Vaginal Microbiome and Its Relationship with Assisted Reproduction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
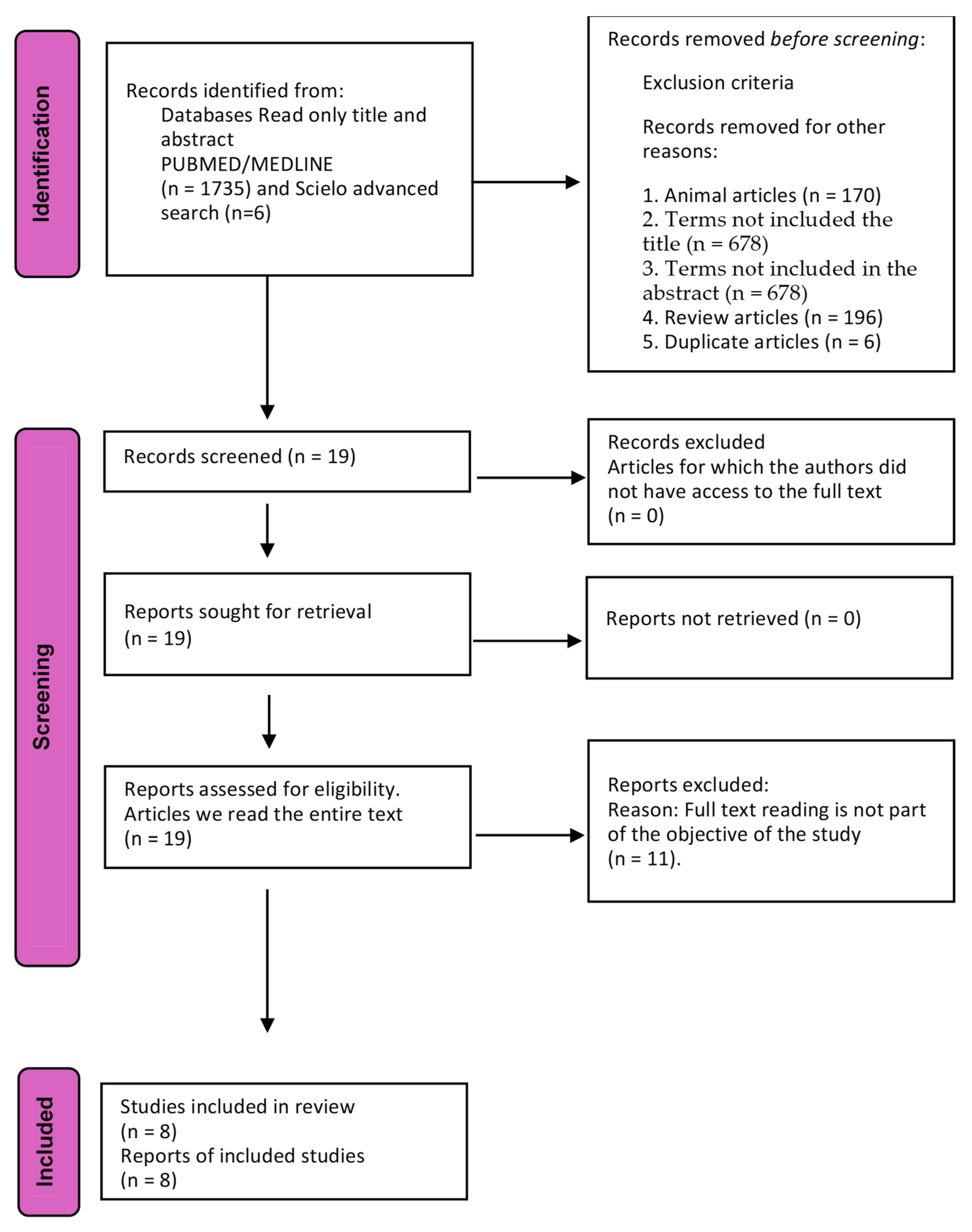
2. Materials and Methods
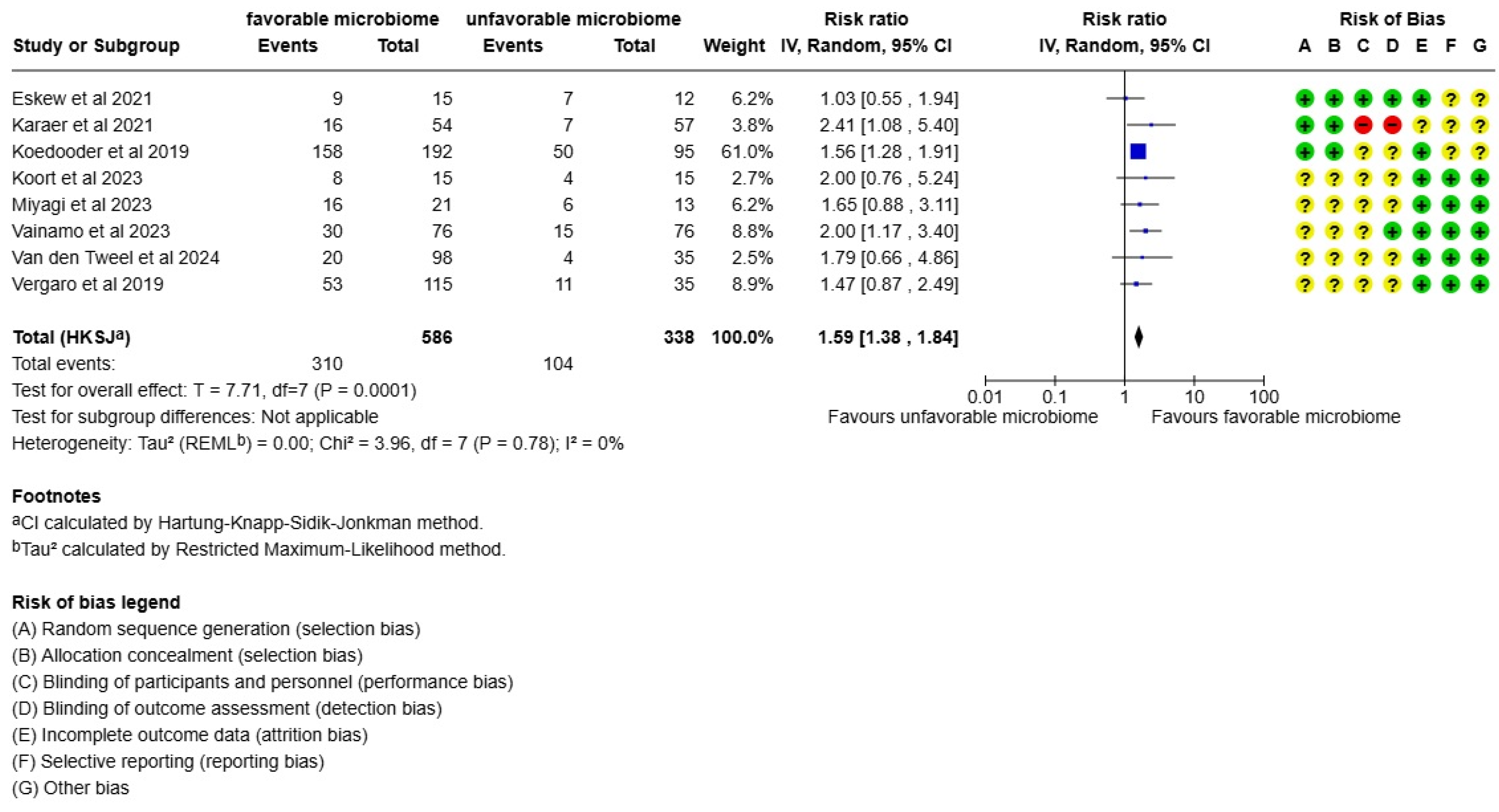
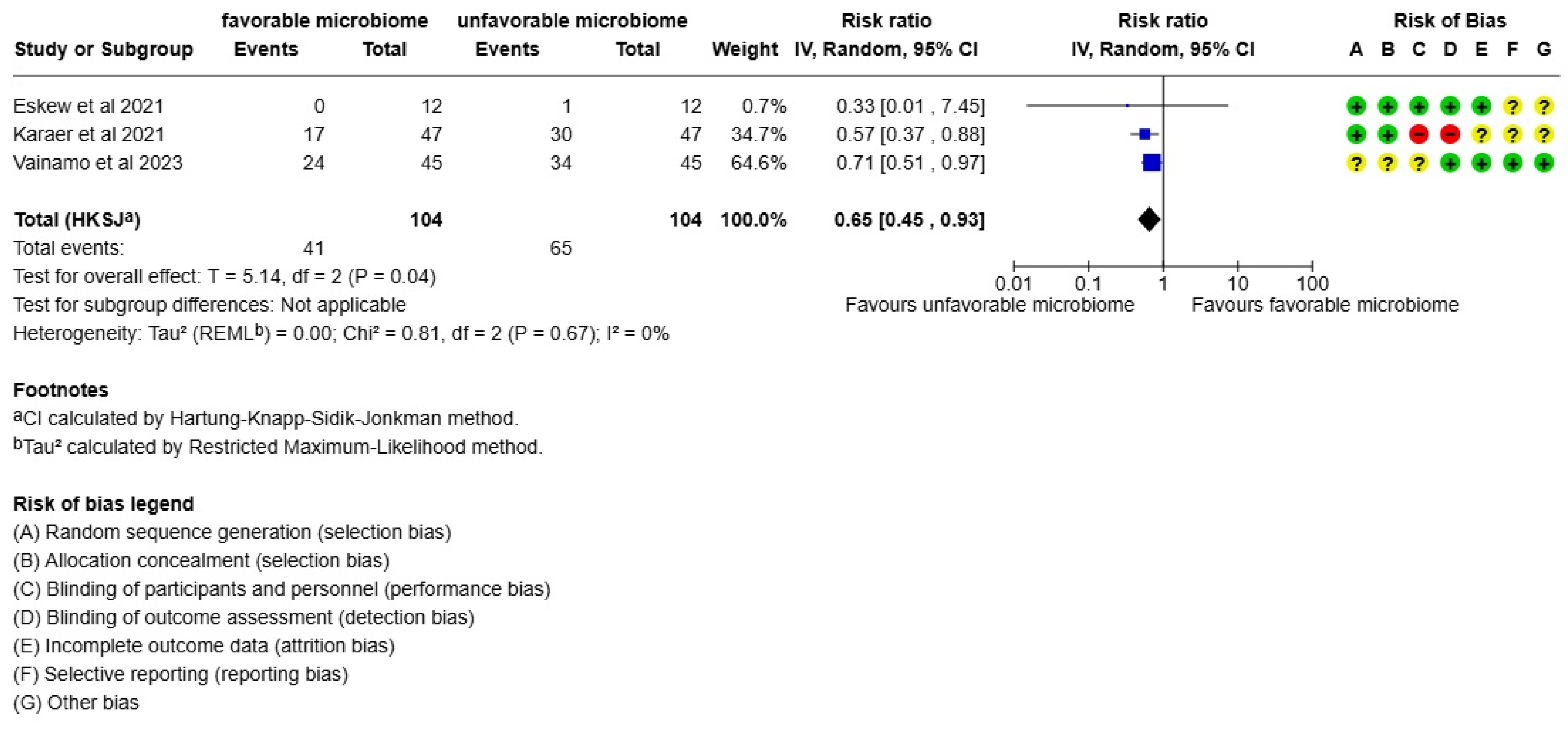
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IVF | In vitro fertilization |
| ART | Assisted reproductive technology |
| NGS | Next-generation sequencing |
| STI | Sexually transmitted infection |
| CTS | Community state type |
References
- Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Saiz, I.C.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; Rugescu, I.; et al. ART in Europe, 2019: Results Generated from European Registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar] [CrossRef]
- Piscopo, R.C.C.P.; Guimarães, R.V.; Ueno, J.; Ikeda, F.; Di Bella, Z.I.K.J.; Girão, M.J.B.C.; Samama, M. Increased Prevalence of Endocervical Mycoplasma and Ureaplasma Colonization in Infertile Women with Tubal Factor. J. Bras. Reprod. Assist. 2020, 24, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Marconi, C.; El-Zein, M.; Ravel, J.; Ma, B.; Lima, M.D.; Carvalho, N.S.; Alves, R.R.F.; Parada, C.M.G.L.; Leite, S.H.M.; Giraldo, P.C.; et al. Characterization of the Vaginal Micron Women of Reproductive Age from 5 Regions in Brazil. Sex Transm. Dis. 2020, 47, 562–569. [Google Scholar] [CrossRef]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; Van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; Van De Wijgert, J.H.H.M. Cervicovaginal Microbiome Dysbiosis Is Associated with Proteome Changes Related to Alterations of the Cervicovaginal Mucosal Barrier. Mucosal. Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Tinelli, R.; Lepera, A.; Alfonso, R.; Indraccolo, U.; Marrocchella, S.; Greco, P.; Resta, L. Prevalence of Chronic Endometritis in Repeated Unexplained Implantation Failure and the IVF Success Rate after Antibiotic Therapy. Hum. Reprod. 2015, 30, 323–330. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, X.; Chen, P.; Wang, Y.; Li, W.; Huang, R. The Endometrial Microbiota Profile Influenced Pregnancy Outcomes in Patients with Repeated Implantation Failure: A Retrospective Study. J. Reprod. Immunol. 2023, 155, 103782. [Google Scholar] [CrossRef]
- Qing, X.; Xie, M.; Liu, P.; Feng, O.; Leng, H.; Guo, H.; Zhang, Y.; Ma, Y.; Zheng, W. Correlation between Dysbiosis of Vaginal Microecology and Endometriosis: A Systematic Review and Meta-Analysis. PLoS ONE 2024, 19, e0306780. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Aasmets, O.; Arffman, R.K.; Laru, J.; Rossi, H.R.; Salumets, A.A.; Piltonen, T.T.; Org, E. The Reproductive Tract Microbiome in Women with Polycystic Ovary Syndrome and across Different Menstrual Cycle Phases. Hum. Reprod. 2025, 40, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Louwers, Y.V.; Laven, J.S.E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef]
- Riganelli, L.; Iebba, V.; Piccioni, M.; Illuminati, I.; Bonfiglio, G.; Neroni, B.; Calvo, L.; Gagliardi, A.; Levrero, M.; Merlino, L.; et al. Structural Variations of Vaginal and Endometrial Microbiota: Hints on Female Infertility. Front. Cell. Infect. Microbiol. 2020, 10, 350. [Google Scholar] [CrossRef]
- Su, W.; Gong, C.; Zhong, H.; Yang, H.; Chen, Y.; Wu, X.; Jin, J.; Xi, H.; Zhao, J. Vaginal and Endometrial Microbiome Dysbiosis Associated with Adverse Embryo Transfer Outcomes. Reprod. Biol. Endocrinol. 2024, 22, 111. [Google Scholar] [CrossRef]
- Foteinidou, P.; Exindari, M.; Chatzidimitriou, D.; Gioula, G. Endometrial Microbiome and Its Correlation to Female Infertility: A Systematic Review and Meta-Analysis. Acta Microbiol. Hell. 2024, 69, 14–28. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef] [PubMed]
- Tsonis, O.; Gkrozou, F.; Paschopoulos, M. Microbiome Affecting Reproductive Outcome in ARTs. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102036. [Google Scholar] [CrossRef]
- Sobstyl, A.; Chałupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. [Google Scholar] [CrossRef]
- Zhao, C.; Wei, Z.; Yang, J.; Zhang, J.; Yu, C.; Yang, A.; Zhang, M.; Zhang, L.; Wang, Y.; Mu, X.; et al. Characterization of the Vaginal Microbiome in Women with Infertility and Its Potential Correlation with Hormone Stimulation during In Vitro Fertilization Surgery. mSystems 2020, 5, e00450-20. [Google Scholar] [CrossRef]
- Carosso, A.; Revelli, A.; Gennarelli, G.; Canosa, S.; Cosma, S.; Borella, F.; Tancredi, A.; Paschero, C.; Boatti, L.; Zanotto, E.; et al. Controlled Ovarian Stimulation and Progesterone Supplementation Affect Vaginal and Endometrial Microbiota in IVF Cycles: A Pilot Study. J. Assist. Reprod. Genet. 2020, 37, 2315–2326. [Google Scholar] [CrossRef]
- van den Tweel, M.M.; van den Munckhof, E.H.A.; van der Zanden, M.; Molijn, A.; van Lith, J.M.M.; Boers, K.E. The Vaginal Microbiome Changes During Various Fertility Treatments. Reprod. Sci. 2024, 31, 1593–1600. [Google Scholar] [CrossRef]
- Eslami, M.; Naderian, R.; Ahmadpour, A.; Shushtari, A.; Maleki, S.; Mohammadian, P.; Amiri, A.; Janbazi, M.; Memarian, M.; Yousefi, B. Microbiome Structure in Healthy and Pregnant Women and Importance of Vaginal Dysbiosis in Spontaneous Abortion. Front. Cell. Infect. Microbiol. 2024, 14, 1401610. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Zhang, M.; Fang, J.; Zheng, Y.; Jiang, C.; Pan, M. Microbiomic Insights into the Unique Effects of Vaginal Microbiota on Preterm Birth in Chinese Pregnant Women. Front. Microbiol. 2025, 16, 1560528. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Kim, S.Y.; Lee, Y.J.; Park, J.E. RoBANS 2: A Revised Risk of Bias Assessment Tool for Nonrandomized Studies of Interventions. Korean J. Fam. Med. 2023, 44, 249–260. [Google Scholar] [CrossRef]
- Väinämö, S.; Saqib, S.; Kalliala, I.; Kervinen, K.; Luiro, K.; Niinimäki, M.; Halttunen-Nieminen, M.; Virtanen, S.; Nieminen, P.; Salonen, A.; et al. Longitudinal Analysis of Vaginal Microbiota during IVF Fresh Embryo Transfer and in Early Pregnancy. Microbiol. Spectr. 2023, 11, e01650-23. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable Association Discovery in Population-Scale Meta-Omics Studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Eskew, A.M.; Stout, M.J.; Bedrick, B.S.; Riley, J.K.; Herter, B.N.; Gula, H.; Jungheim, E.S.; Wylie, K.M. Association of Vaginal Bacterial Communities and Reproductive Outcomes with Prophylactic Antibiotic Exposure in a Subfertile Population Undergoing in Vitro Fertilization: A Prospective Exploratory Study. F S Sci. 2021, 2, 71–79. [Google Scholar] [CrossRef]
- Karaer, A.; Doğan, B.; Günal, S.; Tuncay, G.; Arda Düz, S.; Ünver, T.; Tecellioğlu, N. The Vaginal Microbiota Composition of Women Undergoing Assisted Reproduction: A Prospective Cohort Study. BJOG 2021, 128, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morré, S.A.; De Jonge, J.D.; Poort, L.; Cuypers, W.J.S.S.; Beckers, N.G.M.; Broekmans, F.J.M.; et al. The Vaginal Microbiome as a Predictor for Outcome of in Vitro Fertilization with or without Intracytoplasmic Sperm Injection: A Prospective Study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef]
- Miyagi, M.; Mekaru, K.; Tanaka, S.E.; Arai, W.; Ashikawa, K.; Sakuraba, Y.; Nakamura, R.; Oishi, S.; Akamine, K.; Aoki, Y. Endometrial and Vaginal Microbiomes Influence Assisted Reproductive Technology Outcomes. J. Bras. Reprod. Assist. 2023, 27, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, P.; Tiscornia, G.; Barragán, M.; García, D.; Rodriguez, A.; Santaló, J.; Vassena, R. Vaginal Microbiota Profile at the Time of Embryo Transfer Does Not Affect Live Birth Rate in IVF Cycles with Donated Oocytes. Reprod. Biomed. Online 2019, 38, 883–891. [Google Scholar] [CrossRef]
- Koort, K.; Sõsa, K.; Türk, S.; Lapp, E.; Talving, E.; Karits, P.; Rosenstein, K.; Jaagura, M.; Sekavin, A.; Sõritsa, D.; et al. Lactobacillus Crispatus-Dominated Vaginal Microbiome and Acinetobacter-Dominated Seminal Microbiome Support Beneficial ART Outcome. Acta Obstet. Gynecol. Scand. 2023, 102, 921–934. [Google Scholar] [CrossRef]
- van den Tweel, M.M.; van den Munckhof, E.H.A.; van der Zanden, M.; Molijn, A.C.; van Lith, J.M.M.; Le Cessie, S.; Boers, K.E. Bacterial Vaginosis in a Subfertile Population Undergoing Fertility Treatments: A Prospective Cohort Study. J. Assist. Reprod. Genet. 2024, 41, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic Celicanin, M.; Haahr, T.; Humaidan, P.; Skafte-Holm, A. Vaginal Dysbiosis-the Association with Reproductive Outcomes in IVF Patients: A Systematic Review and Meta-Analysis. Curr. Opin. Obstet. Gynecol. 2024, 36, 155–164. [Google Scholar] [CrossRef]
- Skafte-holm, A.; Humaidan, P.; Bernabeu, A.; Lledo, B.; Jensen, J.S.; Haahr, T. The Association between Vaginal Dysbiosis and Reproductive Outcomes in Sub-fertile Women Undergoing Ivf-treatment: A Systematic Prisma Review and Meta-analysis. Pathogens 2021, 10, 295. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The Vaginal Microbiome and the Risk of Preterm Birth: A Systematic Review and Network Meta-Analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Haahr, T.; Zacho, J.; Bräuner, M.; Shathmigha, K.; Skov Jensen, J.; Humaidan, P. Reproductive Outcome of Patients Undergoing in Vitro Fertilisation Treatment and Diagnosed with Bacterial Vaginosis or Abnormal Vaginal Microbiota: A Systematic PRISMA Review and Meta-Analysis. BJOG 2019, 126, 200–207. [Google Scholar] [CrossRef]
- Lafioniatis, A.; Samara, A.A.; Makaritsis, P.K.; Dafopoulos, S.; Sotiriou, S.; Dafopoulos, K. Understanding the Role of Female Genital Tract Microbiome in Recurrent Implantation Failure. J. Clin. Med. 2024, 13, 3173. [Google Scholar] [CrossRef]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The Endometrial Microbiota and Early Pregnancy Loss. Hum. Reprod. 2024, 39, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Ishimwe, J.A. Maternal Microbiome in Preeclampsia Pathophysiology and Implications on Offspring Health. Physiol. Rep. 2021, 9, e14875. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gin, C.; Fettweis, J.; Foxman, B.; Gelaye, B.; MacIntyre, D.A.; Subramaniam, A.; Fraser, W.; Tabatabaei, N.; Callahan, B. Meta-Analysis Reveals the Vaginal Microbiome Is a Better Predictor of Earlier than Later Preterm Birth. BMC Biol. 2023, 21, 199. [Google Scholar] [CrossRef]
- Balla, B.; Illés, A.; Tobiás, B.; Pikó, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kósa, J.P. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 13227. [Google Scholar] [CrossRef]
- Wright, M.L.; Fettwels, J.M.; Silberg, J.L.; Neale, M.C.; Serrano, M.G.; Jimenez, N.L.; Prom-Wormley, E.; Girerd, P.H.; Borzellece, J.R., Jr.; Strauss, J.F.; et al. Vaginal microbiome Lactobacillus crispatus in heritable among European American women. Commu Biol. 2021, 4, 872. [Google Scholar] [CrossRef]
- Incognito, G.G.; Ronsini, C.; Palmara, V.; Romeo, P.; Vizzielli, G.; Restaino, S.; La Verde, M.; De Tommasi, O.; Palumbo, M.; Cianci, S. The Interplay Between Cervicovaginal Microbiota Diversity, Lactobacillus Profiles and Human Papillomavirus in Cervical Cancer: A Systematic Review. Healthcare 2025, 13, 599. [Google Scholar] [CrossRef]
- Gerede, A.; Nikolettos, K.; Vavoulidis, E.; Margioula-Siarkou, C.; Petousis, S.; Giourga, M.; Fotinopoulos, P.; Salagianni, M.; Stavros, S.; Dinas, K.; et al. Vaginal Microbiome and Pregnancy Complications: A Review. J. Clin. Med. 2024, 13, 3875. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.V.; Monteiro, P.B.; de Moura, G.A.; Santos, N.O.; Fontanezi, C.T.B.; de Almeida Gomes, I.; Teixeira, C.A. Vaginal Microbioma and the Presence of Lactobacillus spp. as Interferences in Female Fertility: A Review System. J. Bras. Reprod. Assist. 2023, 27, 496–506. [Google Scholar] [CrossRef]
- Dube, R.; Kar, S.S. Genital Microbiota and Outcome of Assisted Reproductive Treatment—A Systematic Review. Life 2022, 12, 1867. [Google Scholar] [CrossRef]
- Golob, J.L.; Oskotsky, T.T.; Tang, A.S.; Roldan, A.; Chung, V.; Ha, C.W.Y.; Wong, R.J.; Flynn, K.J.; Parraga-Leo, A.; Wibrand, C.; et al. Microbiome preterm birth DREAM challenge: Crowdsourcing machine learning approaches to advance preterm birth research. Cell Rep. Med. 2024, 5, 101350. [Google Scholar] [CrossRef]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef]
- Mandar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutskov, K.; Nolvak, H.; Preem, J.K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef]
- Borovkova, N.; Korrovits, P.; Ausmees, K.; Türk, S.; Jõers, K.; Punab, M.; Mändar, R. Influence of sexual intercourse on genital tract microbiota in infertile couples. Anaerobe 2011, 17, 414–418. [Google Scholar] [CrossRef] [PubMed]

| Author and Year (Alphabetic Order) | Country | Age Year (sd) | BMI Index k/mg2 (sd) | AMH | Abundance of Lactobacillus ssp. | Causes of Infertility | Detection Method | Main Inclusion Criteria | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Eskew et al., 2021 [27] | USA | 32.27 (3.8) | 30.94 (8.1) | 2.16 (1.15) | The importance of timing in the assessment of vaginal microbiome to determine its associations with reproductive outcomes | Male factor (33.3%) Unexplained (6.7%) Tubal factor (13.3%) Endometriosis (6.7%) Ovulatory dysfunction (6.7%) PCOS (26.7%) PAIN (6.7%) | 16S rRN bacterial community structure is not predictive for embryo transfer | Molecular feature of microbiome analysis | Bacterial community structure is not predictive for embryo transfer |
| Karaer et al., 2021 [28] | Turkey | 23–29 | 20.0–29.9 | No data in the article | The abundance of Lactobacillus was lower in women who failed to become pregnant | Male factor (37.03%) Unexplained (45.37%) Others such as poor ovarian reserve and tubal factor (17.60%) | 16S rRNA | Molecular feature of microbiome analysis | The high abundance of the genus Streptococcus was greater in the non-pregnancy group |
| Koedooder et al., 2019 [29] | The Netherlands | The majority of participants were from 30–35 years old | Most participants had a BMI from 20–29.9 | No data in the article | There was a low abundance of Lactobacillus in women who failed to become pregnant and a high abundance of Lactobacillus in pregnant women | Male factor (71.6%) Other factors (17%) | 16S rRNA | Molecular feature of microbiome analysis | Without a favorable microbiome, the implantation and subsequent embryo development seem to be compromised |
| Koort et al., 2023 [32] | Estonia | In the ART group, the average age of women was 34.1 and in the control group, it was 32.2 | In the ART group, 75% of participants had a BMI below 25 (k/mg2) and 25% had a BMI above 25 (k/mg2) | No data in the article | Bacterial vaginosis community and with L. iners-predominant and L. gasseri-predominant microbiome had a lower ART success rate than women with L. crispatus-predominant microbiome | No data in the article | 16S rRNA and Nugent score | Molecular feature of microbiome analysis | Disturbed microbiome in the reproductive tract in both partners may be one of the reasons for ART failure |
| Miyagi et al., 2023 [30] | Japan | 35.6 (34.1) | 22.7 (22.5) | 3.53 (0.61) | Pregnant women present significantly higher proportions of Lactobacillus spp. | Fallopian tube factor (47.6%) Male factor (23.8%) Endometriosis (33.3%) | 16S rRNA | Molecular feature of microbiome analysis | The balance between Lactobacillus and pathological bacterial abundance was associated with pregnancy from ART |
| Vainamo et al., 2023 [24] | Finland | 32.9 | 24.9 | 2.6 (1.9) | Role of L. crispatus in the success of IVF-ET | Endometriosis (33.3%) Male factor (6.7%) Tubal factor (16.7%) Anovulation (13.3%) Unexplained (30%) | 16S rRNA | Molecular feature of microbiome analysis | Most women who achieved pregnancy had an indication of holding a reservoir of this beneficial Lactobacillus in their reproductive tract |
| van den Tweel et al., 2024 [33] | The Netherlands | 34 | 27.3 | - | There was a tendency of more miscarriages based on positive BV status or community state type groups III and IV | Male factor (34%) Tubal factor (5%) Hormonal (8.5%) Endometriosis (15%) Unknown (34%) | 16S rRNA | Molecular feature of microbiome analysis | Bacterial vaginosis does not significantly impact ongoing pregnancy rates but could affect miscarriage rates |
| Vergaro et al., 2019 [31] | Spain | 41.5 | 24.4 | 24.4 | Higher proportion of samples dominated by L. crispatus in women achieving live birth | No data in article | 16S rRNA | Molecular feature of microbiome analysis | Vaginal microbiota profile on the day of the embryo transfer is not related to live birth rate in women receiving oocytes |
| Taxon | Coefficient | p Value |
|---|---|---|
| Lactobacillus crispatus | 1.802 | 0.003 |
| Streptococcus anginosus | −0.708 | 0.060 |
| Lactobacillus sp. | 0.402 | 0.605 |
| Limosilactobacillus sp. | −0.135 | 0.542 |
| Lactobacillus iners | 0.425 | 0.656 |
| Lactobacillus jensenii | −0.269 | 0.705 |
| Fannyhessea vaginae | −0.320 | 0.374 |
| Anaerococcus sp. | 0 | 0.999 |
| Dialister sp. | 0 | 0.999 |
| Gardnerella sp. | 0 | 0.999 |
| Enterococcus sp. | 0 | 0.999 |
| Anaerococcus prevotii | 0 | 0.999 |
| Anaerococcus tetradius | 0 | 0.999 |
| Dialister succinatiphilus | 0 | 0.999 |
| Enterococcus faecalis | 0 | 0.999 |
| Escherichia coli | 0 | 0.999 |
| Finegoldia magna | 0 | 0.999 |
| Gardnerella swidsinskii | 0 | 0.999 |
| Gardnerella vaginalis | 0 | 0.999 |
| Lactobacillus gasseri | 0 | 0.999 |
| Limosilactobacillus coleohominis | 0 | 0.999 |
| Megasphaera lornae | 0 | 0.999 |
| Parvimonas micra | 0 | 0.999 |
| Peptostreptococcus anaerobius | 0 | 0.999 |
| Peptostreptococcus stomatis | 0 | 0.999 |
| Porphyromonas asaccharolytica | 0 | 0.999 |
| Porphyromonas uenonis | 0 | 0.999 |
| Prevotella amnii | 0 | 0.999 |
| Prevotella bivia | 0 | 0.999 |
| Streptococcus agalactiae | 0 | 0.999 |
| Streptococcus anginosus | 0 | 0.999 |
| Streptococcus dysgalactiae | 0 | 0.999 |
| Streptococcus hominis | 0 | 0.999 |
| Veillonella atypica | 0 | 0.999 |
| Veillonella dispar | 0 | 0.999 |
| Veillonella ratti | 0 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samama, M.; Ueno, J.; Carvalho de Arruda Veiga, E.; Piscopo, R.C.C.P.; Ikeda, F.; Pires de Lemos, N.; Tadeu Bidinotto, L.; Guimarães da Silva, M.; Jarmy Di Bella, Z.; Entezami, F. Vaginal Microbiome and Its Relationship with Assisted Reproduction: A Systematic Review and Meta-Analysis. Life 2025, 15, 1382. https://doi.org/10.3390/life15091382
Samama M, Ueno J, Carvalho de Arruda Veiga E, Piscopo RCCP, Ikeda F, Pires de Lemos N, Tadeu Bidinotto L, Guimarães da Silva M, Jarmy Di Bella Z, Entezami F. Vaginal Microbiome and Its Relationship with Assisted Reproduction: A Systematic Review and Meta-Analysis. Life. 2025; 15(9):1382. https://doi.org/10.3390/life15091382
Chicago/Turabian StyleSamama, Marise, Joji Ueno, Eduardo Carvalho de Arruda Veiga, Rita C. C. P. Piscopo, Fabio Ikeda, Nina Pires de Lemos, Lucas Tadeu Bidinotto, Márcia Guimarães da Silva, Zsuzsanna Jarmy Di Bella, and Frida Entezami. 2025. "Vaginal Microbiome and Its Relationship with Assisted Reproduction: A Systematic Review and Meta-Analysis" Life 15, no. 9: 1382. https://doi.org/10.3390/life15091382
APA StyleSamama, M., Ueno, J., Carvalho de Arruda Veiga, E., Piscopo, R. C. C. P., Ikeda, F., Pires de Lemos, N., Tadeu Bidinotto, L., Guimarães da Silva, M., Jarmy Di Bella, Z., & Entezami, F. (2025). Vaginal Microbiome and Its Relationship with Assisted Reproduction: A Systematic Review and Meta-Analysis. Life, 15(9), 1382. https://doi.org/10.3390/life15091382









