Safety, Pharmacokinetics, and Food Effect of the RORα Agonist TB-840, a Novel Candidate for Metabolic Dysfunction-Associated Steatohepatitis (MASH): A Randomized First-in-Human Study in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Determination of Plasma and Urine Concentrations of TB-840
2.4. Pharmacokinetics Analysis
2.5. Safety and Tolerability Assessments
2.6. Statistical Analysis
3. Results
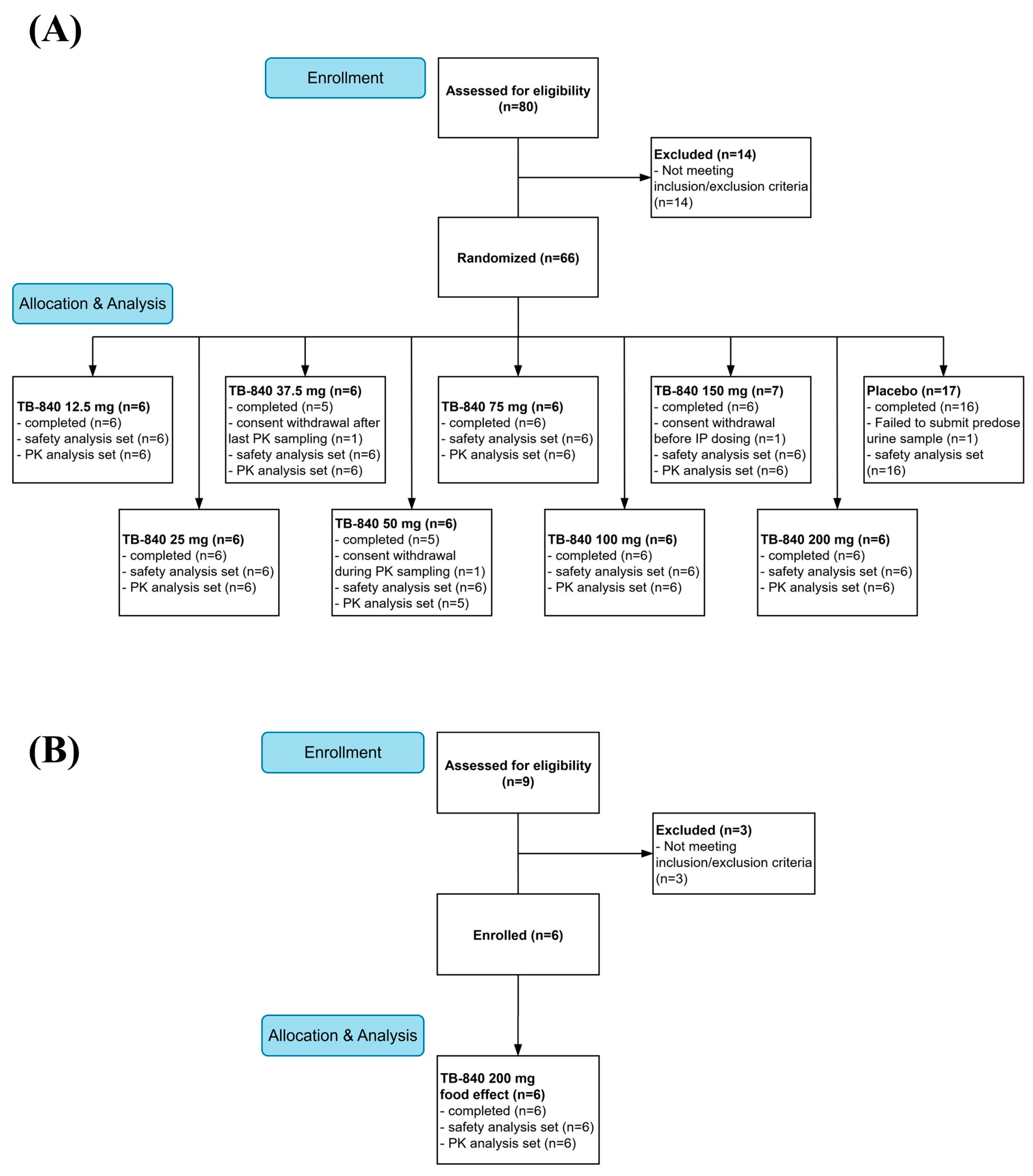
3.1. Participants
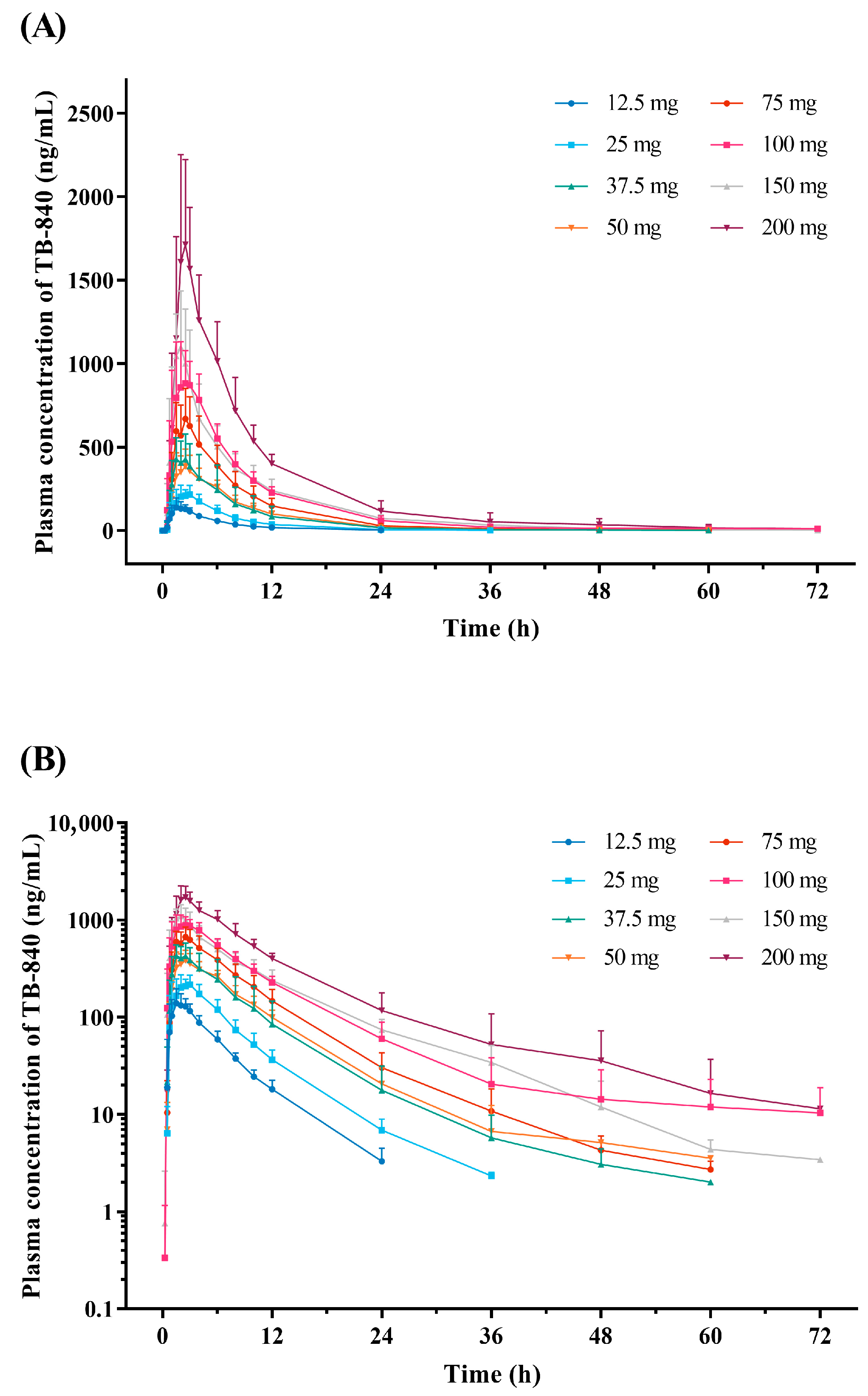
3.2. Pharmacokinetics
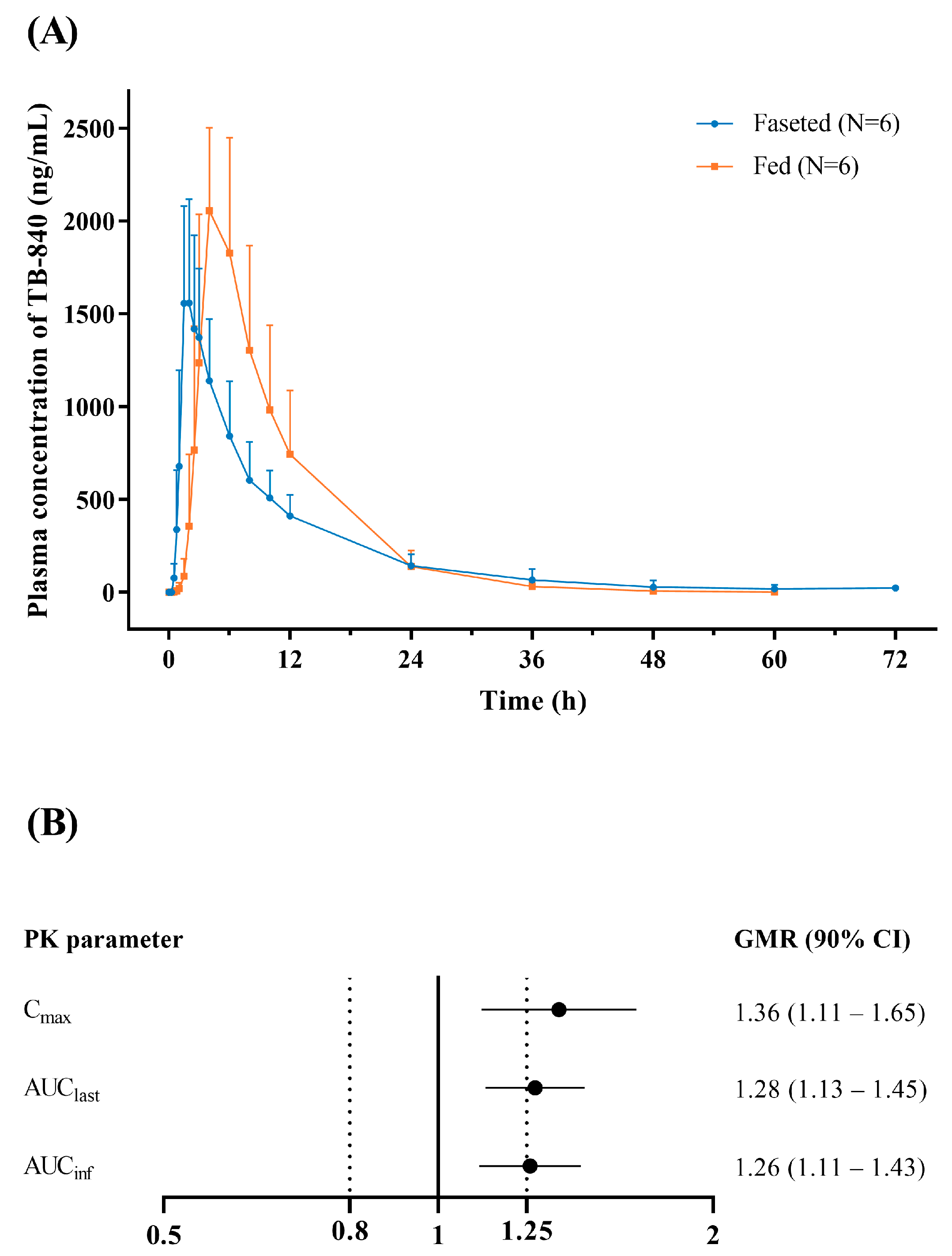
3.3. Effect of Food
3.4. Safety and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Dufour, J.F.; Anstee, Q.M.; Bugianesi, E.; Harrison, S.; Loomba, R.; Paradis, V.; Tilg, H.; Wong, V.W.; Zelber-Sagi, S. Current therapies and new developments in NASH. Gut 2022, 71, 2123–2134. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef]
- Altayar, O.; Noureddin, N.; Thanda Han, M.A.; Murad, M.H.; Noureddin, M. Fibrosis Changes in the Placebo Arm of NASH Clinical Trials. Clin. Gastroenterol. Hepatol. 2019, 17, 2387. [Google Scholar] [CrossRef]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Allen, A.M.; Dubourg, J.; Noureddin, M.; Alkhouri, N. Challenges and opportunities in NASH drug development. Nat. Med. 2023, 29, 562–573. [Google Scholar] [CrossRef]
- Keam, S.J. Resmetirom: First Approval. Drugs 2024, 84, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Kokkorakis, M.; Boutari, C.; Hill, M.A.; Kotsis, V.; Loomba, R.; Sanyal, A.J.; Mantzoros, C.S. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: Trials, opportunities, and challenges. Metabolism 2024, 154, 155835. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Lawitz, E.J.; Coste, A.; Poordad, F.; Alkhouri, N.; Loo, N.; McColgan, B.J.; Tarrant, J.M.; Nguyen, T.; Han, L.; Chung, C.; et al. Acetyl-CoA Carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1983–1991.e3. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M.; Parks, E.J.; Gaballah, A.H.; Bingham, K.; Hammoud, G.M.; Kemble, G.; Buckley, D.; McCulloch, W.; Manrique-Acevedo, C. Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males with Metabolic Abnormalities. Hepatology 2020, 72, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal 2009, 7, e003. [Google Scholar] [CrossRef]
- Ou, Z.; Shi, X.; Gilroy, R.K.; Kirisci, L.; Romkes, M.; Lynch, C.; Wang, H.; Xu, M.; Jiang, M.; Ren, S.; et al. Regulation of the human hydroxysteroid sulfotransferase (SULT2A1) by RORalpha and RORgamma and its potential relevance to human liver diseases. Mol. Endocrinol. 2013, 27, 106–115. [Google Scholar] [CrossRef]
- Kim, E.J.; Yoon, Y.S.; Hong, S.; Son, H.Y.; Na, T.Y.; Lee, M.H.; Kang, H.J.; Park, J.; Cho, W.J.; Kim, S.G.; et al. Retinoic acid receptor-related orphan receptor alpha-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology 2012, 55, 1379–1388. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, H.J.; Kim, E.J.; Kim, K.S.; Hong, S.; Park, H.G.; Lee, M.O. RORα Decreases Oxidative Stress Through the Induction of SOD2 and GPx1 Expression and Thereby Protects Against Nonalcoholic Steatohepatitis in Mice. Antioxid. Redox Sign. 2014, 21, 2083–2094. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, Y.H.; Na, H.; Kim, J.Y.; Kim, T.; Kim, H.J.; Shin, C.; Lee, J.W.; Lee, M.O. Liver-specific deletion of RORalpha aggravates diet-induced nonalcoholic steatohepatitis by inducing mitochondrial dysfunction. Sci. Rep. 2017, 7, 16041. [Google Scholar] [CrossRef]
- Han, Y.H.; Kim, H.J.; Na, H.; Nam, M.W.; Kim, J.Y.; Kim, J.S.; Koo, S.H.; Lee, M.O. RORalpha Induces KLF4-Mediated M2 Polarization in the Liver Macrophages that Protect against Nonalcoholic Steatohepatitis. Cell Rep. 2017, 20, 124–135. [Google Scholar] [CrossRef]
- Han, Y.H.; Shin, K.O.; Kim, J.Y.; Khadka, D.B.; Kim, H.J.; Lee, Y.M.; Cho, W.J.; Cha, J.Y.; Lee, B.J.; Lee, M.O. A maresin 1/RORalpha/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2019, 129, 1684–1698. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yang, I.S.; Kim, H.J.; Yoon, J.Y.; Han, Y.H.; Seong, J.K.; Lee, M.O. RORalpha contributes to the maintenance of genome ploidy in the liver of mice with diet-induced nonalcoholic steatohepatitis. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E118–E131. [Google Scholar] [CrossRef]
- Park, Y.; Hong, S.; Lee, M.; Jung, H.; Cho, W.-J.; Kim, E.-J.; Son, H.-Y.; Lee, M.-O.; Park, H.-g. N-methylthioureas as new agonists of retinoic acid receptor-related orphan receptor. Arch. Pharmacal Res. 2012, 35, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Estimating the Maximum Safe Starting dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; US Food and Drug Administration: Silver Spring, MD, USA, 2005; pp. 1–27.
- Nematisouldaragh, D.; Kirshenbaum, E.; Uzonna, M.; Kirshenbaum, L.; Rabinovich-Nikitin, I. The Role of Retinoic-Acid-Related Orphan Receptor (RORs) in Cellular Homeostasis. Int. J. Mol. Sci. 2024, 25, 11340. [Google Scholar] [CrossRef]
- Farahani, S.; Solgi, L.; Bayat, S.; Abedin Do, A.; Zare-Karizi, S.; Safarpour Lima, B.; Mirfakhraie, R. RAR-related orphan receptor A: One gene with multiple functions related to migraine. CNS Neurosci. Ther. 2020, 26, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Li, K.; Hu, X.; Fan, S.; Gao, Y.; Xue, X.; Bu, Y.; Zhang, H.; Wang, Y.; Wei, C.; et al. Sleep Deprivation Activates a Conserved Lactate-H3K18la-RORalpha Axis Driving Neutrophilic Inflammation Across Species. Adv. Sci. 2025, 12, e04028. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, G.; Hao, H.; Wang, H. FXR agonists for MASH therapy: Lessons and perspectives from obeticholic acid. Med. Res. Rev. 2024, 44, 568–586. [Google Scholar] [CrossRef]
- Coleman, C.I.; Limone, B.; Sobieraj, D.M.; Lee, S.; Roberts, M.S.; Kaur, R.; Alam, T. Dosing frequency and medication adherence in chronic disease. J. Manag. Care Pharm. 2012, 18, 527–539. [Google Scholar] [CrossRef]
- Sabatini, S.; Gastaldelli, A. Metabolic effects and mechanism of action of the pan-PPAR agonist lanifibranor. J. Hepatol. 2025, 82, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Trauner, M. FXR agonists in NASH treatment. J. Hepatol. 2023, 79, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Oh, D.; Kim, H.J.; Chambugong, M.; Kim, M.H.; Lee, M.O.; Park, H.G. An RORalpha agonist, ODH-08, inhibits fibrogenic activation of hepatic stellate cells via suppression of SMAD3. Life Sci. 2024, 340, 122443. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Single Ascending Dose (N = 66) | Food Effect (N = 6) |
|---|---|---|
| Age (years) | 26.9 ± 5.3 | 27.2 ± 3.4 |
| Male sex | 66 (100) | 6 (100) |
| Height (cm) | 174.7 ± 5.1 | 177.6 ± 4.7 |
| Weight (kg) | 71.7 ± 8.6 | 75.3 ± 9.4 |
| BMI (kg/m2) | 23.5 ± 2.5 | 23.8 ± 2.3 |
| Pharmacokinetic Parameters | 12.5 mg (N = 6) | 25 mg (N = 6) | 37.5 mg (N = 6) | 50 mg (N = 5) | 75 mg (N = 6) | 100 mg (N = 6) | 150 mg (N = 6) | 200 mg (N = 6) |
|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | 167.9 ± 37.1 | 268.5 ± 54.6 | 474.9 ± 120.1 | 428.0 ± 86.5 | 731.4 ± 155.4 | 985.1 ± 147.3 | 1172.9 ± 330.2 | 1803.1 ± 443.4 |
| AUClast (h∙ng/mL) | 839 ± 98 | 1526 ± 188 | 3290 ± 1715 | 3347 ± 249 | 5292 ± 1231 | 8299 ± 1216 | 8782 ± 1399 | 14,718 ± 1800 |
| AUCinf (h∙ng/mL) | 863 ± 102 | 1557 ± 203 | 3316 ± 1712 | 3382 ± 265 | 5330 ± 1244 | 8420 ± 1278 | 8823 ± 1395 | 14,794 ± 1815 |
| Tmax (h) | 1.7 (0.7–2.9) | 2.5 (1.5–3.0) | 1.8 (1.5–2.5) | 2.0 (1.5–2.5) | 1.8 (1.5–2.5) | 2.2 (1.5–4.0) | 1.7 (1.5–2.5) | 2.3 (2.0–4.0) |
| t1/2 (h) | 4.8 ± 0.9 | 5.1 ± 0.9 | 5.8 ± 1.5 | 5.9 ± 1.8 | 6.7 ± 2.2 | 9.7 ± 7.1 | 7.2 ± 2.7 | 6.4 ± 1.6 |
| CL/F (L/h) | 14.6 ± 1.6 | 16.3 ± 2.0 | 13.4 ± 5.1 | 14.9 ± 1.1 | 14.7 ± 3.5 | 12.1 ± 2.0 | 17.4 ± 2.7 | 13.7 ± 1.9 |
| Vd/F (L) | 101.8 ± 21.8 | 121.2 ± 28.7 | 111.0 ± 47.0 | 124.6 ± 32.3 | 138.8 ± 45.9 | 162.4 ± 106.3 | 184.1 ± 85.2 | 127.0 ± 38.9 |
| fe (%) | 0.13 ± 0.04 | 0.13 ± 0.07 | 0.09 ± 0.04 | 0.12 ± 0.06 | 0.14 ± 0.07 | 0.1 ± 0.04 | 0.14 ± 0.06 | 0.14 ± 0.03 |
| CLR (L/h) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Ae (μg) | 16.1 ± 5.6 | 32.2 ± 17.8 | 35.6 ± 16.5 | 62.0 ± 31.1 | 108.0 ± 49.8 | 97.7 ± 35.1 | 203.5 ± 96.8 | 289.1 ± 56.9 |
| Pharmacokinetic Parameters | Gradients | 95% Confidence Intervals |
|---|---|---|
| Cmax | 0.8367 | 0.7571–0.9162 |
| AUClast | 1.0165 | 0.9391–1.0939 |
| AUCinf | 1.0091 | 0.9316–1.0866 |
| Pharmacokinetic Parameters | Fasted State (N = 6) | Fed State (N = 6) |
|---|---|---|
| Cmax (ng/mL) | 1741.7 ± 329.0 | 2353.2 ± 412.7 |
| AUClast (h∙ng/mL) | 14,703 ± 3990 | 18,858 ± 5466 |
| AUCinf (h∙ng/mL) | 14,921 ± 4105 | 18,891 ± 5470 |
| Tmax (h) | 1.8 (1.5–3.0) | 4.0 (3.0–6.0) |
| t1/2 (h) | 9.9 ± 6.3 | 5.0 ± 0.5 |
| CL/F (L/h) | 14.3 ± 3.8 | 11.4 ± 3.2 |
| Vd/F (L) | 205.4 ± 163.2 | 81.3 ± 20.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, I.; Lee, S.-R.; Kim, H.J.; Kim, Y.; Lee, S.W. Safety, Pharmacokinetics, and Food Effect of the RORα Agonist TB-840, a Novel Candidate for Metabolic Dysfunction-Associated Steatohepatitis (MASH): A Randomized First-in-Human Study in Healthy Volunteers. Life 2025, 15, 1410. https://doi.org/10.3390/life15091410
Hwang I, Lee S-R, Kim HJ, Kim Y, Lee SW. Safety, Pharmacokinetics, and Food Effect of the RORα Agonist TB-840, a Novel Candidate for Metabolic Dysfunction-Associated Steatohepatitis (MASH): A Randomized First-in-Human Study in Healthy Volunteers. Life. 2025; 15(9):1410. https://doi.org/10.3390/life15091410
Chicago/Turabian StyleHwang, Inyoung, Shi-Ra Lee, Heung Jae Kim, Yun Kim, and Sang Won Lee. 2025. "Safety, Pharmacokinetics, and Food Effect of the RORα Agonist TB-840, a Novel Candidate for Metabolic Dysfunction-Associated Steatohepatitis (MASH): A Randomized First-in-Human Study in Healthy Volunteers" Life 15, no. 9: 1410. https://doi.org/10.3390/life15091410
APA StyleHwang, I., Lee, S.-R., Kim, H. J., Kim, Y., & Lee, S. W. (2025). Safety, Pharmacokinetics, and Food Effect of the RORα Agonist TB-840, a Novel Candidate for Metabolic Dysfunction-Associated Steatohepatitis (MASH): A Randomized First-in-Human Study in Healthy Volunteers. Life, 15(9), 1410. https://doi.org/10.3390/life15091410






