Transcriptomic Survey of How Acetate Addition Affected the Growth in Nannochloropsis oceanica (Suda & Miyashita) R. E. Lee
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions of N. oceanica
2.2. Experimental Design and Sampling
2.3. Library Construction and On-Board Sequencing
2.4. Transcriptome Gene Differential Expression and Enrichment Analysis
3. Results and Discussion
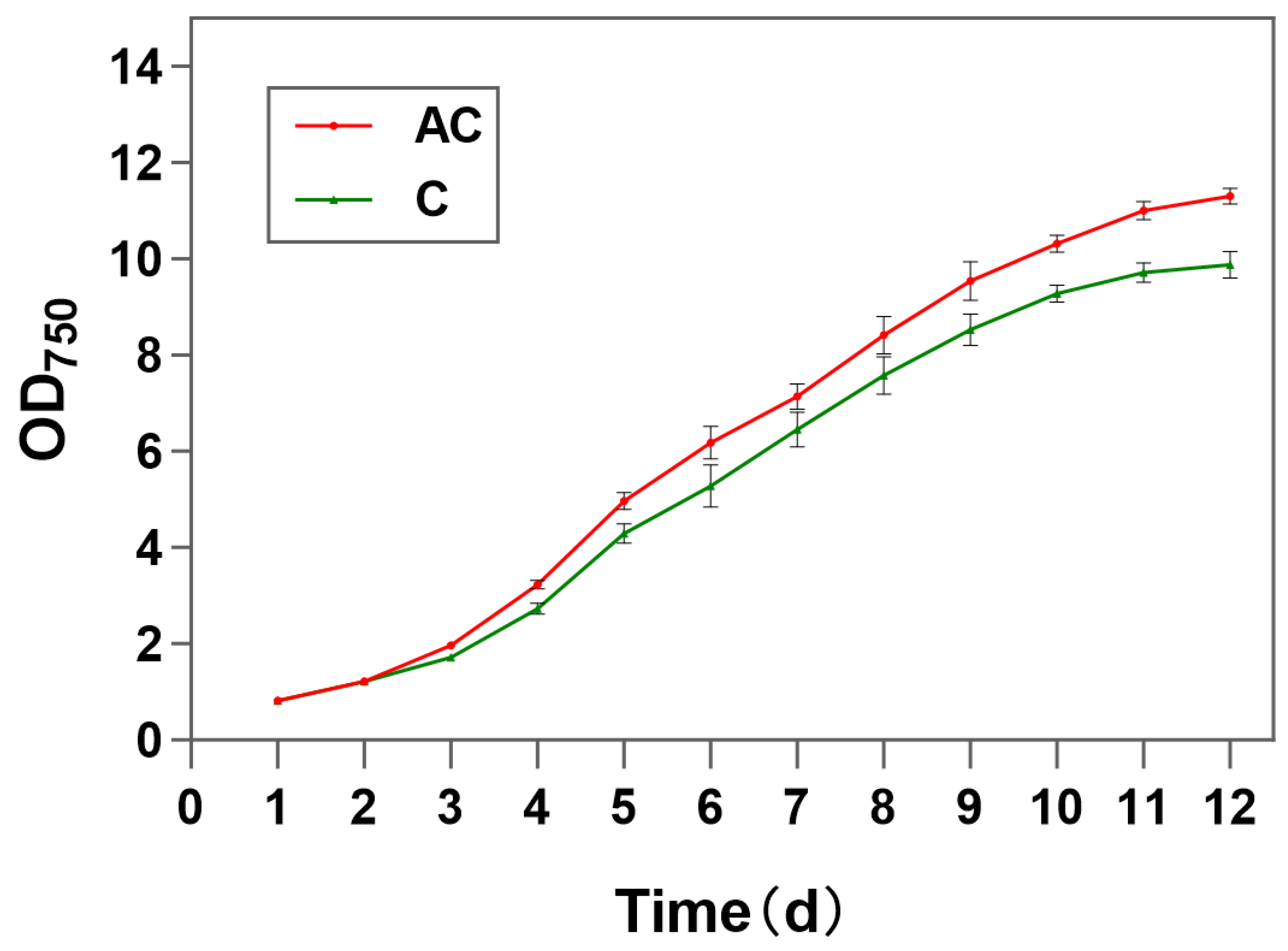
3.1. Physiological Changes in Response to by Sodium Acetate
3.2. Transcriptome Data of N. oceanica from Illumina Sequencing
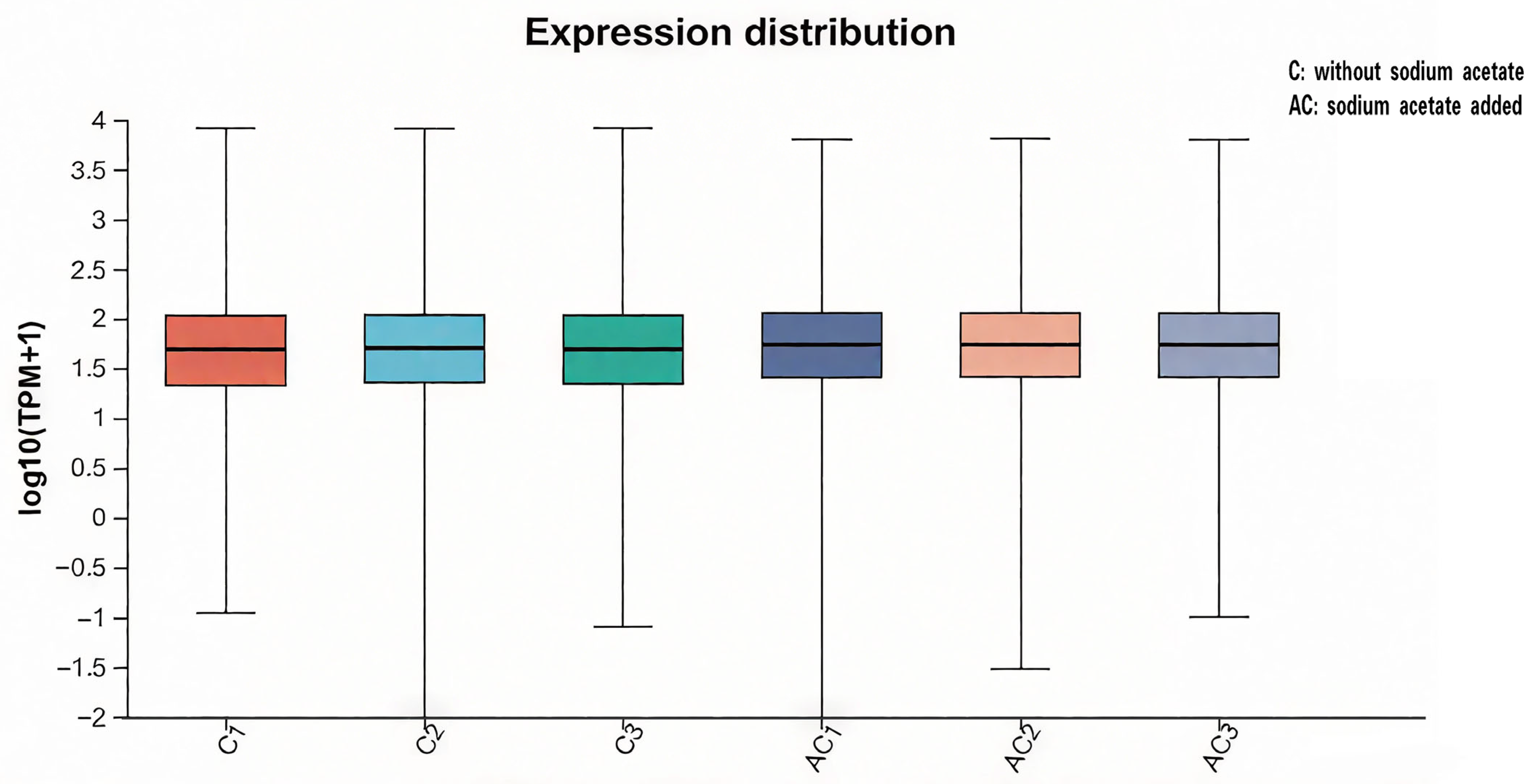
3.3. Gene Expression Distribution Analysis
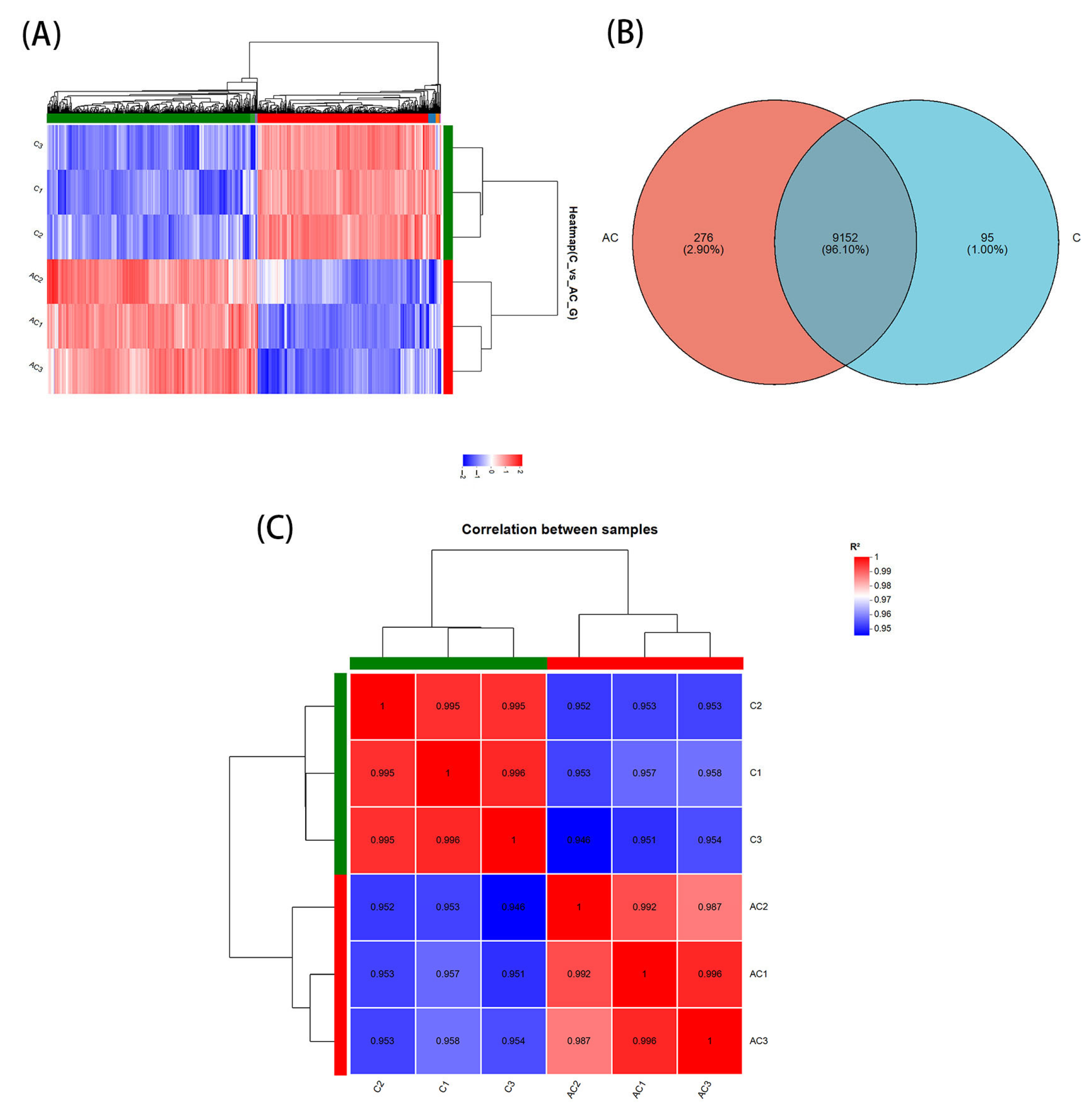
3.4. Identification of DEGs Under Sodium Acetate Conditions in N. oceanica
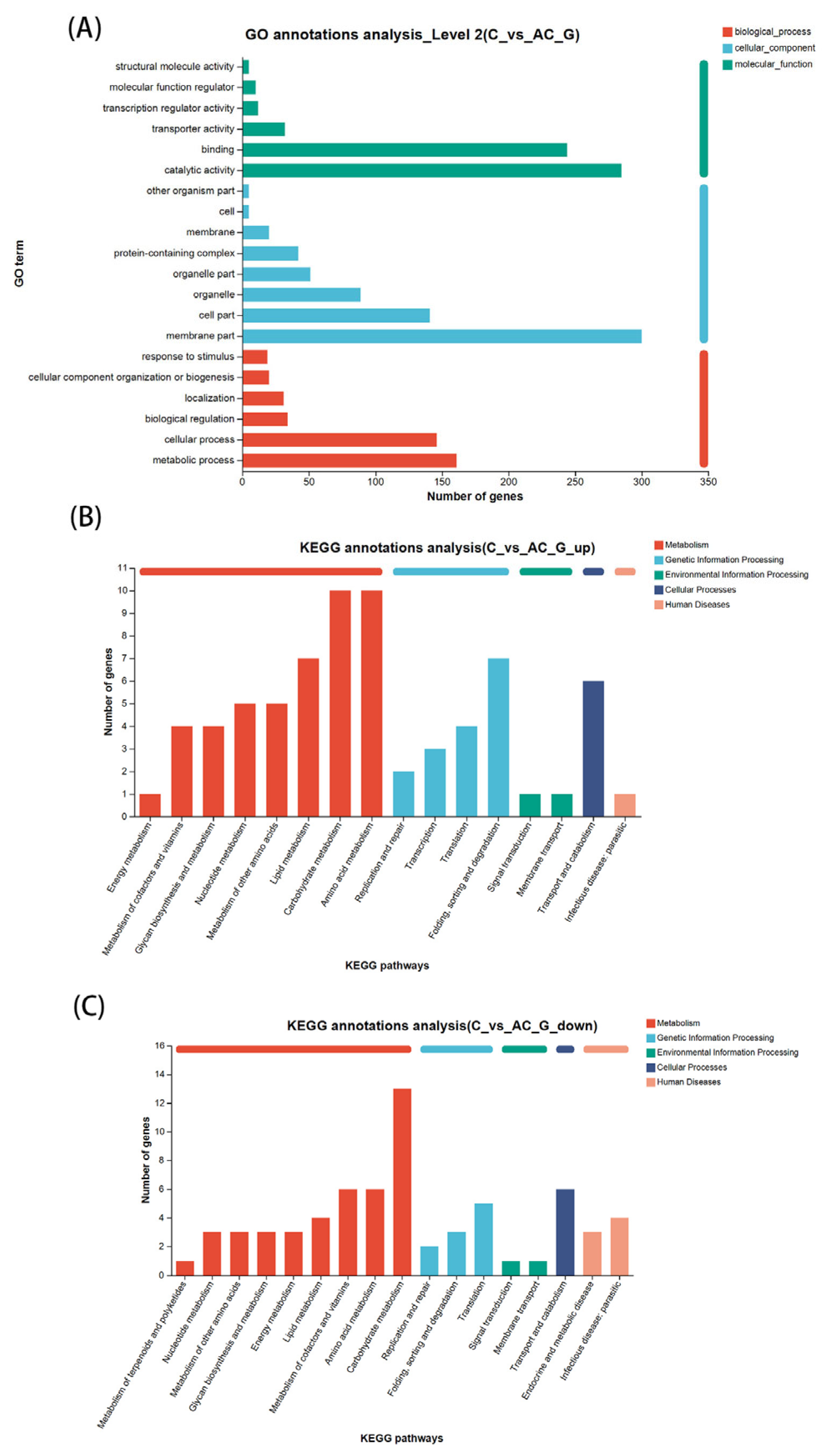
3.5. Functional Enrichment of Differential Expressed Gene by GO and KEGG
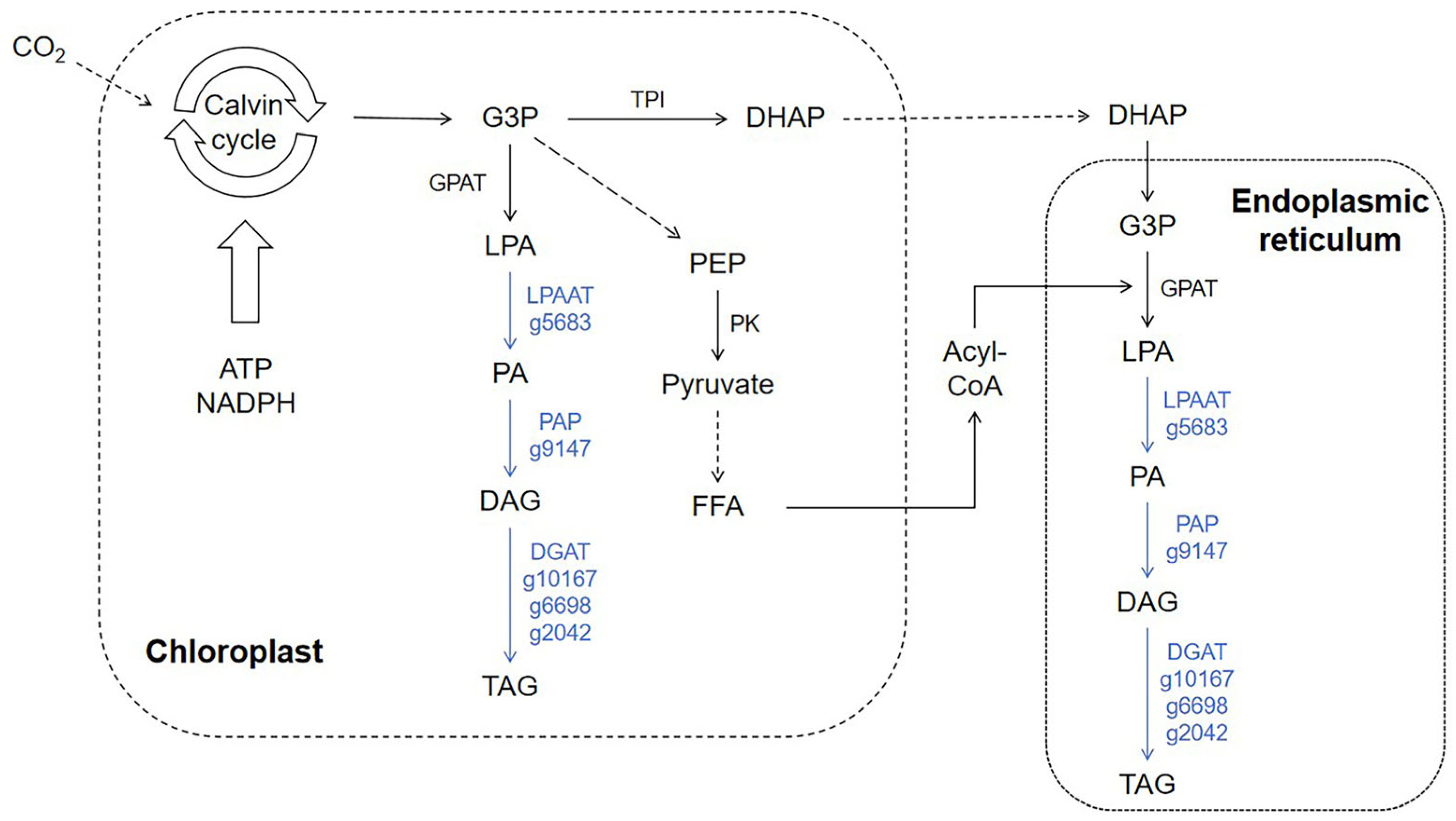
3.6. The Impact of Adding Sodium Acetate on the Lipid Metabolism of N. oceanica
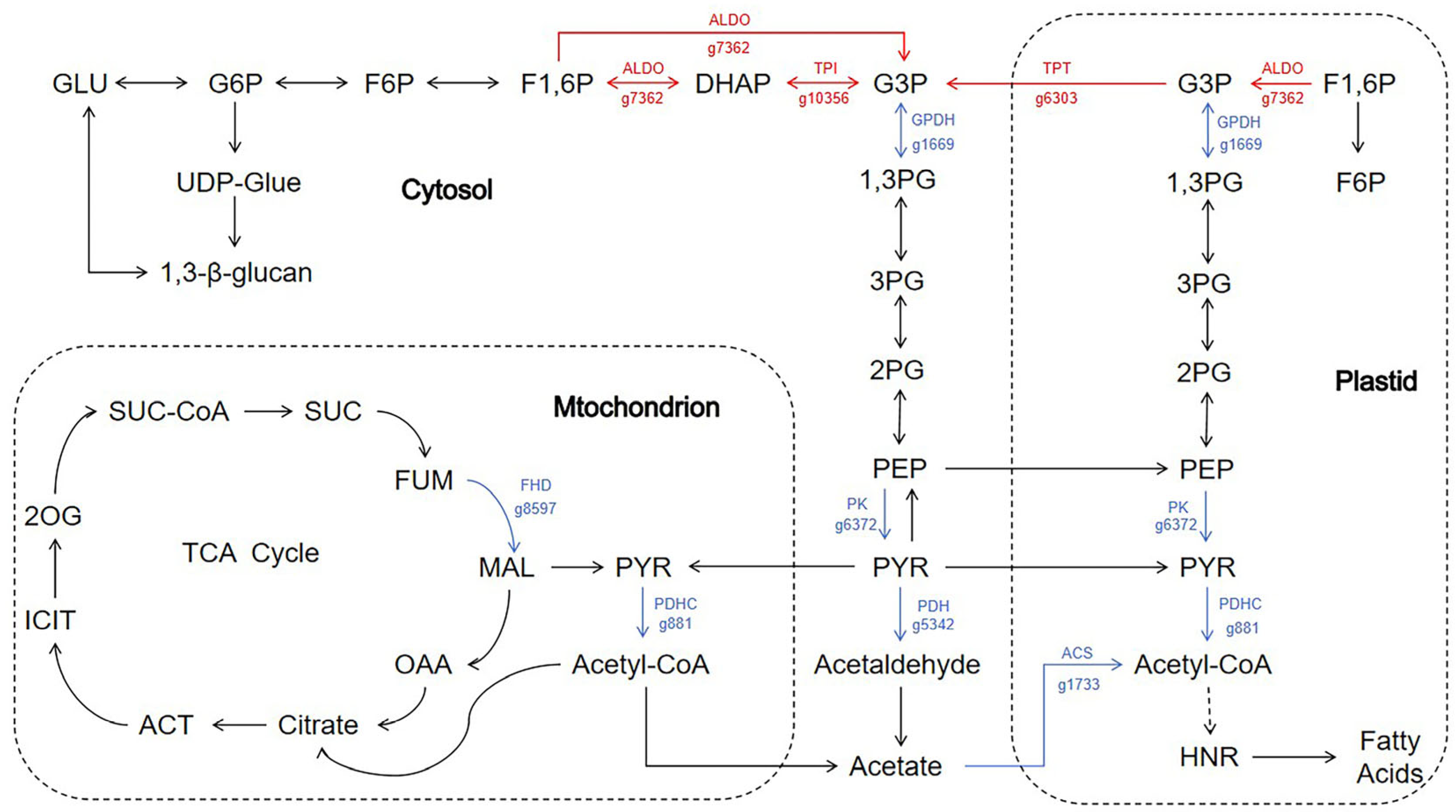
3.7. The Impact of Adding Sodium Acetate on Carbon Metabolism in N. oceanica
3.8. The Impact of Adding Sodium Acetate on Fatty Acid Synthesis and Lipase in N. oceanica
3.9. The Impact of Adding Sodium Acetate on Carbon Fixation in N. oceanica
3.10. Photosynthesis Affected in Response to Sodium Acetate Addition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEGs | Differential expression unigenes |
| G3P | Glyceraldehyde-3-phosphate |
| LPA | Lysophosphatidic acid |
| PA | Phosphatidic acid |
| GPAT | Glycerol-3-phosphate acyltransferase |
| DAG | Diacylglycerol |
| TAG | Triacylglycerol |
| LPAAT | Lysophosphatidic acid acyltransferase |
| PAP | Phosphatidate phosphatase |
| DGAT | Diacylglycerol acyltransferase |
| F6P | Fructose 6-phosphate |
| F1,6P | Fructose 1,6-bisphosphate |
| DHAP | Phosphates dihydroxyacetone |
| PEP | Phosphoenolpyruvate |
| PYR | Pyruvate |
| FUM | Fumarate |
| MAL | Malate |
| ALDO | Fructose-1,6-bisphosphate aldolase |
| TPI | Triphosphate-isomerase |
| TPT | Triosephosphate/phosphate translocator |
| GPDH | Glycerol-3-phosphate dehydrogenase |
| PK | Pyruvate kinase |
| PDH | Pyruvate dehydrogenase |
| FHD | Fumarate hydratase |
| PDHC | Pyruvate dehydrogenase complex |
| ACS | Acetyl-CoA synthetase |
References
- Yun, H.S.; Kim, Y.S.; Yoon, H.S. Effect of Different Cultivation Modes (Photoautotrophic, Mixotrophic, and Heterotrophic) on the Growth of Chlorella sp. and Biocompositions. Front. Bioeng. Biotechnol. 2021, 9, 774143. [Google Scholar] [CrossRef]
- Li, Y.; Tian, W.; Fu, Z.; Ye, W.; Zhang, X.; Zhang, Z.; Sun, D. Mechanisms of Sodium-Acetate-Induced DHA Accumulation in a DHA-Producing Microalga, Crypthecodinium sp. SUN. Mar. Drugs 2022, 20, 508. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Fan, K.W.; Jiang, Y.; Zhong, Y.; Sun, Z.; Chen, F. Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresour. Technol. 2010, 101, 8658–8663. [Google Scholar] [CrossRef] [PubMed]
- Tambat, V.S.; Patel, A.K.; Singhania, R.R.; Vadrale, A.P.; Tiwari, A.; Chen, C.W.; Dong, C.D. Sustainable mixotrophic microalgae refinery of astaxanthin and lipid from Chlorella zofingiensis. Bioresour. Technol. 2023, 387, 129635. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, W.; Wang, J.; He, Y.; Yang, S.; Sun, H. A comprehensive review on the heterotrophic production of bioactive compounds by microalgae. World J. Microbiol. Biotechnol. 2024, 40, 210. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; He, W.; Liang, H.; Hong, T.; Li, T.; Du, H. Transcriptome analysis reveals the molecular mechanism of differences in growth between photoautotrophy and heterotrophy in Chlamydomonas reinhardtii. Front. Plant Sci. 2024, 15, 1407915. [Google Scholar] [CrossRef]
- Hu, Q.; Song, M.; Huang, D.; Hu, Z.; Wu, Y.; Wang, C. Haematococcus pluvialis Accumulated Lipid and Astaxanthin in a Moderate and Sustainable Way by the Self-Protection Mechanism of Salicylic Acid Under Sodium Acetate Stress. Front. Plant Sci. 2021, 12, 763742. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Ko, S.-R.; Oh, H.-M.; Kim, H.-S. Lipid droplet synthesis is limited by acetate availability in starchless mutant of Chlamydomonas reinhardtii. FEBS Lett. 2013, 587, 370–377. [Google Scholar] [CrossRef]
- Yee, W. Feasibility of various carbon sources and plant materials in enhancing the growth and biomass productivity of the freshwater microalgae Monoraphidium griffithii NS16. Bioresour. Technol. 2015, 196, 1–8. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Qin, S.; Zeng, M.; Jiang, Y.; Hu, L.; Xiao, P.; Hao, W.; Hu, Z.; Lei, A.; et al. Growth and lipid accumulation by different nutrients in the microalga Chlamydomonas reinhardtii. Biotechnol. Biofuels 2018, 11, 40. [Google Scholar] [CrossRef]
- Byeon, H.; An, Y.; Kim, T.; Rayamajhi, V.; Lee, J.; Shin, H.; Jung, S. Effects of Four Organic Carbon Sources on the Growth and Astaxanthin Accumulation of Haematococcus lacustris. Life 2023, 14, 29. [Google Scholar] [CrossRef]
- Senousy, H.H.; El-Sheekh, M.M.; Khairy, H.M.; El-Sayed, H.S.; Mahmoud, G.A.; Hamed, A.A. Biodiesel Production from the Marine Alga Nannochloropsis oceanica Grown on Yeast Wastewater and the Effect on Its Biochemical Composition and Gene Expression. Plants 2023, 12, 2898. [Google Scholar] [CrossRef] [PubMed]
- Canini, D.; Martini, F.; Cazzaniga, S.; Miotti, T.; Pacenza, B.; D’Adamo, S.; Ballottari, M. Genetic engineering of Nannochloropsis oceanica to produce canthaxanthin and ketocarotenoids. Microb. Cell Fact. 2024, 23, 322. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.; Regan, F. In-situ lipid and fatty acid extraction methods to recover viable products from Nannochloropsis sp. Sci. Total Environ. 2020, 748, 142464. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, L.; Zheng, J.; Shao, S.; Pan, Y.; Hu, H.; Shen, L.; Wang, W.; Zhou, W.; Liu, J. Turning the industrially relevant marine alga Nannochloropsis red: One move for multifaceted benefits. New Phytol. 2024, 244, 1467–1481. [Google Scholar] [CrossRef]
- Chen, X.; Khatiwada, J.R.; Chio, C.; Shrestha, S.; Kognou, A.L.M.; Fan, L.; Qin, W. Low-cost cultivation of Nannochloropsis oceanica in newly designed photobioreactors and its productivity trends in semi-continuous cultivation under inland outdoor conditions. Bioresour. Technol. 2024, 402, 130829. [Google Scholar] [CrossRef]
- Ma, X.-N.; Chen, T.-P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef]
- Lin, W.; Li, P.; Liao, Z.; Luo, J. Detoxification of ammonium to Nannochloropsis oculata and enhancement of lipid production by mixotrophic growth with acetate. Bioresour. Technol. 2017, 227, 404–407. [Google Scholar] [CrossRef]
- Lintner, M.; Balzano, S.; Keul, N.; Heinz, P.; Manecki, M.; Klimek, A.; Wanek, W.; Cyran, N.; Gruber, D.; Schmidt, K.; et al. Biosorption of heavy metals by microalgae: Hazardous side effects for marine organisms. Chemosphere 2025, 372, 144080. [Google Scholar] [CrossRef]
- Wei, L.; You, W.; Gong, Y.; El Hajjami, M.; Liang, W.; Xu, J.; Poetsch, A. Transcriptomic and proteomic choreography in response to light quality variation reveals key adaption mechanisms in marine Nannochloropsis oceanica. Sci. Total Environ. 2020, 720, 137667. [Google Scholar] [CrossRef]
- Zhao, R.; Yin, S.Y.; Jiang, C.H.; Xue, J.N.; Liu, C.; Cai, X.H.; Xing, Y.P.; Kang, T.G. Comparison of chloroplast genomes of medicinal plants in Aristolochiaceae. Zhongguo Zhong Yao Za Zhi 2022, 47, 2932–2937. [Google Scholar] [CrossRef]
- O’Neill, E.A.; Fehrenbach, G.; Murphy, E.; Alencar, S.A.; Pogue, R.; Rowan, N.J. Use of next generation sequencing and bioinformatics for profiling freshwater eukaryotic microalgae in a novel peatland integrated multi-trophic aquaculture (IMTA) system: Case study from the Republic of Ireland. Sci. Total Environ. 2022, 851, 158392. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Zhang, D.; Cui, X.; Zhou, J.; Li, J.; Wei, Y.; Bu, D. Integrated Omics Approach to Discover Differences in the Metabolism of a New Tibetan Desmodesmus sp. in Two Types of Sewage Treatments. Metabolites 2023, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Kubatko, L.S. Comparative Performance of Popular Methods for Hybrid Detection using Genomic Data. Syst. Biol. 2021, 70, 891–907. [Google Scholar] [CrossRef]
- Robertson, D.S.; Wason, J.M.S.; Ramdas, A. Online multiple hypothesis testing. Stat. Sci. 2023, 38, 557–575. [Google Scholar] [CrossRef]
- Albanese, D.; Donati, C. Genome Recovery, Functional Profiling, and Taxonomic Classification from Metagenomes. Methods Mol. Biol. 2021, 2242, 153–172. [Google Scholar] [CrossRef]
- Sewe, S.O.; Silva, G.; Sicat, P.; Seal, S.E.; Visendi, P. Trimming and Validation of Illumina Short Reads Using Trimmomatic, Trinity Assembly, and Assessment of RNA-Seq Data. Methods Mol. Biol. 2022, 2443, 211–232. [Google Scholar] [CrossRef]
- Yue, L.I.; Yunlong, W.; Longling, O. Comparative analyses on the transcriptome among free-living zooxanthellae under different phosphate concentrations. J. Fish. Sci. China 2022, 29, 585–595. [Google Scholar]
- Zhang, X.; Zhang, Y.; Chen, Z.; Gu, P.; Li, X.; Wang, G. Exploring cell aggregation as a defense strategy against perchlorate stress in Chlamydomonas reinhardtii through multi-omics analysis. Sci. Total Environ. 2023, 905, 167045. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, J.; Yang, C.; Ai, F.; Yin, Y.; Guo, H. Differential impacts of organic and inorganic phosphorus on the growth and phosphorus utilization of Microcystis aeruginosa. Sci. Total Environ. 2024, 951, 175392. [Google Scholar] [CrossRef]
- Wayne, L.L.; Gachotte, D.J.; Graupner, P.R.; Adelfinskaya, Y.; McCaskill, D.G.; Metz, J.G.; Zirkle, R.; Walsh, T.A. Plant and algal lysophosphatidic acid acyltransferases increase docosahexaenoic acid accumulation at the sn-2 position of triacylglycerol in transgenic Arabidopsis seed oil. PLoS ONE 2021, 16, e0256625. [Google Scholar] [CrossRef]
- Duan, J.L.; Han, Y.; Liu, X.Y.; Liu, M.Y.; Sun, Y.C.; Ma, J.Y.; Sun, X.D.; Wang, Y.; Tan, M.M.; Gong, B.; et al. Membranal phosphatidylglycerol enhances oxygen diffusion and release from cyanobacteria. Water Res. 2025, 282, 123782. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Y.; Hao, H.; Wang, R.; He, Z.; Tian, R.; Liu, G. Identification of phosphatidic acid interacting proteins in Ganoderma lingzhi. Sheng Wu Gong Cheng Xue Bao 2021, 37, 3293–3299. [Google Scholar] [CrossRef]
- Ukey, R.; Carmon, T.; Hardman, D.; Hill, N.; Fakas, S. The Yarrowia lipolytica PAH1 homologue contributes but is not required for triacylglycerol biosynthesis during growth on glucose. Yeast 2020, 37, 93–102. [Google Scholar] [CrossRef]
- Orive-Milla, N.; Delmulle, T.; de Mey, M.; Faijes, M.; Planas, A. Metabolic engineering for glycoglycerolipids production in E. coli: Tuning phosphatidic acid and UDP-glucose pathways. Metab. Eng. 2020, 61, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Li, G.; Wan, S.; Batistič, O.; Sun, M.; Zhang, Y.; Scott, R.; Qi, B. Protein S-acyl transferase 15 is involved in seed triacylglycerol catabolism during early seedling growth in Arabidopsis. J. Exp. Bot. 2019, 70, 5205–5216. [Google Scholar] [CrossRef] [PubMed]
- Imbs, A.B.; Dembitsky, V.M. Coral Lipids. Mar. Drugs 2023, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Hao, Y.; Cai, G.; Shi, X.; Li, R.; Wang, J. Cloning and functional characterization of a lysophosphatidic acid acyltransferase gene from Perilla frutescens. Sheng Wu Gong Cheng Xue Bao 2022, 38, 3014–3028. [Google Scholar] [CrossRef]
- Zhu, L.; Feng, S.; Li, Y.; Sun, X.; Sui, Q.; Chen, B.; Qu, K.; Xia, B. Physiological and transcriptomic analysis reveals the toxic and protective mechanisms of marine microalga Chlorella pyrenoidosa in response to TiO(2) nanoparticles and UV-B radiation. Sci. Total Environ. 2024, 912, 169174. [Google Scholar] [CrossRef]
- Gurrieri, L.; Fermani, S.; Zaffagnini, M.; Sparla, F.; Trost, P. Calvin-Benson cycle regulation is getting complex. Trends Plant Sci. 2021, 26, 898–912. [Google Scholar] [CrossRef]
- Sobanski, T.; Suraweera, A.; Burgess, J.T.; Richard, I.; Cheong, C.M.; Dave, K.; Rose, M.; Adams, M.N.; O’Byrne, K.J.; Richard, D.J.; et al. The fructose-bisphosphate, Aldolase A (ALDOA), facilitates DNA-PKcs and ATM kinase activity to regulate DNA double-strand break repair. Sci. Rep. 2023, 13, 15171. [Google Scholar] [CrossRef]
- Xue, L.-L.; Chen, H.-H.; Jiang, J.-G. Implications of glycerol metabolism for lipid production. Prog. Lipid Res. 2017, 68, 12–25. [Google Scholar] [CrossRef]
- Myers, T.D.; Palladino, M.J. Newly discovered roles of triosephosphate isomerase including functions within the nucleus. Mol. Med. 2023, 29, 18. [Google Scholar] [CrossRef] [PubMed]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. A Publ. Protein Soc. 2019, 28, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Baek, H.; Kim, S.; Kim, Y.; Kim, J.; Kim, J.W. Microalgae-Derived Microparticles Improve Immunomodulation via Combined Glycolysis and MAPK Activation. Langmuir 2025, 41, 8619–8626. [Google Scholar] [CrossRef]
- Driver, T.; Trivedi, D.K.; McIntosh, O.A.; Dean, A.P.; Goodacre, R.; Pittman, J.K. Two Glycerol-3-Phosphate Dehydrogenases from Chlamydomonas Have Distinct Roles in Lipid Metabolism. Plant Physiol. 2017, 174, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, X.; Yang, Y.; Dong, J.; Gao, Z.; Qian, P.; Deng, X. Growth and metabolites of Chlorella sorokiniana regulated by sodium acetate. Microbiol. China 2021, 48, 3580–3587. [Google Scholar]
- Meloni, M.; Fanti, S.; Tedesco, D.; Gurrieri, L.; Trost, P.; Fermani, S.; Lemaire, S.D.; Zaffagnini, M.; Henri, J. Characterization of chloroplast ribulose-5-phosphate-3-epimerase from the microalga Chlamydomonas reinhardtii. Plant Physiol. 2024, 194, 2263–2277. [Google Scholar] [CrossRef]
- Li, Y.H.; Peng, L.W.; Wang, X.Q.; Zhang, L. Reduction in chloroplastic ribulose-5-phosphate-3-epimerase decreases photosynthetic capacity in Arabidopsis. Front. Plant Sci. 2022, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Tang, S.; Ben, L.; Zhang, X.; Dong, G.; Zhou, J.; Lin, L.; Yang, Z.; Zhou, Y.; et al. A Dual-localized Fructose Bisphosphate Aldolase is Essential for Chloroplast Development and Carbon Metabolism in Rice. Rice 2025, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.-W.; Zhu, M.; et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef] [PubMed]
- He, S.-C.; Zhang, Z.-Y.; Han, Y.-Q.; Miao, L.; Zhang, C.-Y.; Yu, A.-Q. Research Progress in the Production of Polyunsaturated Fatty Acids by Yarrowia lipolytica Cell Factories. Biotechnol. Bull. 2024, 40, 72–85. [Google Scholar] [CrossRef]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the Functions and Ecophysiological Relevance of the Diverse Carbonic Anhydrases in Microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef]
- Malerba, M.E.; Marshall, D.J.; Palacios, M.M.; Raven, J.A.; Beardall, J. Cell size influences inorganic carbon acquisition in artificially selected phytoplankton. New Phytol. 2021, 229, 2647–2659. [Google Scholar] [CrossRef]
- Zhu, H.; Ye, Z.; Xu, Z.; Wei, L. Transcriptomic Analysis Reveals the Effect of Urea on Metabolism of Nannochloropsis oceanica. Life 2024, 14, 797. [Google Scholar] [CrossRef]
- Lauersen, K.J.; Willamme, R.; Coosemans, N.; Joris, M.; Kruse, O.; Remacle, C. Peroxisomal microbodies are at the crossroads of acetate assimilation in the green microalga Chlamydomonas reinhardtii. Algal Res. 2016, 16, 266–274. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Qiao, H.; Lin, A.; Wang, G. Immobilization of Chlorella sorokiniana GXNN 01 in alginate for removal of N and P from synthetic wastewater. Bioresour. Technol. 2012, 114, 26–32. [Google Scholar] [CrossRef]
- Yu, X.; Ye, X.; Hu, C.; Xu, N.; Sun, X. Sodium acetate can promote the growth and astaxanthin accumulation in the unicellular green alga Haematococcus pluvialis as revealed by a proteomics approach. J. Oceanol. Limnol. 2022, 40, 2052–2067. [Google Scholar] [CrossRef]
| Sample | Total Reads | Total Mapped |
|---|---|---|
| C1 | 19,791,288 | 18,315,060 (92.54%) |
| C2 | 15,363,420 | 14,281,880 (92.96%) |
| C3 | 16,002,066 | 14,894,103 (93.08%) |
| AC1 | 18,369,538 | 17,061,148 (92.88%) |
| AC2 | 18,476,094 | 17,239,512 (93.91%) |
| AC3 | 17,748,090 | 17,239,512 (93.91%) |
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Error Rates (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|---|
| C1 | 20,078,104 | 3,031,793,704 | 19,791,288 | 2,956,576,263 | 0.0267 | 97.24 | 92.67 | 54.74 |
| C2 | 15,592,272 | 2,354,433,072 | 15,363,420 | 2,299,530,630 | 0.0267 | 97.27 | 92.71 | 54.89 |
| C3 | 16,247,216 | 2,453,329,616 | 16,002,066 | 2,386,477,217 | 0.0264 | 97.35 | 92.93 | 54.78 |
| AC1 | 18,598,860 | 2,808,427,860 | 18,369,538 | 2,745,983,768 | 0.0267 | 97.26 | 92.7 | 55.02 |
| AC2 | 18,774,698 | 2834,979,398 | 18,476,094 | 2,756,361,229 | 0.0267 | 97.25 | 92.68 | 55.03 |
| AC3 | 18,024,086 | 2,721,636,986 | 17,748,090 | 2,652,452,542 | 0.0267 | 97.25 | 92.68 | 54.98 |
| Gene ID | Gene Name | Abbreviation | Fold Change (AC vs. C; Fold) |
|---|---|---|---|
| g5683 | Acyltransferase | LPAAT | 5.7 ↓ |
| g9147 | PA-phosphatase-like phosphoesterase | PAP | 13.8 ↓ |
| g10167 | Mono-or diacylglycerol acyltransferase type 2 | DGAT | 5.1 ↓ |
| g6698 | Diacylglycerol acyltransferase, putative | 5.9 ↓ | |
| g2042 | Type 2 diacylglycerol acyltransferase | 6.9 ↓ |
| Gene ID | Gene Name | Abbreviation | Fold Change (AC vs. C; Fold) |
|---|---|---|---|
| g7362 | Fructose-1,6-bisphosphate aldolase | ALDO | 7.9 ↑ |
| g10356 | Triosephosphate isomerase | TPI | 4.1 ↑ |
| g6303 | Triosephosphate/phosphate translocator | TPT | 5.2 ↑ |
| g8597 | Fumarate hydratase class II | FHD | 16.5 ↓ |
| g881 | Dihydrolipoamide acetyltransferase, pyruvate dehydrogenase complex component E2 | PDHC | 5.2 ↓ |
| g1733 | Acetyl CoA synthetase | ACS | 31.4 ↓ |
| g5342 | Pyruvate dehydrogenase | PDH | 154.22 ↓ |
| g1669 | Glycerol-3-phosphate dehydrogenase | GPDH | 4.3 ↓ |
| g6372 | Pyruvate kinase | PK | 10.0 ↓ |
| g10046 | Pyruvate dehydrogenase kinase | PDK | 7.4 ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhu, H.; Su, H.; Wei, L. Transcriptomic Survey of How Acetate Addition Affected the Growth in Nannochloropsis oceanica (Suda & Miyashita) R. E. Lee. Life 2025, 15, 1398. https://doi.org/10.3390/life15091398
Wu Y, Zhu H, Su H, Wei L. Transcriptomic Survey of How Acetate Addition Affected the Growth in Nannochloropsis oceanica (Suda & Miyashita) R. E. Lee. Life. 2025; 15(9):1398. https://doi.org/10.3390/life15091398
Chicago/Turabian StyleWu, Yikai, Han Zhu, Hang Su, and Li Wei. 2025. "Transcriptomic Survey of How Acetate Addition Affected the Growth in Nannochloropsis oceanica (Suda & Miyashita) R. E. Lee" Life 15, no. 9: 1398. https://doi.org/10.3390/life15091398
APA StyleWu, Y., Zhu, H., Su, H., & Wei, L. (2025). Transcriptomic Survey of How Acetate Addition Affected the Growth in Nannochloropsis oceanica (Suda & Miyashita) R. E. Lee. Life, 15(9), 1398. https://doi.org/10.3390/life15091398






