The Relationship Between Radiotherapy-Induced Pain Response Score and Pain Biomarkers TRPV1, β-Endorphin (bEP), Neurotensin (NT), and Orexin A (OXA) in Patients with Bone Metastases
Abstract
1. Introduction
2. Methods
2.1. Patient Selection and Radiotherapy
2.2. Determination of Biochemical Pain Biomarkers
2.3. Statistical Analysis
3. Results
| Pre-RT Parameters | Neurotensin | β-Endorphin | TRPV1 | Orexin A | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| NEU | −0.08 | 0.40 | −0.18 | 0.06 | 0.20 | 0.04 | 0.13 | 0.20 |
| LEU | −0.02 | 0.86 | −0.23 | 0.02 | −0.05 | 0.59 | 0.20 | 0.04 |
| PLT | 0.10 | 0.29 | −0.22 | 0.03 | −0.05 | 0.59 | 0.27 | 0.01 |
| LDH | −0.11 | 0.28 | 0.05 | 0.61 | 0.03 | 0.75 | −0.16 | 0.11 |
| ALP | −0.16 | 0.10 | 0.13 | 0.18 | 0.04 | 0.70 | 0.10 | 0.33 |
| NLR | −0.08 | 0.45 | 0.10 | 0.28 | 0.16 | 0.11 | −0.11 | 0.26 |
| Post-RT Parameters | Neurotensin | β-Endorphin | TRPV1 | Orexin A | ||||
| r | p | r | p | r | p | R | p | |
| NEU | −0.02 | 0.90 | 0.14 | 0.26 | −0.05 | 0.71 | 0.07 | 0.57 |
| LEU | 0.21 | 0.09 | −0.11 | 0.41 | −0.07 | 0.57 | −0.06 | 0.65 |
| LDH | 0.06 | 0.62 | −0.08 | 0.51 | 0.09 | 0.50 | 0.04 | 0.75 |
| ALP | −0.04 | 0.78 | −0.03 | 0.80 | −0.01 | 0.95 | 0.12 | 0.36 |
| PLT | 0.00 | 0.98 | 0.02 | 0.86 | −0.15 | 0.23 | 0.09 | 0.49 |
| NLR | −0.27 | 0.03 | 0.24 | 0.053 | 0.01 | 0.92 | 0.05 | 0.67 |
| Radiotherapy Parameters | Post-RT Biomarkers | |||||||
|---|---|---|---|---|---|---|---|---|
| Neurotensin | β-Endorphin | TRPV1 | Orexin A | |||||
| r | p | r | p | r | p | r | p | |
| Interval days | 0.03 | 0.83 | −0.14 | 0.25 | 0.14 | 0.28 | 0.12 | 0.34 |
| Daily dose | −0.17 | 0.18 | −0.05 | 0.67 | 0.03 | 0.81 | 0.04 | 0.79 |
| Total dose | 0.12 | 0.34 | −0.05 | 0.67 | 0.10 | 0.45 | 0.07 | 0.58 |
| GTV volume | −0.23 | 0.06 | −0.04 | 0.75 | −0.05 | 0.68 | −0.06 | 0.61 |
| Min dose | 0.21 | 0.10 | 0.00 | 0.99 | 0.09 | 0.47 | −0.03 | 0.80 |
| Max dose | −0.07 | 0.61 | −0.03 | 0.81 | −0.04 | 0.75 | 0.04 | 0.75 |
| Mean dose | 0.19 | 0.14 | −0.15 | 0.25 | 0.08 | 0.51 | −0.14 | 0.26 |
| Dose rate | −0.22 | 0.08 | −0.14 | 0.27 | −0.10 | 0.43 | 0.03 | 0.84 |
| MU | 0.02 | 0.85 | −0.03 | 0.79 | 0.02 | 0.88 | −0.03 | 0.79 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoveidaei, A.; Karimi, M.; Khalafi, V.; Fazeli, P.; Hoveidaei, A.H. Impacts of radiation therapy on quality of life and pain relief in patients with bone metastases. World J. Orthop. 2024, 15, 841–849. [Google Scholar] [CrossRef]
- Colosia, A.; Njue, A.; Bajwa, Z.; Dragon, E.; Robinson, R.L.; Sheffield, K.M.; Thakkar, S.; Richiemer, S.H. The Burden of Metastatic Cancer-Induced Bone Pain: A Narrative Review. J. Pain Res. 2022, 15, 3399–3412. [Google Scholar] [CrossRef]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, 166–191. [Google Scholar] [CrossRef]
- Witteman, C.; Renooij, S. Evaluation of a verbal–numerical probability scale. Int. J. Approx. Reason. 2003, 33, 117–131. [Google Scholar] [CrossRef]
- Herr, K.A.; Garand, L. Assessment and measurement of pain in older adults. Clin. Geriatr. Med. 2001, 17, 457–478. [Google Scholar] [CrossRef]
- Sung, Y.T.; Wu, J.S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. Methods 2018, 50, 1694–1715. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.S.; Bernardes, L.B.; Trevisan, G. TRP channels in cancer pain. Eur. J. Pharmacol. 2021, 904, 174185. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2024, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.J. Treatment of cancer pain. Lancet 2011, 377, 2236–2247. [Google Scholar] [CrossRef]
- De Felice, F.; Piccioli, A.; Musio, D.; Tombolini, V. The role of radiation therapy in bone metastases management. Oncotarget 2017, 8, 25691–25699. [Google Scholar] [CrossRef]
- Konopka-Filippow, M.; Politynska, B.; Wojtukiewicz, A.M.; Wojtukiewicz, M.Z. Cancer Pain: Radiotherapy as a Double-Edged Sword. Int. J. Mol. Sci. 2025, 26, 5223. [Google Scholar] [CrossRef]
- Wang, W.L.; Hao, Y.H.; Pang, X.; Tang, Y.L. Cancer pain: Molecular mechanisms and management. Mol. Biomed. 2025, 6, 45. [Google Scholar] [CrossRef]
- Caraceni, A.; Cherny, N.; Fainsinger, R.; Kaasa, S.; Poulain, P.; Radbruch, L.; De Conno, F. Pain measurement tools and methods in clinical research in palliative care: Recommendations of an Expert Working Group of the European Association of Palliative Care. J. Pain Symptom Manag. 2002, 23, 239–255. [Google Scholar] [CrossRef]
- Fogliata, A.; Vanetti, E.; Albers, D.; Brink, C.; Clivio, A.; Knöös, T.; Nicolini, G.; Cozzi, L. On the dosimetric behaviour of photon dose calculation algorithms in the presence of simple geometric heterogeneities: Comparison with Monte Carlo calculations. Phys. Med. Biol. 2007, 52, 1363–1385. [Google Scholar] [CrossRef]
- Rakici, S.Y.; Cinar, Y. Dual-Isocentric Volumetric Modulated Arc Therapy in Synchronous Bilateral Breast Cancer Irradiation: A Dosimetric Study. J. Radiat. Cancer Res. 2020, 11, 188. [Google Scholar] [CrossRef]
- Caraceni, A.; Shkodra, M. Cancer Pain Assessment and Classification. Cancers 2019, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Valderas, J.; Kotzeva, A.; Espallargues, M.; Guyatt, G.; Ferrans, C.; Halyard, M.; Revicki, D.; Symonds, T.; Parada, A.; Alonso, J. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual. Life Res. 2008, 17, 179–193. [Google Scholar] [CrossRef]
- Rivera, S.C.; Kyte, D.G.; Aiyegbusi, O.L.; Slade, A.L.; McMullan, C.; Calvert, M.J. The impact of patient-reported outcome (PRO) data from clinical trials: A systematic review and critical analysis. Health Qual. Life Outcomes 2019, 17, 156. [Google Scholar] [CrossRef]
- Gutiérrez Bayard, L.; Salas Buzón, M.d.C.; Angulo Paín, E.; de Ingunza Barón, L. Radiation therapy for the management of painful bone metastases: Results from a randomized trial. Rep. Pract. Oncol. Radiother. 2014, 19, 405–411. [Google Scholar] [CrossRef]
- Marazzi, F.; Orlandi, A.; Manfrida, S.; Masiello, V.; Di Leone, A.; Massaccesi, M.; Moschella, F.; Franceschini, G.; Bria, E.; Gambacorta, M.A.; et al. Diagnosis and Treatment of Bone Metastases in Breast Cancer: Radiotherapy, Local Approach and Systemic Therapy in a Guide for Clinicians. Cancers 2020, 12, 2390. [Google Scholar] [CrossRef]
- Jayarangaiah, A.; Kemp, A.K.; Theetha Kariyanna, P. Bone Metastasis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Adli, M.; Kuzhan, A.; Alkis, H.; Andic, F.; Yilmaz, M. FDG PET uptake as a predictor of pain response in palliative radiation therapy in patients with bone metastasis. Radiology 2013, 269, 850–856. [Google Scholar] [CrossRef]
- Struck, A.F.; Muzahir, S.; Hall, L.T. (18)F-FDG PET/CT and pain in metastatic bone cancer. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 287–292. [Google Scholar] [PubMed]
- Rades, D. Dose-Fractionation Schedules for Radiotherapy of Bone Metastases. Breast Care 2010, 5, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Barrantes, R.; Marchant, I.; Olivero, P. TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration. Neural Regen. Res. 2016, 11, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Hchicha, K.; Korb, M.; Badraoui, R.; Naïli, H. A novel sulfate-bridged binuclear copper (II) complex: Structure, optical, ADMET and in vivo approach in a murine model of bone metastasis. New J. Chem. 2021, 45, 13775–13784. [Google Scholar] [CrossRef]
- Erin, N.; Szallasi, A. Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells. Biomolecules 2023, 13, 983. [Google Scholar] [CrossRef]
- Mystakidou, K.; Befon, S.; Hondros, K.; Kouskouni, E.; Vlahos, L. Continuous Subcutaneous Administration of High-Dose Salmon Calcitonin in Bone Metastasis: Pain Control and Beta-Endorphin Plasma Levels. J. Pain Symptom Manag. 1999, 18, 323–330. [Google Scholar] [CrossRef]
- Rakici, S.Y.; Levent, T.; Celebi, E.O.; Ufuk, Y.; Engin, D.; Medeni, A.; and Mercantepe, T. Radioprotective effect of endogenous melatonin secretion associated with the circadian rhythm in irradiated rats. Int. J. Radiat. Biol. 2019, 95, 1236–1241. [Google Scholar] [CrossRef]
- Argueta, D.A.; Aich, A.; Lei, J.; Kiven, S.; Nguyen, A.; Wang, Y.; Gu, J.; Zhao, W.; Gupta, K. β-endorphin at the intersection of pain and cancer progression: Preclinical evidence. Neurosci. Lett. 2021, 744, 135601. [Google Scholar] [CrossRef]
- Oldenburger, E.; Brown, S.; Willmann, J.; van der Velden, J.M.; Spałek, M.; van der Linden, Y.M.; Kazmierska, J.; Menten, J.; Andratschke, N.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 173, 240–253. [Google Scholar] [CrossRef]
- Alain, C.; Pascal, N.; Valérie, G.; Thierry, V. Orexins/Hypocretins and Cancer: A Neuropeptide as Emerging Target. Molecules 2021, 26, 4849. [Google Scholar] [CrossRef]
- Thio, Q.; Goudriaan, W.A.; Janssen, S.J.; Paulino Pereira, N.R.; Sciubba, D.M.; Rosovksy, R.P.; Schwab, J.H. Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with bone metastases. Br. J. Cancer 2018, 119, 737–743. [Google Scholar] [CrossRef]
- Yang, G.; Chang, J.S.; Byun, H.K.; Cho, Y.; Koom, W.S.; Kim, J.S.; Beom, S.H.; Kim, H.S.; Kim, T.I.; Yang, S.Y.; et al. Interaction Between Neutrophil-to-Lymphocyte Ratio, Radiotherapy Fractionation/Technique, and Risk of Development of Distant Metastasis in Locally Advanced Rectal Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111 (Suppl. S3), e83. [Google Scholar] [CrossRef]
- Zucker, A.; Winter, A.; Lumley, D.; Karwowski, P.; Jung, M.K.; Kao, J. Prognostic role of baseline neutrophil-to-lymphocyte ratio in metastatic solid tumors. Mol. Clin. Oncol. 2020, 13, 25. [Google Scholar] [CrossRef]
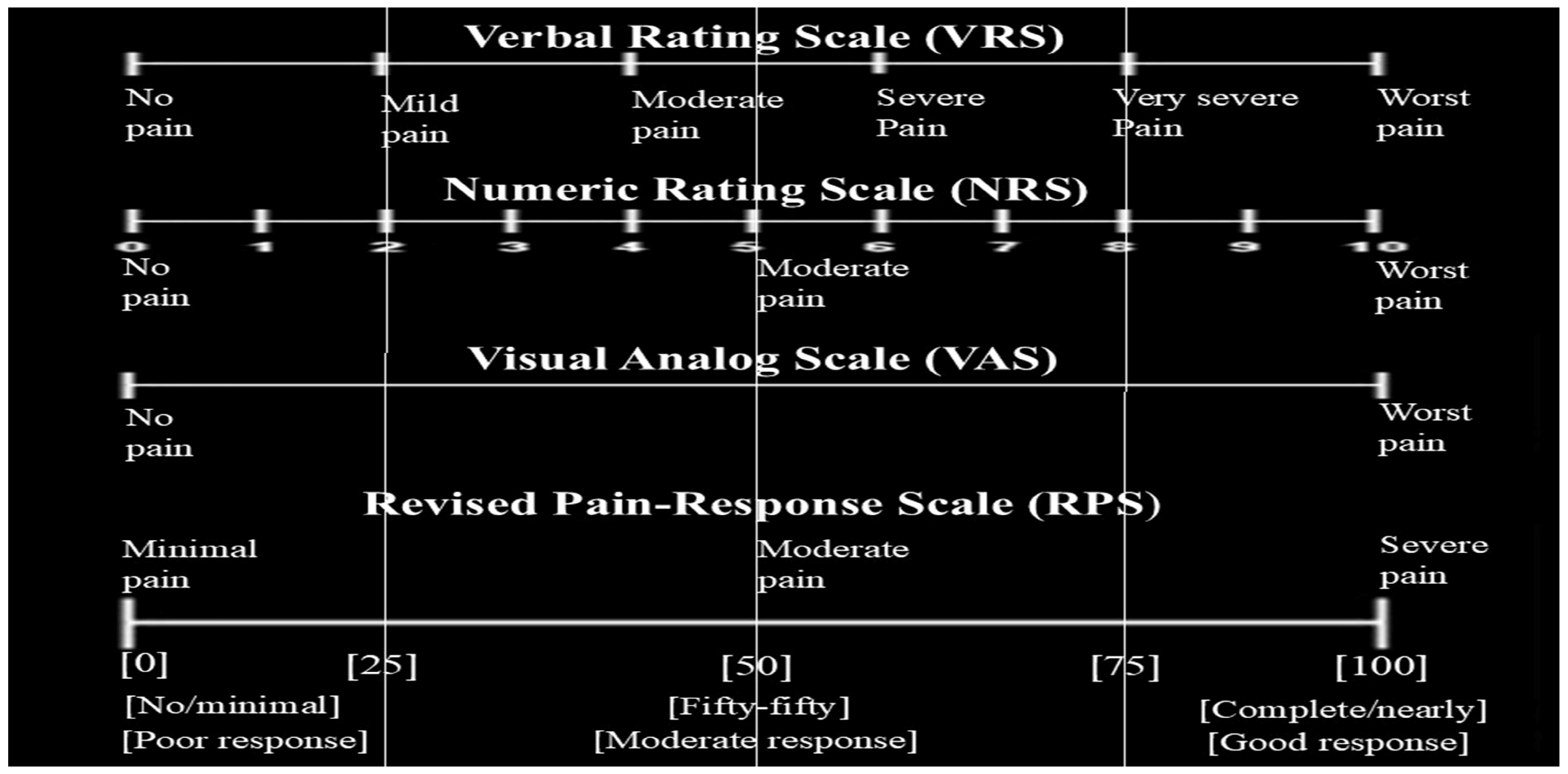
| General Parameters | Pain Response After Radiotherapy | |||||
|---|---|---|---|---|---|---|
| Poor Response (n = 47) | Moderate Response (n = 11) | Good Response (n = 47) | p | |||
| Age | Mean ± SD | 63.1 ± 13.1 | 66.1 ± 14.5 | 67.7 ± 12.2 | 0.243 | |
| Median (min–max) | (37–87) | (35–87) | (46–85) | |||
| Gender | Male | 30 (63.8) | 8 (72.7) | 25 (53.2) | 0.380 | |
| Female | 17 (36.2) | 3 (27.3) | 22 (46.8) | |||
| Diagnosis | Prostate cancer | 8 (17.0) | 0 (0.0) | 15 (31.9) | 0.043 | |
| Breast cancer | 12 (25.5) | 2 (18.2) | 17 (36.2) | |||
| Lung cancer | 12 (25.5) | 5 (45.5) | 7 (14.9) | |||
| Other | 15 (31.9) | 4 (36.4) | 8 (17.0) | |||
| Histopathological cancer type | Adenocarcinoma | 22 (46.8) | 4 (36.4) | 21 (44.7) | 0.796 | |
| Squamous cell carcinoma | 4 (8.5) | 1 (9.1) | 2 (4.3) | |||
| Neuroendocrine carcinoma | 2 (4.3) | 1 (9.1) | 2 (4.3) | |||
| Infiltrative ductal carcinoma | 12 (25.5) | 2 (18.2) | 17 (36.2) | |||
| Other | 7 (14.9) | 3 (27.3) | 5 (10.6) | |||
| Differentiation | Unknown | 24 (51.1) | 9 (81.8) | 20 (42.6) | 0.098 | |
| Good | 0 (0.0) | 0 (0.0) | 2 (4.3) | |||
| Moderate | 6 (12.8) | 0 (0.0) | 12 (25.5) | |||
| Poor | 17 (36.2) | 2 (18.2) | 13 (27.7) | |||
| Perineural invasion | Unknown | 33 (70.2) | 11(100.0) | 28 (59.6) | ||
| No | 7 (14.9) | 0 (0.0) | 7 (14.9) | 0.112 | ||
| Yes | 7 (14.9) | 0 (0.0) | 12 (25.5) | |||
| Second primary cancer | No | 40 (85.1) | 10 (90.9) | 38 (80.9) | 0.680 | |
| Yes | 7 (14.9) | 1 (9.1) | 9 (11.1) | |||
| Pain intensity | Unknown | 0 (0.0) | 2 (18.2) | 4 (8.5) | 0.001 | |
| Mild | 6 (12.8) | 0 (0.0) | 9 (19.1) | |||
| Moderate | 16 (34.0) | 0 (0.0) | 23 (48.9) | |||
| Severe | 25 (53.2) | 9 (81.8) | 11 (23.4) | |||
| Metastasis location | Appendicular | 6 (12.8) | 1 (9.1) | 11 (23.4) | 0.561 | |
| Axial | 17 (36.2) | 4 (36.4) | 12 (25.5) | |||
| Mixt | 24 (51.1) | 6 (54.5) | 24 (51.1) | |||
| Metastasis type | Increased FDG activity | 1 (2.1) | 1 (9.1) | 9 (19.1) | 0.001 | |
| Soft tissue-lytic-destructive- | 9 (19.1) | 6 (54.6) | 4 (8.5) | |||
| Lytic | 12 (25.5) | 3 (27.3) | 6 (12.8) | |||
| Mixt | 11 (23.0) | 1 (9.1) | 4 (8.5) | |||
| Sclerotic | 14 (29.8) | 0 (0.0) | 24 (51.1) | |||
| Number of bone metastases | Multiple | 31 (66.0) | 8 (72.7) | 24 (51.1) | 0.223 | |
| Oligo | 16 (34.0) | 3 (27.3) | 23 (48.9) | |||
| Extra-bone metastasis | Unknown | 4 (8.5) | 0 (0.0) | 0 (0.0) | 0.089 | |
| Yes | 27 (57.4) | 6 (54.5) | 21 (44.7) | |||
| No | 16 (34.0) | 5 (45.5) | 26 (55.3) | |||
| Time between diagnosis and month of metastasis | <3 | 23 (48.9) | 9 (81.8) | 29 (61.7) | 0.670 | |
| 3–11 | 8 (17.0) | 0 (0.0) | 6 (12.8) | |||
| 12–35 | 4 (8.5) | 1 (9.1) | 3 (6.4) | |||
| 36–59 | 6 (12.8) | 0 (0.0) | 5 (10.6) | |||
| ≥60 | 6 (12.8) | 1 (9.1) | 4 (8.5) | |||
| Parameters related to PET/CT SUVmax | ||||||
| Primary tumor SUVmax | Median (min–max) | 3.9 (0.0–48.4) | 6.8 (0.0–19.6) | 5.7 (0.0–65.5) | 0.500 | |
| <2 | 17 (36.2) | 2 (18.2) | 13 (27.7) | 0.654 | ||
| 2–10 | 19 (40.4) | 7 (63.6) | 22 (46.8) | |||
| >10 | 11 (23.4) | 2 (18.2) | 12 (25.5) | |||
| Lymph node metastasis SUVmax | Median (min–max) | 2.7 (0.0–59.7) | 1.9(0.0–10.6) | 1.7(0.0–97.3) | 0.916 | |
| <2 | 23 (48.9) | 6 (54.5) | 24 (51.1) | 0.889 | ||
| 2–10 | 15 (31.9) | 4 (36.4) | 17 (36.2) | |||
| >10 | 9 (19.1) | 1 (9.1) | 8 (12.8) | |||
| Bone metastasis SUVmax | Median (min–max) | 9.7 (0.0–39.2) | 6.3 (3.7–22.5) | 9.2(0.0–38.6) | 0.646 | |
| <2 | 2 (4.3) | 0 (0.0) | 3 (6.4) | 0.704 | ||
| 2–10 | 22 (46.8) | 7 (63.6) | 26 (55.3) | |||
| >10 | 23 (48.9) | 4 (36.4) | 18 (38.3) | |||
| Parameters associated with radiotherapy | ||||||
| Previous RT | Any previous RT | Unknown | 2 (4.3) | 0 (0.0) | 1 (2.1) | 0.435 |
| Yes | 18 (38.3) | 2 (18.2) | 12 (25.5) | |||
| No | 27 (57.4) | 9 (81.8) | 34 (72.3) | |||
| Previous primary RT | Unknown | 2 (4.3) | 0 (0.0) | 1 (2.1) | 0.179 | |
| Yes | 14 (29.8) | 1 (9.1) | 6 (12.8) | |||
| No | 31 (66.0) | 10 (90.9) | 40 (85.1) | |||
| Time between previous RT and current RT (months) | Median (min–max) | 29.0 (0–110) | 23 (13–33) | 45 (0–100) | 0.778 | |
| RT interval (days) | 1–5 | 22 (46.8) | 5 (45.5) | 18 (38.3) | 0.754 | |
| 6–9 | 12 (25.5) | 2 (18.2) | 13 (27.7) | |||
| 10–30 | 13 (27.7) | 4 (36.4) | 14 (29.8) | |||
| >30 | 0 (0.0) | 0 (0.0) | 2 (4.3) | |||
| RT daily dose (Gy) | 2 Gy | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0.807 | |
| 3 Gy | 8 (17.0) | 3 (27.3) | 11 (23.4) | |||
| 4 Gy | 1 (2.1) | 0 (0.0) | 2 (4.3) | |||
| ≥5 Gy | 38 (80.9) | 8 (72.7) | 33 (70.2) | |||
| Total RT dose (Gy) | 20 Gy | 6 (12.8) | 0 (0.0) | 3 (6.4) | 0.534 | |
| 25Gy | 33 (70.2) | 8 (72.7) | 32 (68.1) | |||
| ≥30 | 8 (17.0) | 3 (27.3) | 12 (25.5) | |||
| RT target volume: Gros tumor volume (cm3) | <20 | 5 (10.6) | 1 (9.1) | 7 (14.9) | 0.416 | |
| 20–50 | 9 (19.1) | 1 (9.1) | 5 (10.6) | |||
| 51–100 | 5 (10.6) | 1 (9.1) | 7 (14.9) | |||
| 101–500 | 16 (34.0) | 6 (54.5) | 24 (51.1) | |||
| >500 | 12 (25.5) | 2 (18.2) | 4 (8.5) | |||
| Target-covering RT Dose % | Minimum dose | <50 | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0.131 |
| 50–84 | 7 (14.9) | 1 (9.1) | 2 (4.3) | |||
| 85–95 | 25 (53.2) | 5 (45.5) | 28 (59.6) | |||
| 95–100 | 13 (27.7) | 4 (36.4) | 15 (31.9) | |||
| >100 | 2 (4.3) | 0 (0.0) | 2 (4.3) | |||
| Maximum dose | 100–110 | 42(89.4) | 9 (81.8) | 43 (91.5) | 0.640 | |
| >110 | 5 (10.6) | 2 (18.2) | 4 (8.5) | |||
| Mean dose | ≤100 | 7 (14.9) | 0 (0.0) | 9 (19.1) | 0.281 | |
| >100 | 40 (85.1) | 11 (100.0) | 38 (80.9) | |||
| RT Dose rate | 400 | 10 (25.0) | 2 (18.2) | 10 (25.6) | 0.988 | |
| 600 | 30 (75.0) | 9 (81.8) | 29 (74.4) | |||
| RT MU | 300–600 | 2 (4.3) | 0 (0.0) | 5 (10.6) | 0.408 | |
| 601–1000 | 11 (23.4) | 2 (18.2) | 10 (21.3) | |||
| 1001–2000 | 23 (48.9) | 4 (36.4) | 17 (36.2) | |||
| 2000–4000 | 4 (8.5) | 3 (27.3) | 11 (23.4) | |||
| >4000 | 7 (14.9) | 2 (18.2) | 4 (8.5) | |||
| RT technique | 3DCRT | 16 (34.0) | 4 (36.4) | 15 (38.5) | 0.912 | |
| IMRT | 31 (66.0) | 7 (63.6) | 29 (61.7) | |||
| Response status in follow-up | ||||||
| Follow-up period (Month) Mean ± SD | 42.1 ± 39.0 | 16.9 ± 20.4 | 36.4 ± 39.3 | 0.080 | ||
| Median (min-max) | 32 (5–130) | 9 (5–67) | 18 (5–179) | |||
| Survival rate | 70.2% | 63.6% | 80.4% | 0.346 | ||
| Blood Count and Biochemical Parameters | Pain Response After Radiotherapy | ||||
|---|---|---|---|---|---|
| Poor Response (n = 47) | Moderate Response (n = 11) | Good Response (n = 47) | p | ||
| Pre-RT Neu | Median | 4.6 (1.5–19.1) | 5.0 (1.6–10.7) | 3.7 (1.4–11.7) | 0.116 |
| Post-RT Neu | Median | 3.9 (0.8–25.0) | 4.7 (1.4–7.8) | 3.0 (0.7–8.0) | 0.010 |
| p | 0.006 | 0.131 | <0.001 | ||
| Pre-RT Leu | Median | 1.2 (0.2–2.7) | 1.4 (0.5–4.0) | 1.3 (0.3–3.3) | 0.439 |
| Post-RT Leu | Median | 0.9 (0.2–4.7) | 0.9 (0.3–1.4) | 0.8 (0.3–3.5) | 0.426 |
| p | <0.001 | 0.003 | <0.001 | ||
| Pre-RT NLR | Median | 4.0 (1.3–36.7) | 3.3 (0.7–12.1) | 2.6 (1.2–11.4) | 0.036 |
| Post-RT NLR | Median | 3.9 (0.8–37.2) | 5.5 (1.5–18.0) | 3.1 (1.2–10.8) | 0.047 |
| p | 0.005 | 0.021 | 0.002 | ||
| Pre-RT PLT | Median | 259 (69–528) | 374 (190–542) | 271 (130–686) | 0.105 |
| Post-RT PLT | Median) | 204.0 (39–505) | 260.0 (72–434) | 202.0 (57–554) | 0.486 |
| p | 0.001 | 0.021 | <0.001 | ||
| Pre-RT LDH | Median | 221.0 (19–1160) | 175.0 (113–545) | 220.0 (126–642) | 0.201 |
| Post-RT LDH | Median | 234.0 (99–1064) | 188.0 (56–423) | 211.0 (117–904) | 0.200 |
| p | 0.202 | 0.722 | 0.553 | ||
| Pre-RT ALP | Median | 98.0 (35–396) | 125.0 (81–277) | 94.0 (54–1025) | 0.374 |
| Post-RT ALP | Median | 98.0 (27–528) | 121.0 (69–673) | 88.0 (26–1002) | 0.333 |
| p | 0.240 | 0.285 | 0.065 | ||
| Pre-RT tumor markers | Unknown | 20 (42.6) | 3 (27.3) | 8 (17.0) | 0.082 |
| Normal | 15 (31.9) | 4 (36.4) | 17 (36.2) | ||
| High | 12 (25.5) | 4 (36.4) | 22 (46.8) | ||
| Biochemical Pain Biomarkers | Pain Response After Radiotherapy | ||||
|---|---|---|---|---|---|
| Poor Response (n = 47) | Moderate Response (n = 11) | Good Response (n = 47) | p | ||
| Neurotensin | Pre-RT | 609.0 (1.0–2452.0) | 369.9 (116.6–920.0) | 631.4 (39.7–2863.0) | 0.057 |
| Post-RT | 480.0 (100.1–2500.0) | 671.0 (75.1–1195.0) | 400.3 (79.1–1479.0) | 0.828 | |
| p | 0.600 | 0.176 | 0.006 | ||
| β-Endorphin | Pre-RT | 85.5 (32.9–142.8) | 71.6 (25.9–115.5) | 92.1 (18.7–228.8) | 0.514 |
| Post-RT | 36.0 (5.2–200.0) | 36.8 (20.2–58.6) | 49.1 (13.3–135.6) | 0.031 | |
| p | <0.001 | 0.018 | <0.001 | ||
| Transient Receptor Potential Cation Channel Subfamily V-Member 1 | Pre-RT | 309.6 (25.2–1047.1) | 269.1 (78.5–517.5) | 321.7 (48.1–1100.7) | 0.496 |
| Post-RT | 355.6 (86.4–1473.7) | 450.8 (332.6–821.7) | 352.8 (119.3–1510.9) | 0.238 | |
| p | <0.001 | 0.018 | <0.001 | ||
| Orexin A | Pre-RT | 1389.0 (521.0–3311.0) | 1381.0 (602.0–2076.0) | 1449.0 (492.0–3311.0) | 0.721 |
| Post-RT | 1544.0 (492.0–3311.0) | 1675.0 (510.0–2439.0) | 1589.5 (694.0–3703.0) | 0.947 | |
| p | 0.202 | 0.237 | 0.125 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakici, S.Y.; Yilmaz, A.; Karakas, S.M. The Relationship Between Radiotherapy-Induced Pain Response Score and Pain Biomarkers TRPV1, β-Endorphin (bEP), Neurotensin (NT), and Orexin A (OXA) in Patients with Bone Metastases. Life 2025, 15, 1372. https://doi.org/10.3390/life15091372
Rakici SY, Yilmaz A, Karakas SM. The Relationship Between Radiotherapy-Induced Pain Response Score and Pain Biomarkers TRPV1, β-Endorphin (bEP), Neurotensin (NT), and Orexin A (OXA) in Patients with Bone Metastases. Life. 2025; 15(9):1372. https://doi.org/10.3390/life15091372
Chicago/Turabian StyleRakici, Sema Yilmaz, Adnan Yilmaz, and Sibel Mataraci Karakas. 2025. "The Relationship Between Radiotherapy-Induced Pain Response Score and Pain Biomarkers TRPV1, β-Endorphin (bEP), Neurotensin (NT), and Orexin A (OXA) in Patients with Bone Metastases" Life 15, no. 9: 1372. https://doi.org/10.3390/life15091372
APA StyleRakici, S. Y., Yilmaz, A., & Karakas, S. M. (2025). The Relationship Between Radiotherapy-Induced Pain Response Score and Pain Biomarkers TRPV1, β-Endorphin (bEP), Neurotensin (NT), and Orexin A (OXA) in Patients with Bone Metastases. Life, 15(9), 1372. https://doi.org/10.3390/life15091372







