Predictive Model for Estimating the Length of Stay in Hip Arthroplasty Patients by Machine Learning—H.I.P.P.O Score
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Evaluation
2.2. Statistical Analysis
2.3. Algorithm of the Proposed Method
2.4. Ethical Aspects
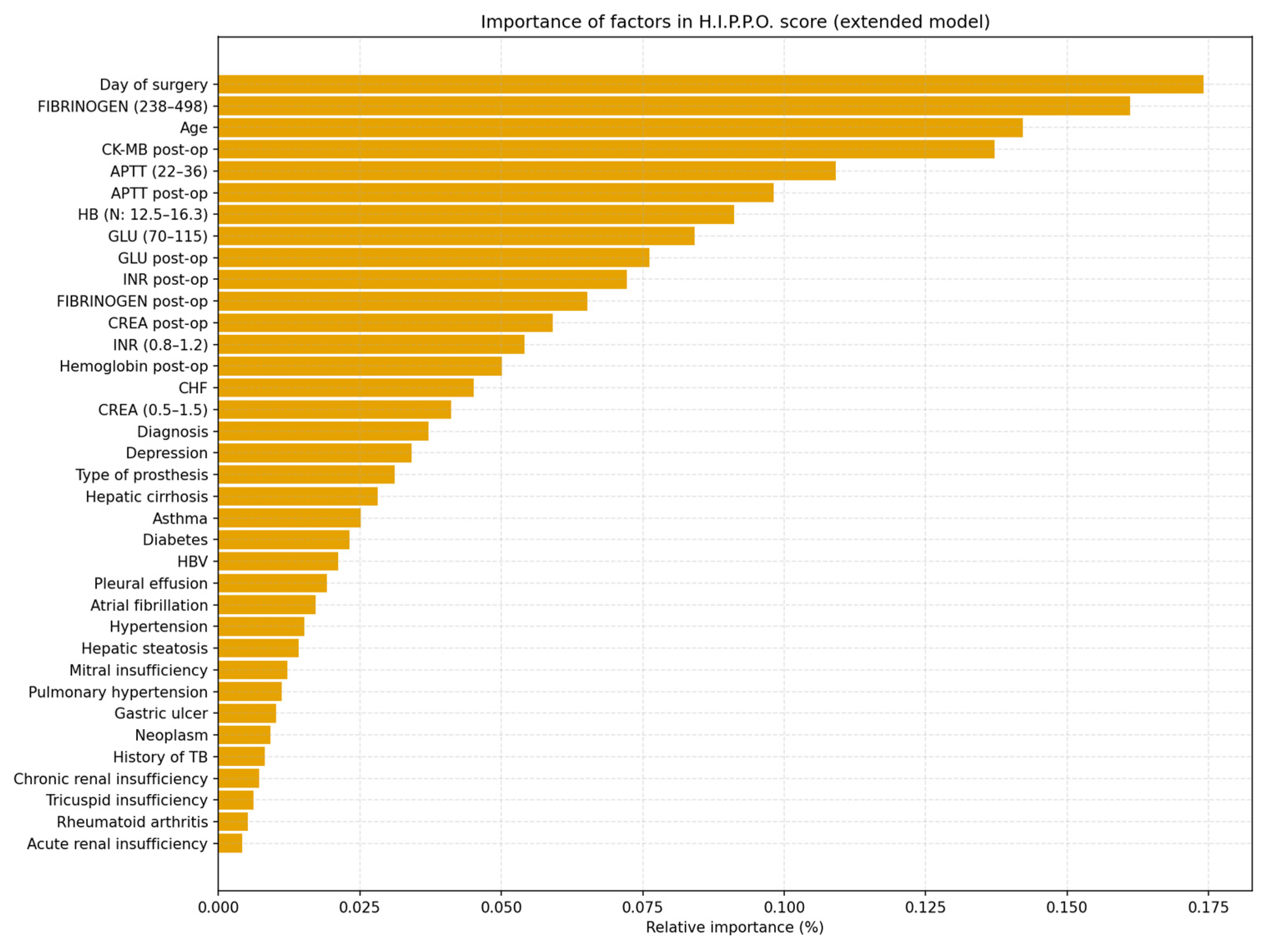
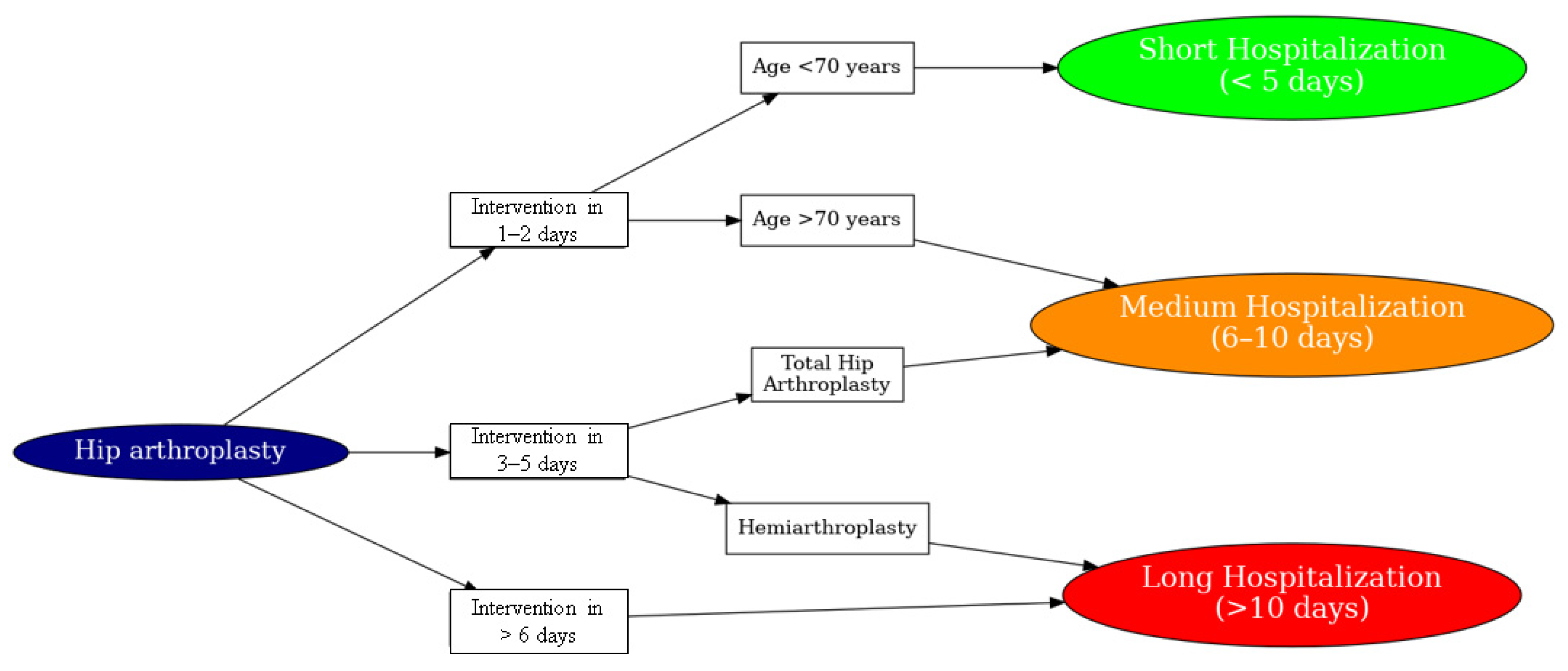
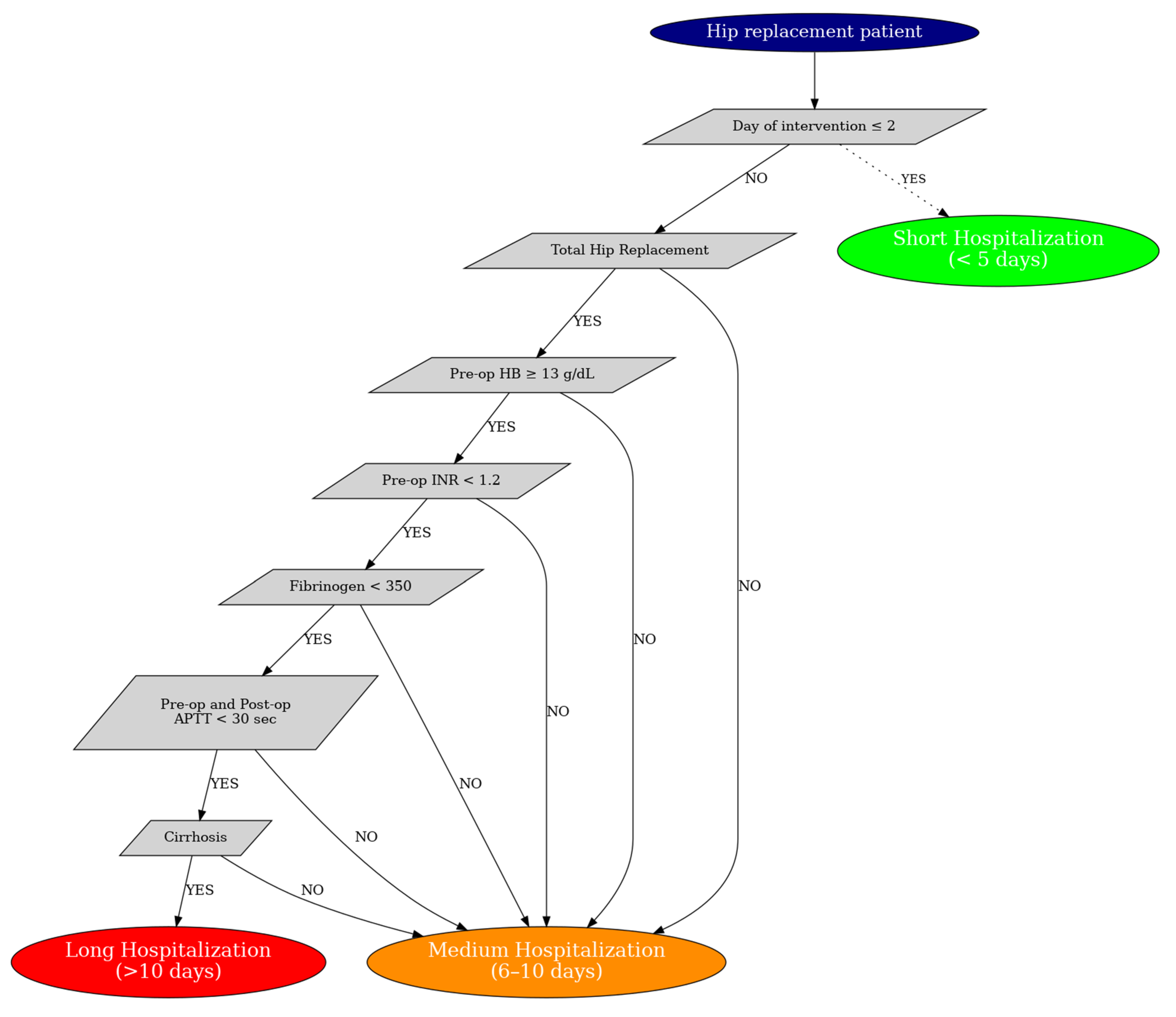
3. H.I.P.P.O Score
Predictive Model and H.I.P.P.O. Score
- The day of the intervention;
- Type of prosthesis (bipolar vs. total);
- Preoperative Fibrinogen;
- Pre and postoperative APTT;
- Preoperative INR;
- Preoperative hemoglobin;
- Presence of liver cirrhosis.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deere, K.; Whitehouse, M.R.; Kunutsor, S.K.; Sayers, A.; Mason, J.; Blom, A.W. How Long Do Revised and Multiply Revised Hip Replacements Last? A Retrospective Observational Study of the National Joint Registry. Lancet Rheumatol. 2022, 4, e468–e479. [Google Scholar] [CrossRef] [PubMed]
- Husted, H.; Otte, K.S.; Kristensen, B.B.; Ørsnes, T.; Kehlet, H. Readmissions after Fast-Track Hip and Knee Arthroplasty. Arch. Orthop. Trauma Surg. 2010, 130, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Kosklin, R.; Lammintakanen, J.; Kivinen, T. Knowledge Management Effects and Performance in Health Care: A Systematic Literature Review. Knowl. Manag. Res. Pract. 2023, 21, 738–748. [Google Scholar] [CrossRef]
- Cafri, G.; Li, L.; Paxton, E.W.; Fan, J. Predicting Risk for Adverse Health Events Using Random Forest. J. Appl. Stat. 2018, 45, 2279–2294. [Google Scholar] [CrossRef]
- Navarro, S.M.; Wang, E.Y.; Haeberle, H.S.; Mont, M.A.; Krebs, V.E.; Patterson, B.M.; Ramkumar, P.N. Machine Learning and Primary Total Knee Arthroplasty: Patient Forecasting for a Patient-Specific Payment Model. J. Arthroplast. 2018, 33, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Sconza, C.; Respizzi, S.; Grappiolo, G.; Monticone, M. The Risk Assessment and Prediction Tool (RAPT) after Hip and Knee Replacement: A Systematic Review. Joints 2019, 7, 041–045. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, P.N.; Navarro, S.M.; Haeberle, H.S.; Karnuta, J.M.; Mont, M.A.; Iannotti, J.P.; Patterson, B.M.; Krebs, V.E. Development and Validation of a Machine Learning Algorithm After Primary Total Hip Arthroplasty: Applications to Length of Stay and Payment Models. J. Arthroplast. 2019, 34, 632–637. [Google Scholar] [CrossRef] [PubMed]
- LeBrun, D.G.; Nguyen, J.T.; Fisher, C.; Tuohy, S.; Lyman, S.; Gonzalez Della Valle, A.; Ast, M.P.; Carli, A.V. The Risk Assessment and Prediction Tool (RAPT) Score Predicts Discharge Destination, Length of Stay, and Postoperative Mobility After Total Joint Arthroplasty. J. Arthroplast. 2023, 38, S121–S129. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Whitaker, B.; Mouat-Hunter, A.; McCrory, B. Predicting Length of Stay Using Machine Learning for Total Joint Replacements Performed at a Rural Community Hospital. PLoS ONE 2022, 17, e0277479. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Zhong, X.; Miley, E.N.; Gray, C.F. Preoperative Prediction and Risk Factor Identification of Hospital Length of Stay for Total Joint Arthroplasty Patients Using Machine Learning. Arthroplast. Today 2023, 22, 101166. [Google Scholar] [CrossRef] [PubMed]
- Klestil, T.; Röder, C.; Stotter, C.; Winkler, B.; Nehrer, S.; Lutz, M.; Klerings, I.; Wagner, G.; Gartlehner, G.; Nussbaumer-Streit, B. Impact of Timing of Surgery in Elderly Hip Fracture Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 13933. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Shin, W.C.; Moon, N.H.; Suh, K.T. Timing of Hip-Fracture Surgery in Elderly Patients: Literature Review and Recommendations. Hip Pelvis 2020, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Essa, S.; Anaqreh, Y.; Abueed, M.; Alrawashdeh, M.; Hussein, N.; Adi, Y.; Batbouta, J.; Alkhatatba, M.; Mohaidat, Z.; Radaideh, A. Impact of Surgical Timing on Mortality and Functional Outcomes in Elderly Hip Fracture Patients: A Retrospective Cohort Study. Acta Inform. Medica 2024, 32, 196. [Google Scholar] [CrossRef] [PubMed]
- Sicat, C.S.; Muthusamy, N.; Singh, V.; Davidovitch, R.I.; Slover, J.D.; Schwarzkopf, R. Impact of Preoperative Anemia Severity on Primary Total Hip Arthroplasty Outcomes. J. Arthroplast. 2022, 37, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Q.; Yang, Y.-Z.; Li, P.-F.; Ma, G.-R.; Zhang, A.-R.; Zhang, H.; Guo, H.-Z. Impact of Preoperative Anemia on Patients Undergoing Total Joint Replacement of Lower Extremity: A Systematic Review and Meta-Analysis. J. Orthop. Surg. 2024, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Margelyte, R.; Redaniel, M.T.; Eyles, E.; Jones, T.; Penfold, C.; Blom, A.; Elliott, A.; Harper, A.; Keen, T.; et al. Risk Factors for Prolonged Length of Hospital Stay Following Elective Hip Replacement Surgery: A Retrospective Longitudinal Observational Study. BMJ Open 2024, 14, e078108. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Gratza, S.; Eckardt, A.; Ilchmann, T. Stepwise Implementation of an Enhanced Recovery Pathway for Elective Total Hip Arthroplasty in a Swiss Hospital: A Cohort Study. Swiss Med. Wkly. 2024, 154, 3537. [Google Scholar] [CrossRef] [PubMed]
- Onggo, J.; Nambiar, M.; McDougall, C.; Hau, R.; Babazadeh, S. Comparing Outcomes of Total Hip Arthroplasty versus Hemiarthroplasty in Neck of Femur Fracture Patients: An Australian Registry Study. Eur. J. Trauma Emerg. Surg. 2023, 49, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Ekhtiari, S.; Gormley, J.; Axelrod, D.E.; Devji, T.; Bhandari, M.; Guyatt, G.H. Total Hip Arthroplasty Versus Hemiarthroplasty for Displaced Femoral Neck Fracture: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Bone Jt. Surg. 2020, 102, 1638–1645. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value | Short (%)/Average | Average (%)/Average | Long (%)/Average | Test | P | Significantly |
|---|---|---|---|---|---|---|---|
| DM | Yes | 25.00% | 9.10% | 19.20% | Chi2 | 0.3408 | Not |
| DM | Not | 75.00% | 90.90% | 80.80% | Chi2 | 0.3408 | Not |
| HT | Yes | 75.00% | 69.10% | 92.30% | Chi2 | 0.0709 | Not |
| HT | Not | 25.00% | 30.90% | 7.70% | Chi2 | 0.0709 | Not |
| CRF | Present | 0% | 5.50% | 15.40% | Chi2 | 0.2618 | Not |
| CRF | Absent | 100.00% | 94.50% | 84.60% | Chi2 | 0.2618 | Not |
| Cirrhosis | Present | 0% | 0% | 11.50% | Chi2 | 0.0294 | Yes |
| Cirrhosis of the Liver | Absent | 100.00% | 100.00% | 88.50% | Chi2 | 0.0294 | Yes |
| CANCER | Present | 0% | 9.10% | 15.40% | Chi2 | 0.5391 | Not |
| CANCER | Absent | 100.00% | 90.90% | 84.60% | Chi2 | 0.5391 | Not |
| Prosthesis type | Bipolar prosthesis | 25.00% | 49.10% | 88.50% | Chi2 | 0.0011 | Yes |
| Prosthesis type | Total arthroplasty | 75.00% | 50.90% | 11.50% | Chi2 | 0.0011 | Yes |
| Age | Average age | 61 | 69.58 | 79.38 | Kruskal | 0.0024 | Yes |
| Intervention day | Average of the day of the intervention | 1.75 | 2.69 | 5.27 | Kruskal | 0 | Yes |
| HB | Pre-op hemoglobin | 14.7 | 12.57 | 12.27 | Kruskal | 0.0119 | Yes |
| HB | Post-op hemoglobin | 11.75 | 11.03 | 11.28 | Kruskal | 0.497 | Not |
| INR | Post-operative INR | 1.88 | 2.47 | 1.3 | Kruskal | 0.3228 | Not |
| INR | Pre-op INR | 0.98 | 1.59 | 1.37 | Kruskal | 0.032 | Yes |
| APTT | Pre-op APTT | 24.28 | 27.7 | 31.15 | Kruskal | 0.0058 | Yes |
| APTT | APTT post-op (sec) | 21 | 26.41 | 32.04 | Kruskal | 0.025 | Yes |
| FIBRINOGEN | Pre-op fibrinogen (mg/dL) | 282.5 | 350.04 | 415.88 | Kruskal | 0.0013 | Yes |
| FIBRINOGEN | Post-op fibrinogen (mg/dL) | 311.32 | 375.89 | 476.46 | Kruskal | 0.2543 | Not |
| GLU | Pre-op blood glucose (mg/dL) | 132.5 | 103.39 | 151.27 | Kruskal | 0.0696 | Not |
| GLU | Post-op blood glucose (mg/dL) | 100.6 | 94.72 | 103.43 | Kruskal | 0.6449 | Not |
| CREATE | Pre-op creatinine (mg/dL) | 0.85 | 1.37 | 1.11 | Kruskal | 0.8367 | Not |
| CREATE | Post-op creatinine (mg/dL) | 2.07 | 2.59 | 2.68 | Kruskal | 0.7822 | Not |
| CK-MB | CK-MB post-op (UI/L) | 11.9 | 13.55 | 46.99 | Kruskal | 0.0354 | Yes |
| CK-MB | CK-MB pre-op | 7.88 | 14.77 | 18.37 | Kruskal | 0.064 | Not |
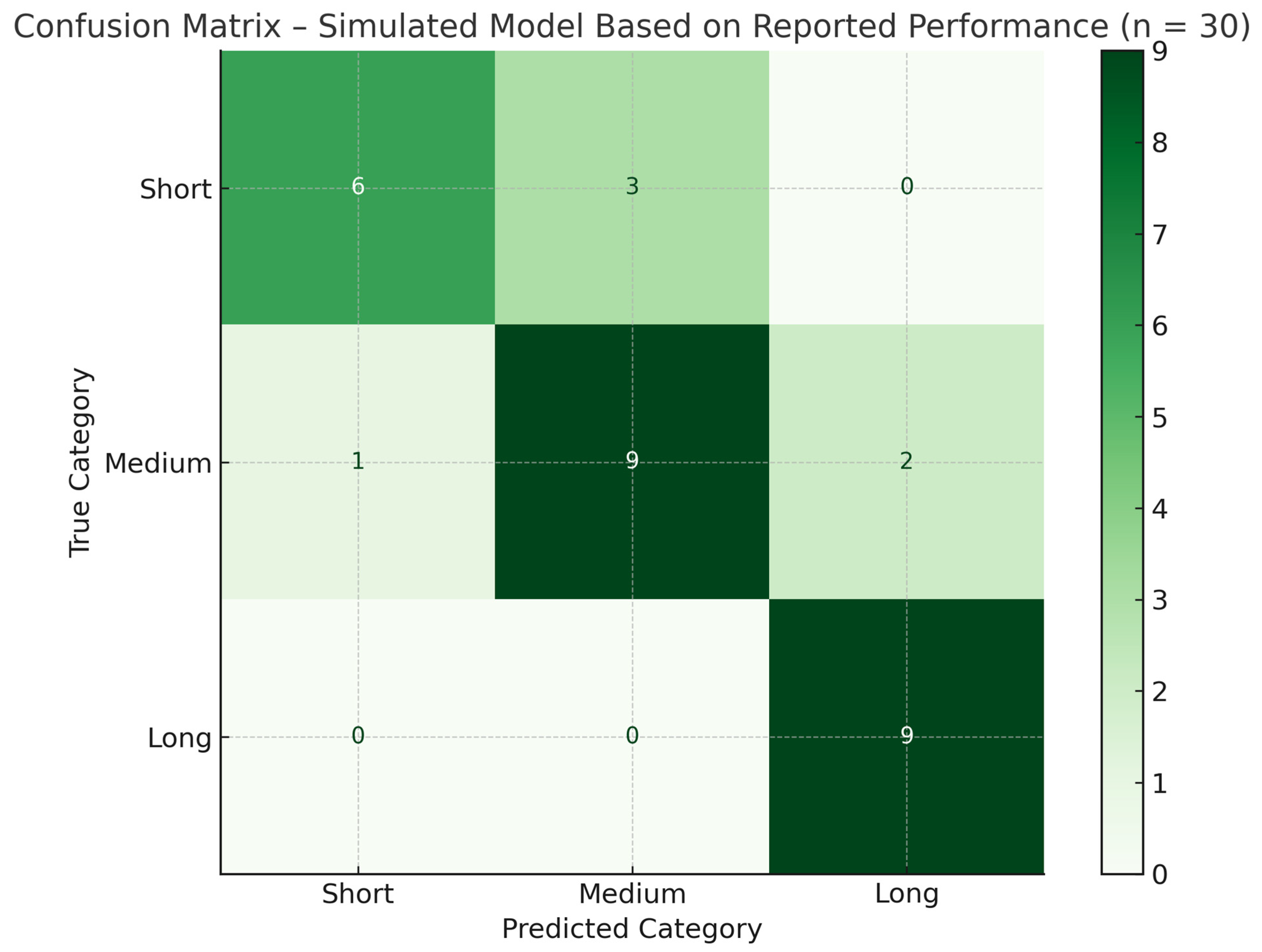
| Precision | Recall | F1 Score | Support | |
|---|---|---|---|---|
| Short hospitalization | 1 | 0.667 | 0.8 | 9 |
| Average hospitalization | 0.75 | 0.75 | 0.75 | 12 |
| Long hospitalization | 0.75 | 1 | 0.857 | 9 |
| Accuracy * | 0.8 | 0.8 | 0.8 | 0.8 |
| AVG Macro | 0.833 | 0.806 | 0.802 | 30 |
| weighted avg | 0.825 | 0.8 | 0.797 | 30 |
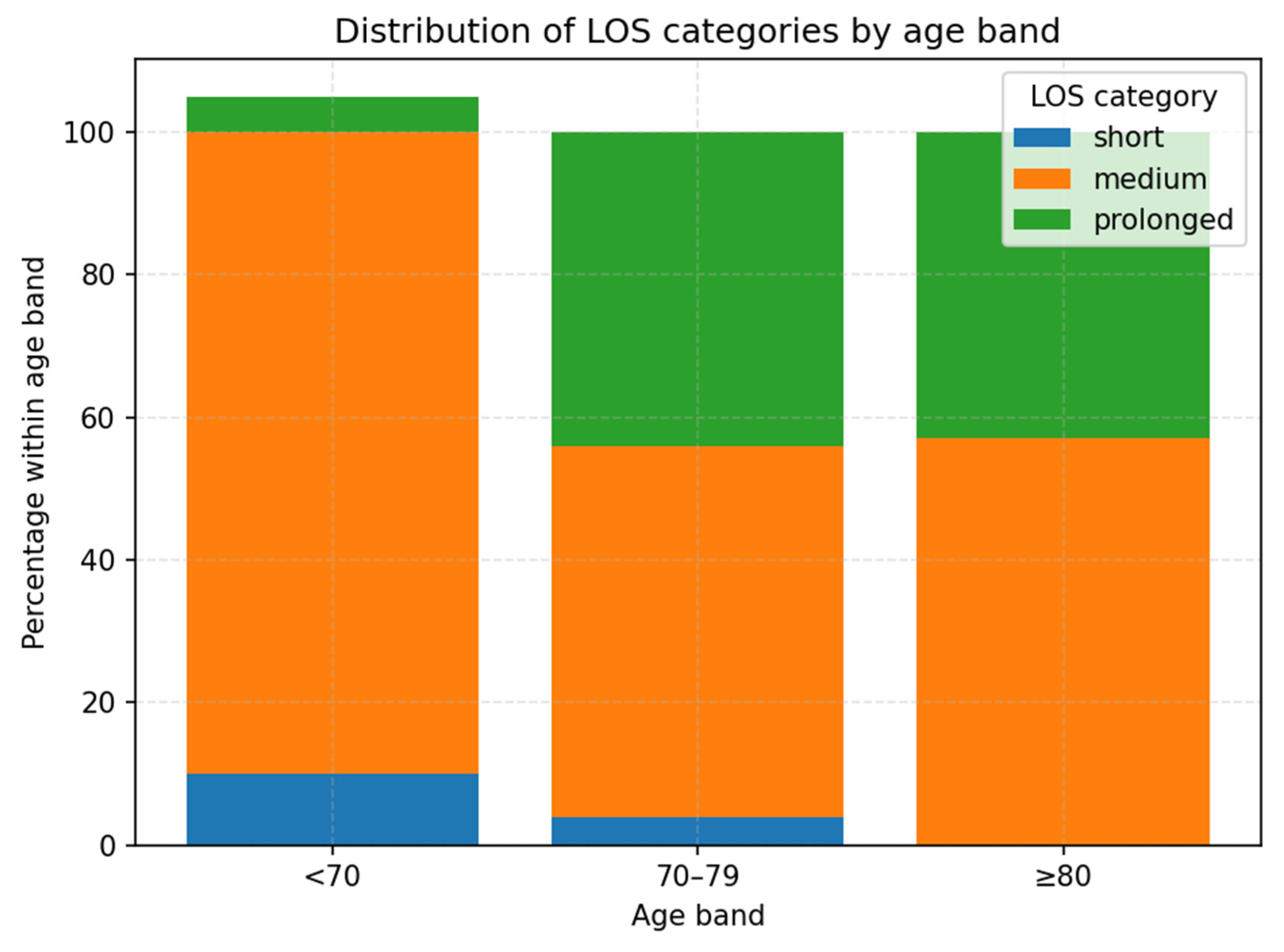
| Age_Band | Short | Medium | Prolonged |
|---|---|---|---|
| <70 | 10 | 83.3 | 6.7 |
| 70–79 | 3.7 | 51.9 | 44.4 |
| >80 | 0 | 57.1 | 42.9 |
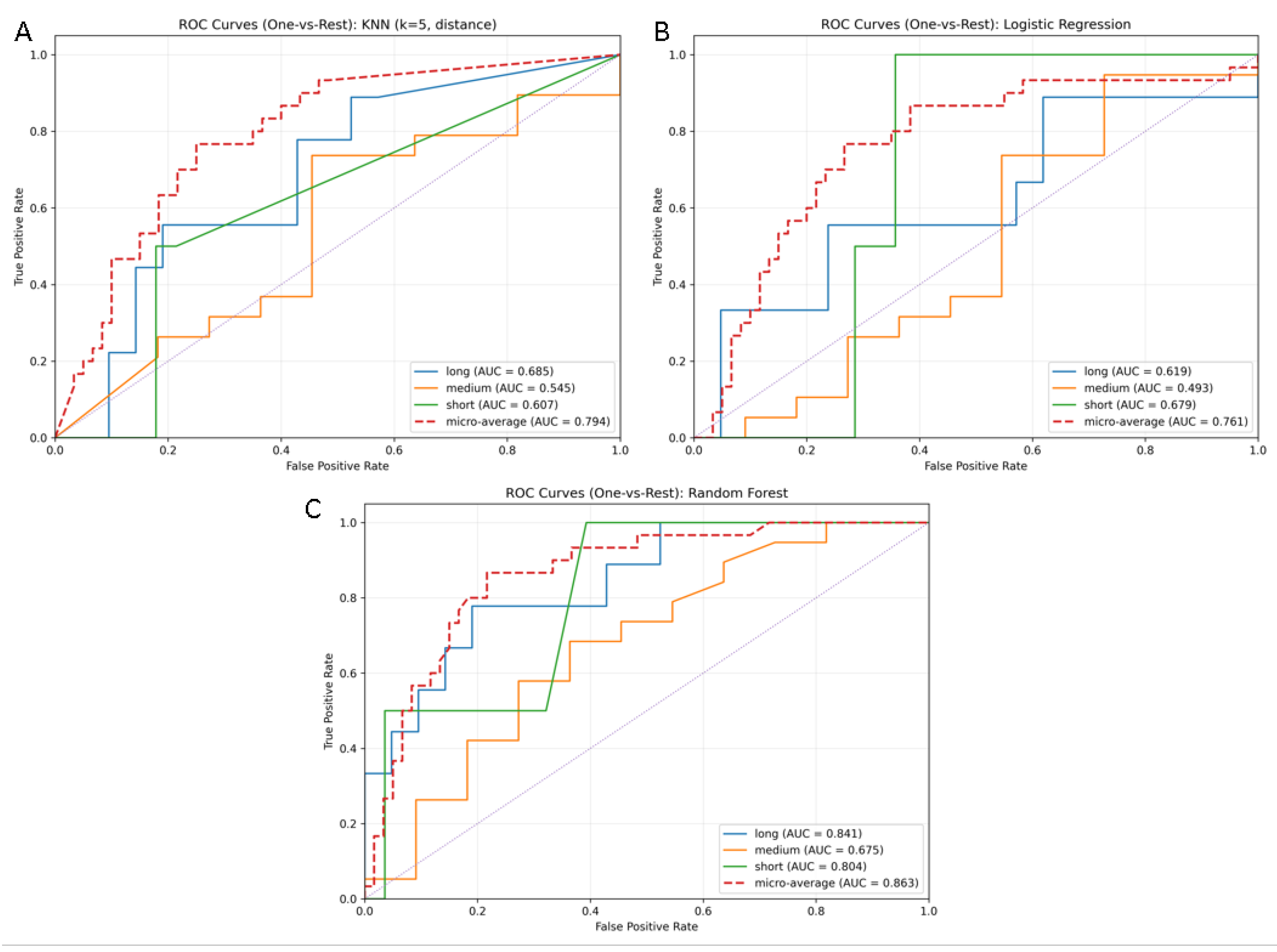
| Model | Accuracy | F1 (Short) | F1 (Medium) | F1 (Long) | F1 (Macro Avg) | F1 (Weighted) |
|---|---|---|---|---|---|---|
| Random Forest | 0.7 | 0 | 0.791 | 0.533 | 0.441 | 0.661 |
| Logistic Regression | 0.6 | 0 | 0.7 | 0.444 | 0.381 | 0.577 |
| KNN | 0.633 | 0 | 0.756 | 0.308 | 0.354 | 0.571 |
| Characteristics | RAPT Score [6] | Ramkumar et al. [7] | H.I.P.P.O. Score |
|---|---|---|---|
| Type of model | Simple clinical score | ML algorithm (naïve Bayes) | Interpretable ML-based custom score |
| Surgical procedure analyzed | Total hip/knee arthroplasty | Primary total hip arthroplasty | Primary total hip arthroplasty |
| Number of patients | Not specified; validated in various populations | 122,334 patients | Single-center cohort |
| Type of data used | Basic clinical data | Preoperative administrative data | Clinical + biological + organizational data |
| Included variables | Age, sex, mobility, social support, independence | Age, sex, race, comorbidity score, illness severity | HB, INR, glucose, APTT, fibrinogen, surgery day, CKMB, etc. |
| Model purpose | Predicting discharge disposition and LOS | Predicting LOS and reimbursement (PSPM) | Predicting hospital LOS |
| Performance (accuracy/AUC) | Variable accuracy; limited for intermediate scores | LOS: AUC = 0.87; accuracy = 82.9% | High accuracy (F1/precision/recall reported per class) |
| Clinical applicability | Very easy to use | Scalable in large systems, less interpretable | Easy to apply in non-digital hospital settings |
| Technical requirements | Minimal (can be applied on paper) | Moderate—requires database access and ML coding | Low—based on routinely collected clinical data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danet, A.; Spiridonica, R.; Iacobescu, G.; Cursaru, A.; Cretu, B.; Serban, B.; Iordache, S.; Corlatescu, A.-D.; Cirstoiu, C. Predictive Model for Estimating the Length of Stay in Hip Arthroplasty Patients by Machine Learning—H.I.P.P.O Score. Life 2025, 15, 1408. https://doi.org/10.3390/life15091408
Danet A, Spiridonica R, Iacobescu G, Cursaru A, Cretu B, Serban B, Iordache S, Corlatescu A-D, Cirstoiu C. Predictive Model for Estimating the Length of Stay in Hip Arthroplasty Patients by Machine Learning—H.I.P.P.O Score. Life. 2025; 15(9):1408. https://doi.org/10.3390/life15091408
Chicago/Turabian StyleDanet, Andrei, Razvan Spiridonica, Georgian Iacobescu, Adrian Cursaru, Bogdan Cretu, Bogdan Serban, Sergiu Iordache, Antonio-Daniel Corlatescu, and Catalin Cirstoiu. 2025. "Predictive Model for Estimating the Length of Stay in Hip Arthroplasty Patients by Machine Learning—H.I.P.P.O Score" Life 15, no. 9: 1408. https://doi.org/10.3390/life15091408
APA StyleDanet, A., Spiridonica, R., Iacobescu, G., Cursaru, A., Cretu, B., Serban, B., Iordache, S., Corlatescu, A.-D., & Cirstoiu, C. (2025). Predictive Model for Estimating the Length of Stay in Hip Arthroplasty Patients by Machine Learning—H.I.P.P.O Score. Life, 15(9), 1408. https://doi.org/10.3390/life15091408






