1.7; control group = 20, age 22.8 ± 1.8. Sex not reported. | To investigate the effects of physical exercise on inflammatory biomarkers in individuals with idiopathic chronic low back pain. | For all three groups, sessions of their specific exercises lasted 30 min, 5 days a week for 4 weeks. | CRP; TNF-α, IL-2; IL-4; IL-6 | Pain reported using the VAS scale | Significant reduction in pain intensity in the virtual reality training group compared to the other groups (p = 0.001). Significant reduction in inflammatory biomarker concentrations such as CRP (p = 0.001), TNF-α (p = 0.001), IL-2 (p = 0.001), IL-4 (p = 0.001), and IL-6 (p = 0.001) in the virtual reality training group compared to the other groups. |
| Authors (Year) | Biomarker | Conclusion |
|---|---|---|
| Bandak et al., (2021) [19] | Knee MRI with and without contrast IL-6, IL-10 | Report the presence of synovitis. No association is identified between the reduction in pain perception and inflammatory activity at the synovial level. This suggests that changes in pain perception may be due to other mechanisms, such as the systemic anti-inflammatory effect and/or improved psychological well-being. Report systemic pro-inflammatory activity. No association is identified between the reduction in pain perception and variations in the concentrations of these biomarkers. The observed pain reduction may be attributed to other mechanisms, such as improved muscle strength, joint range of motion, or enhanced proprioception. |
| Liu et al., (2019) [23] | Functional brain MRI BDNF IFN-γ PD-1 TIM-3 | An association is identified between the reduction in pain perception and functional changes recorded in the brain in areas related to descending pain modulation as well as reward/motivation systems. Physical exercise may modulate brain areas involved in opioid and dopaminergic neurotransmission systems to alleviate pain. Its plasma concentrations have been linked to self-reported pain. No association is identified between the reduction in pain perception and the plasma concentrations of this biomarker, as no changes in its concentration are observed. It has been associated with spinal microglia activation, triggering pain, and has also been linked to systemic inflammatory activity. An association is identified between the reduction in pain perception and the decrease in the concentrations of this inflammatory activity biomarker, reflecting the relationship between perceived pain and systemic inflammatory activity. It plays a role in regulating the immune response by decreasing its activity. An association is identified between the reduction in pain perception and the increase in the concentrations of this immune response biomarker, reflecting the relationship between perceived pain and the level of immune system activation. It is involved in the regulation of the immune system and the inflammatory response. No association is identified between the reduction in pain perception and the plasma concentrations of this biomarker, as no changes in its concentration are observed. |
| Oğuz et al., (2021) [20] | COMP MMP-1, MMP-3 | Report the degradation of articular cartilage. No association is identified between the reduction in pain perception and this biomarker, which does not change in concentration, suggesting that cartilage metabolism may not be affected. Report the degradation of the extracellular matrix in synovial tissue and articular cartilage. No association is identified between the reduction in pain perception and variations in the concentrations of these biomarkers. While MMP-1 remains unchanged after the intervention period, MMP-3 decreases. Physical exercise improves pain perception independently of variations in these biomarkers of articular cartilage. |
| Nambi et al., (2020) [21] | BMP-2, BMP-4, BMP-6, BMP-7 CRP, TNF-α, IL-2, IL-4, IL-6 | Exert an anabolic effect on chondrocytes by activating the synthesis of cartilage matrix components, regulating and promoting the synthesis of proteoglycans and collagen, and playing a positive role in inflammatory cytokines. No association is identified between the reduction in pain perception and the concentration of these biomarkers. Report on the systemic inflammatory response. An association is identified between the reduction in pain perception and the decrease in the concentrations of these biomarkers, reflecting the relationship between perceived pain and systemic inflammatory activity. |
| Nambi et al., (2023) [22] | CRP, TNF-α, IL-2, IL-4, IL-6 | Report on the systemic inflammatory response. An association is identified between the reduction in pain perception and the decrease in the concentrations of these biomarkers, reflecting the relationship between perceived pain and systemic inflammatory activity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Bellot, I.; Peiró, A.M.; Zandonai, T. The Effect of Physical Exercise on Non-Oncological Musculoskeletal Chronic Pain and Its Associated Biomarkers: Systematic Review on Randomized Controlled Trials. Life 2025, 15, 1413. https://doi.org/10.3390/life15091413
Castillo-Bellot I, Peiró AM, Zandonai T. The Effect of Physical Exercise on Non-Oncological Musculoskeletal Chronic Pain and Its Associated Biomarkers: Systematic Review on Randomized Controlled Trials. Life. 2025; 15(9):1413. https://doi.org/10.3390/life15091413
Chicago/Turabian StyleCastillo-Bellot, Israel, Ana María Peiró, and Thomas Zandonai. 2025. "The Effect of Physical Exercise on Non-Oncological Musculoskeletal Chronic Pain and Its Associated Biomarkers: Systematic Review on Randomized Controlled Trials" Life 15, no. 9: 1413. https://doi.org/10.3390/life15091413
APA StyleCastillo-Bellot, I., Peiró, A. M., & Zandonai, T. (2025). The Effect of Physical Exercise on Non-Oncological Musculoskeletal Chronic Pain and Its Associated Biomarkers: Systematic Review on Randomized Controlled Trials. Life, 15(9), 1413. https://doi.org/10.3390/life15091413







