Effects of Endophytic Fungus Setophoma terrestris on Growth of Panax notoginseng and Its Rhizosphere Soil Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design
2.3. Strain Fermentation Treatment
2.3.1. Preparation of Fermentates
2.3.2. Sample Collection and Pretreatment
2.4. Determination of Plant Biomass
2.4.1. Fresh and Dry Weights
2.4.2. Determination of Chlorophyll Content
2.4.3. Determination of Total Saponins
2.5. Determination of Metabolites in Rhizosphere Soil
2.6. Determination of Soil Physicochemical Indicators
2.6.1. Determination of Soil Total Nitrogen (TN) and Total Phosphorus (TP) Contents
2.6.2. Determination of Total Potassium (TK) Content in Soil
2.6.3. Determination of Soil Nitrate Nitrogen (NO3−-N) and Ammonium Nitrogen (NH4+-N) Contents
2.6.4. Determination of Soil Organic Matter (SOM) Content
2.6.5. Determination of Soil pH Value
2.7. Determination of Rhizosphere Soil Microbial Diversity
2.8. Data Analysis
3. Results and Analysis
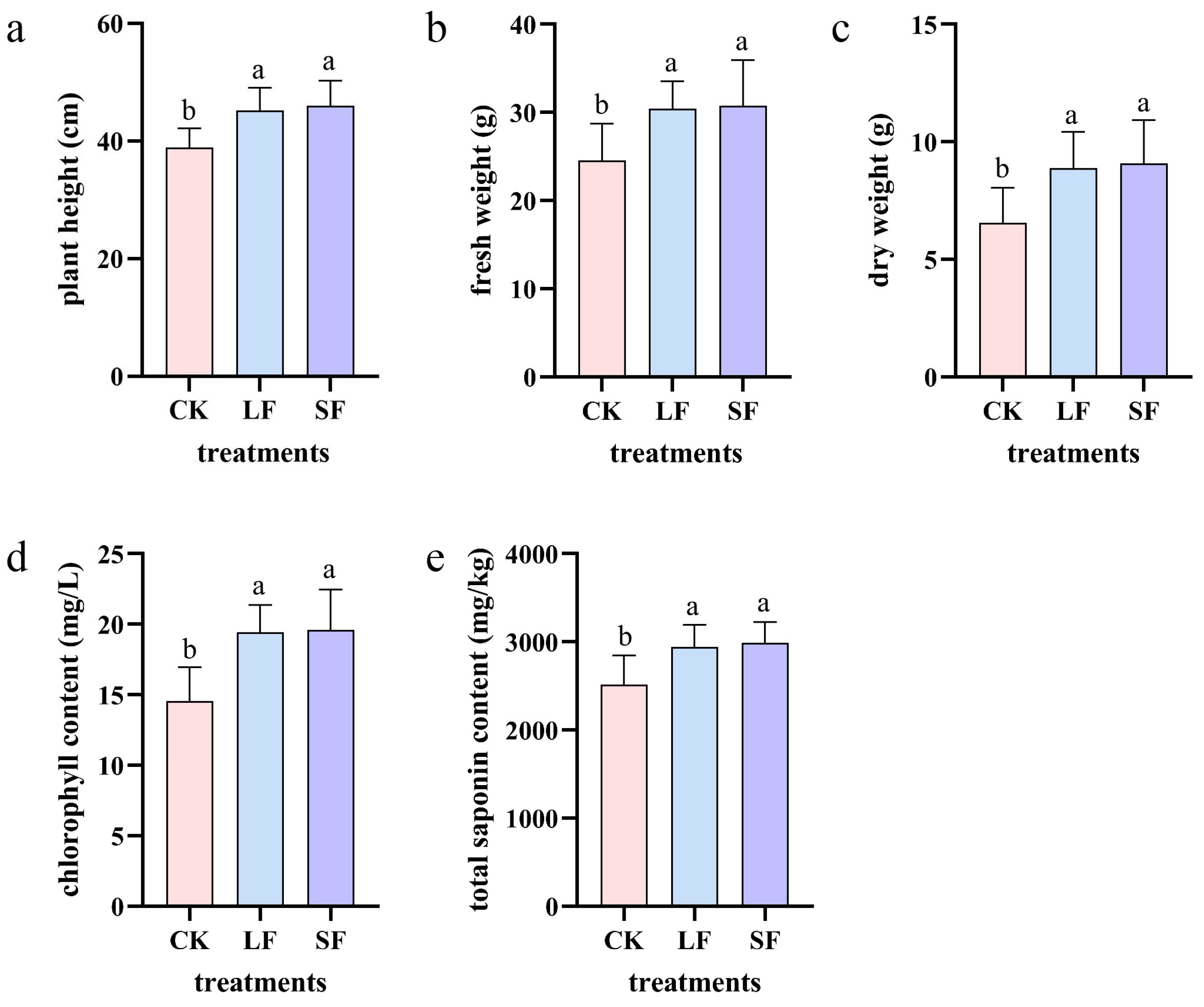
3.1. Biomass
3.1.1. Plant Height
3.1.2. Fresh and Dry Weights
3.1.3. Chlorophyll Content
3.1.4. Total Saponin Content
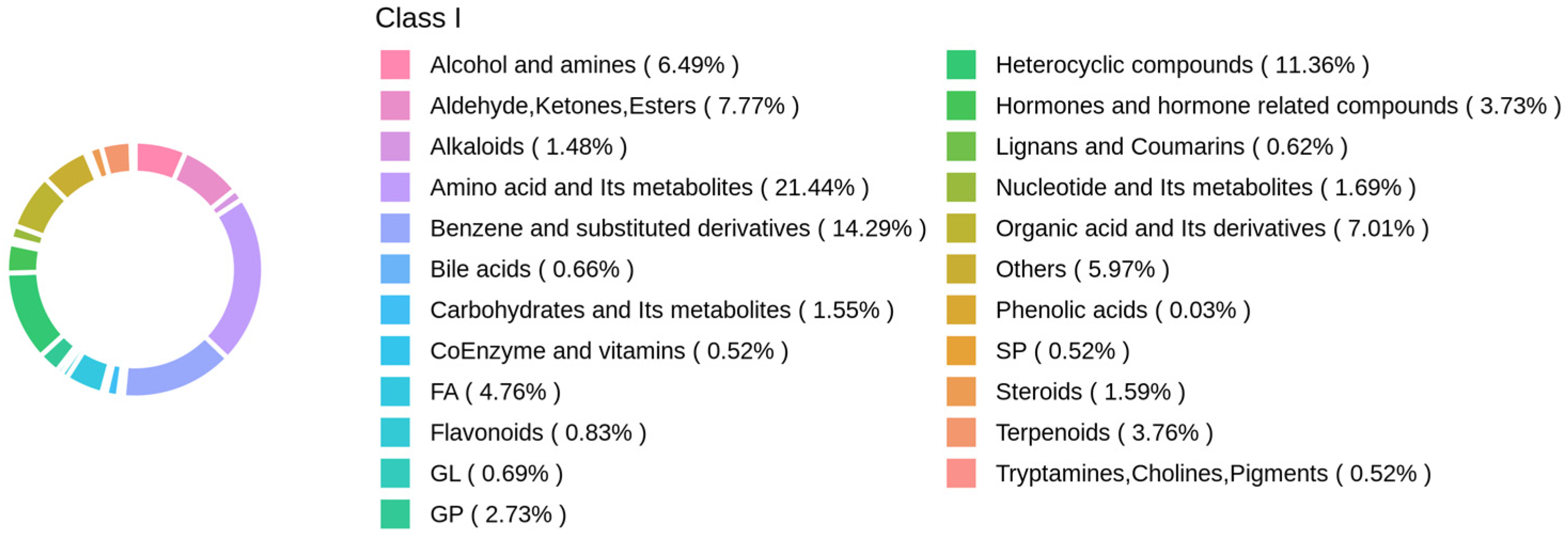
3.2. Annotation and Evaluation of Metabolites in Panax notoginseng Rhizosphere Soil
3.2.1. Qualitative and Quantitative Analysis of Metabolites
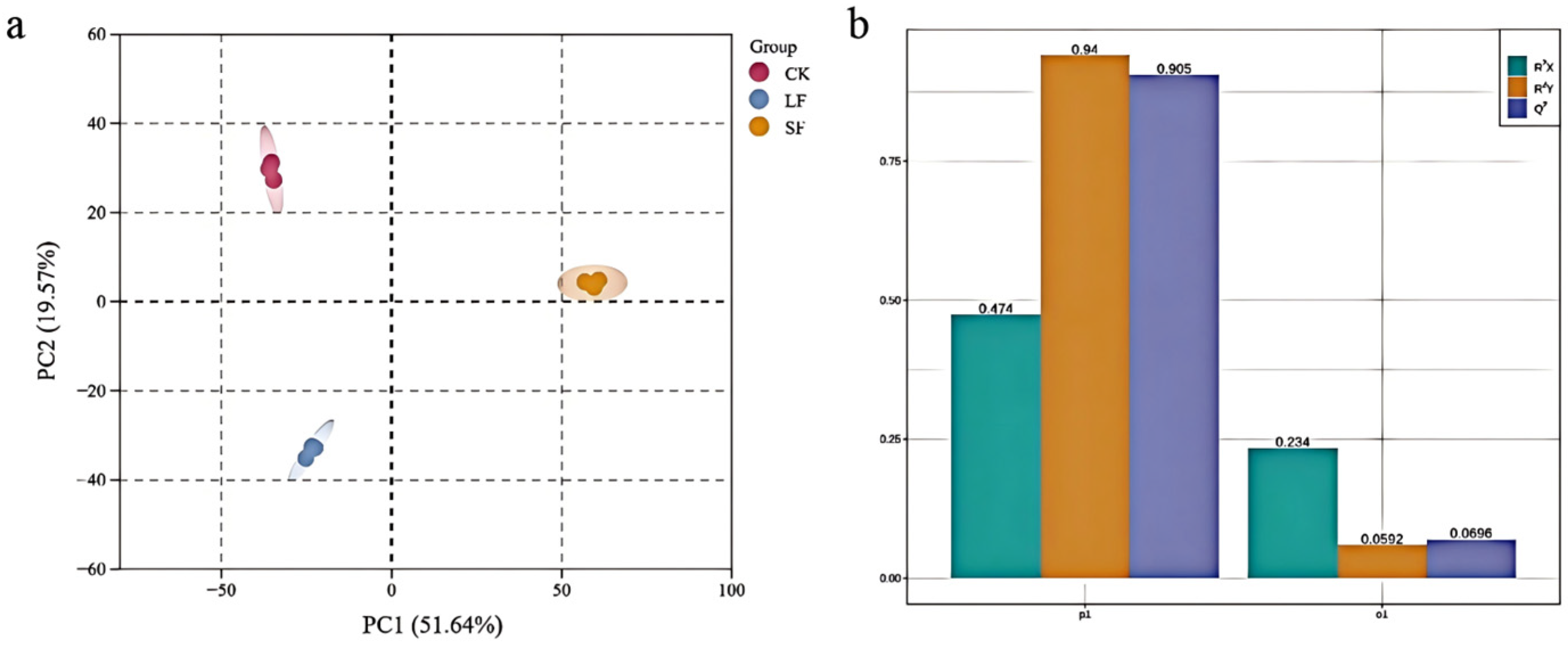
3.2.2. Principal Component Analysis (PCA) and OPLS-DA of Metabolites
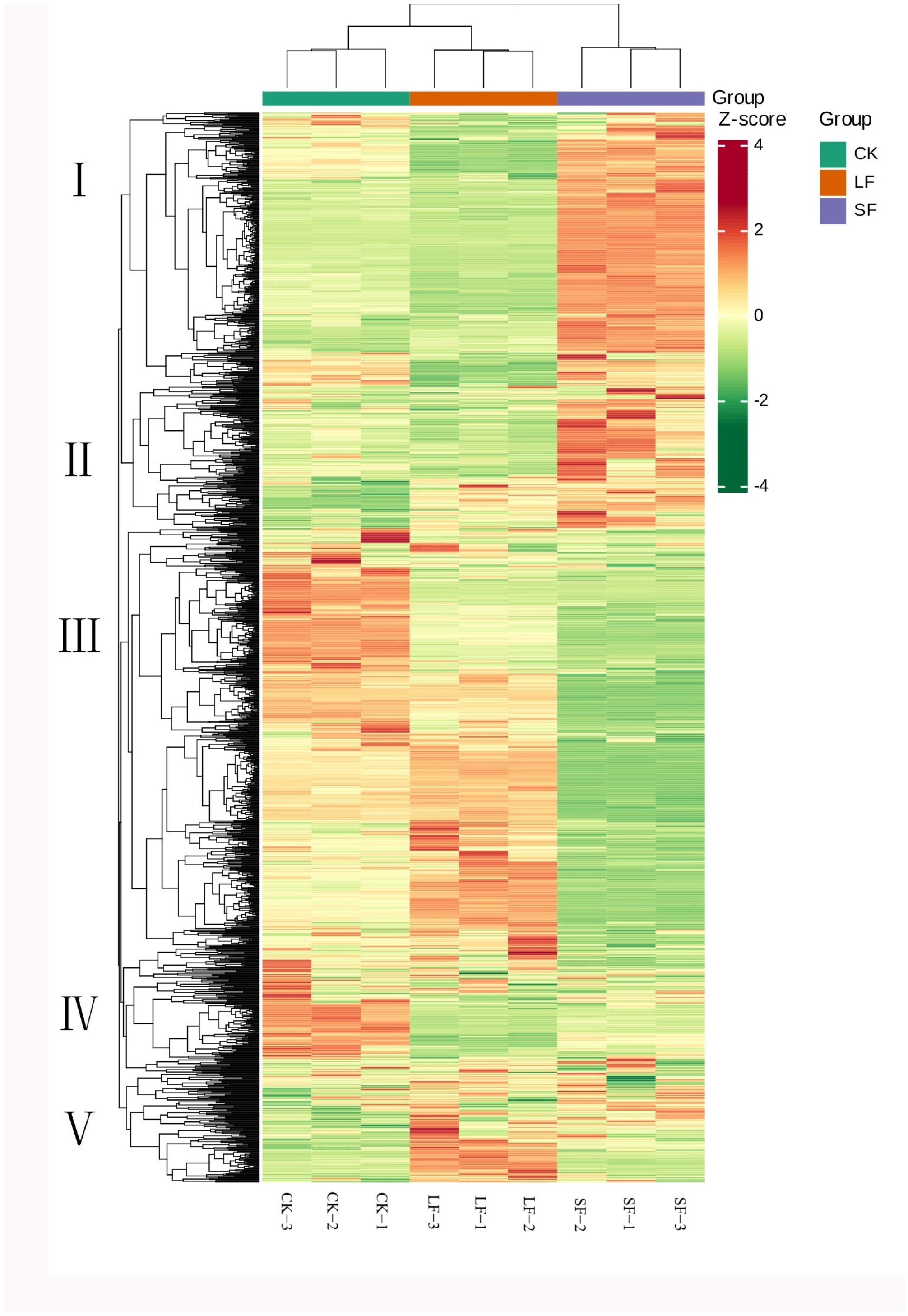
3.2.3. Cluster Heatmap Analysis of Metabolites
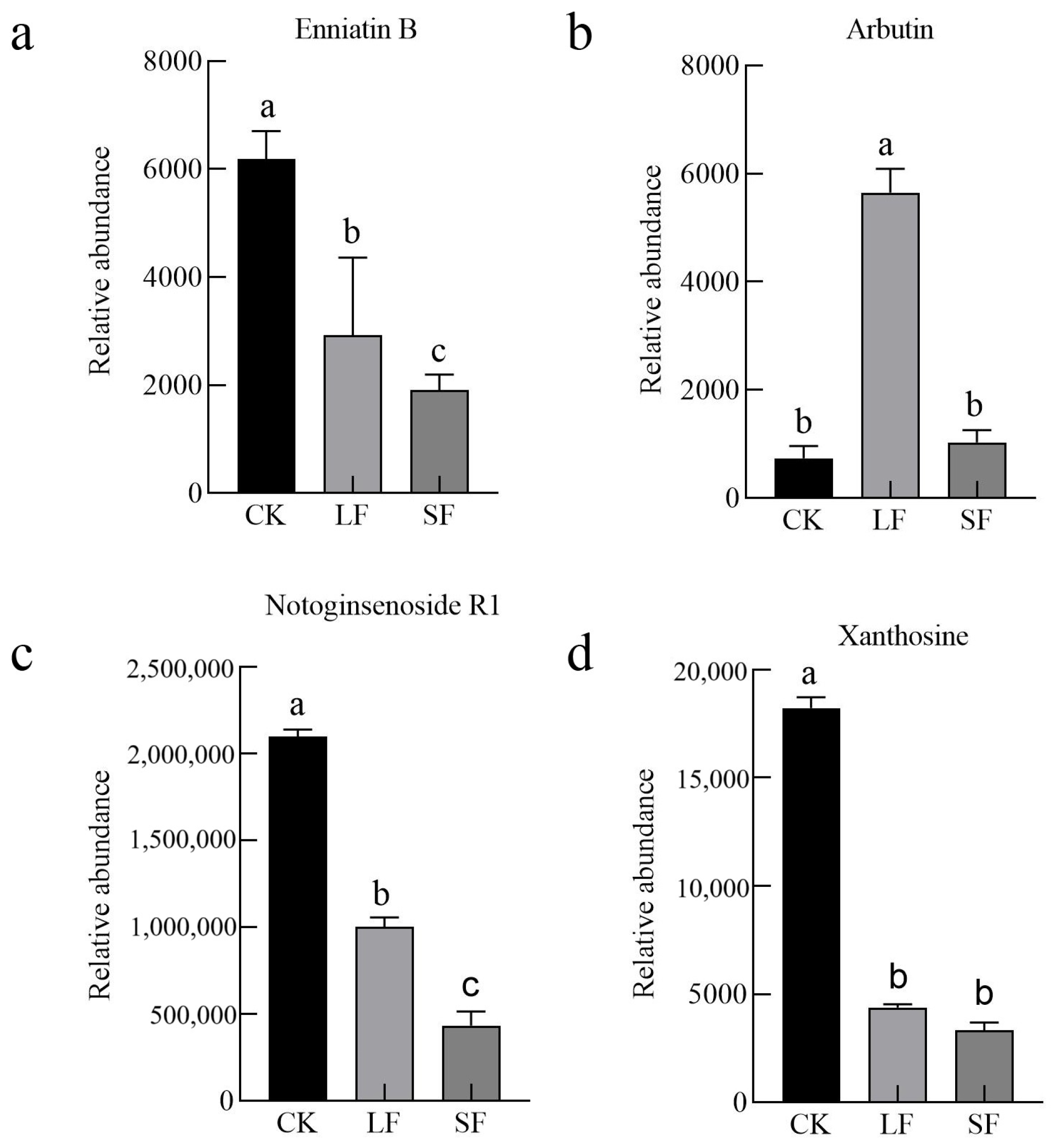
3.2.4. Volcano Plot Analysis of Differential Metabolites
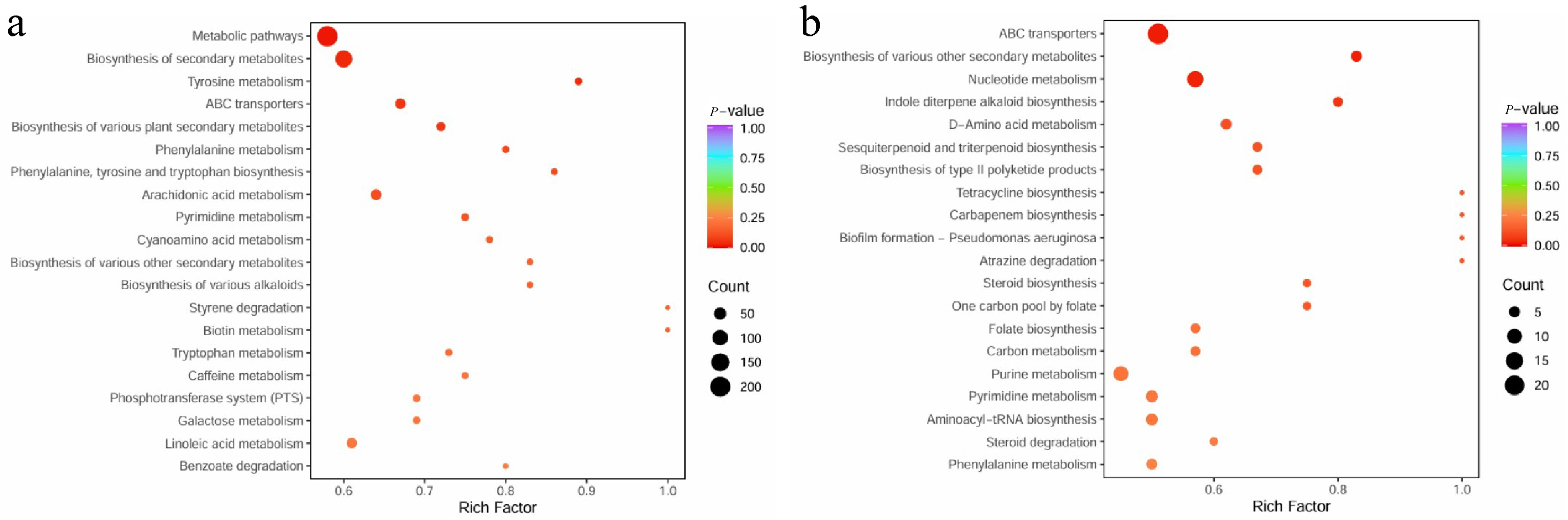
3.2.5. KEGG Functional Annotation
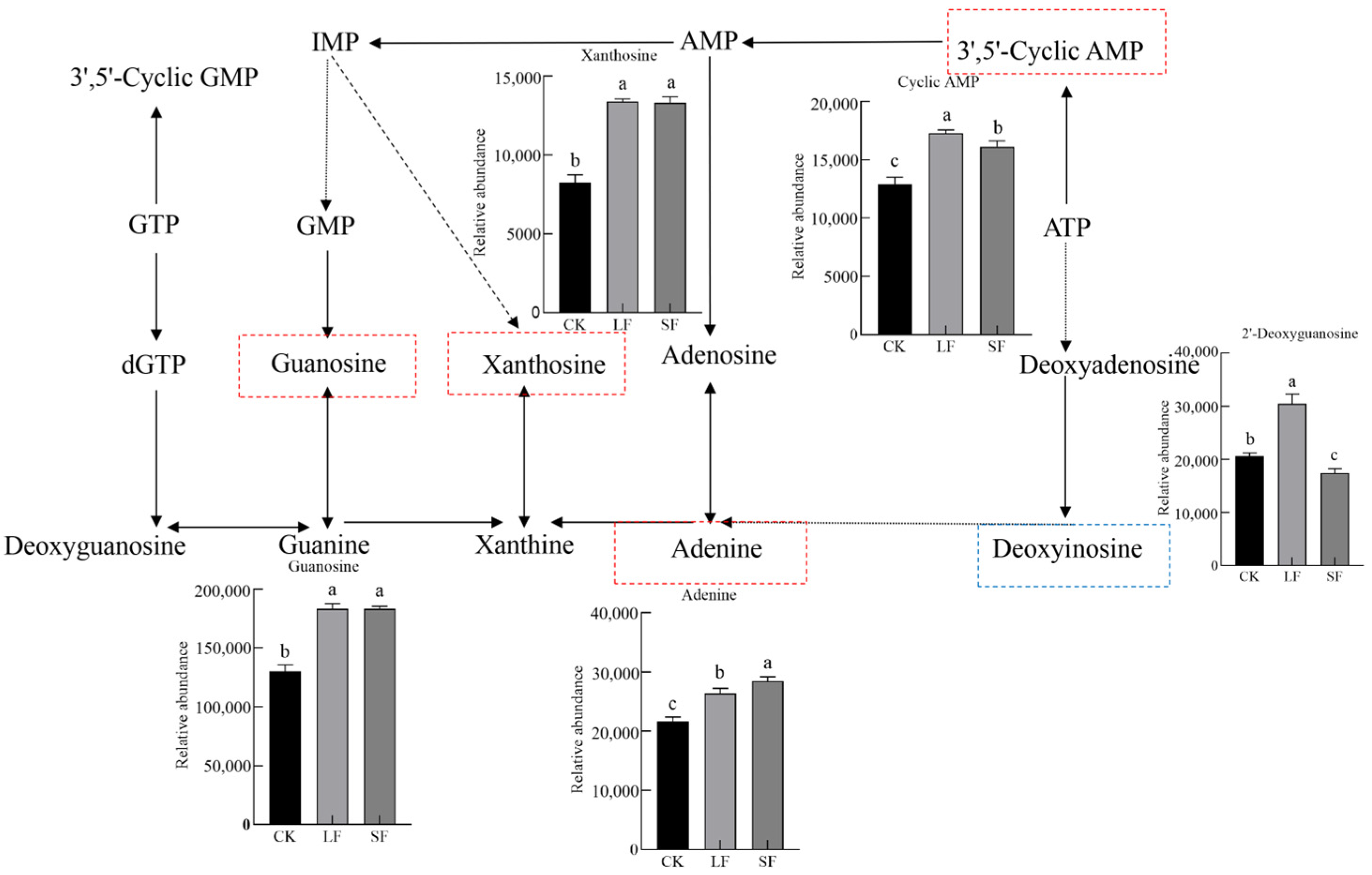
3.2.6. Analysis of Key KEGG Metabolic Pathways
3.3. Annotation and Evaluation of Rhizosphere Soil Microorganisms of Panax notoginseng
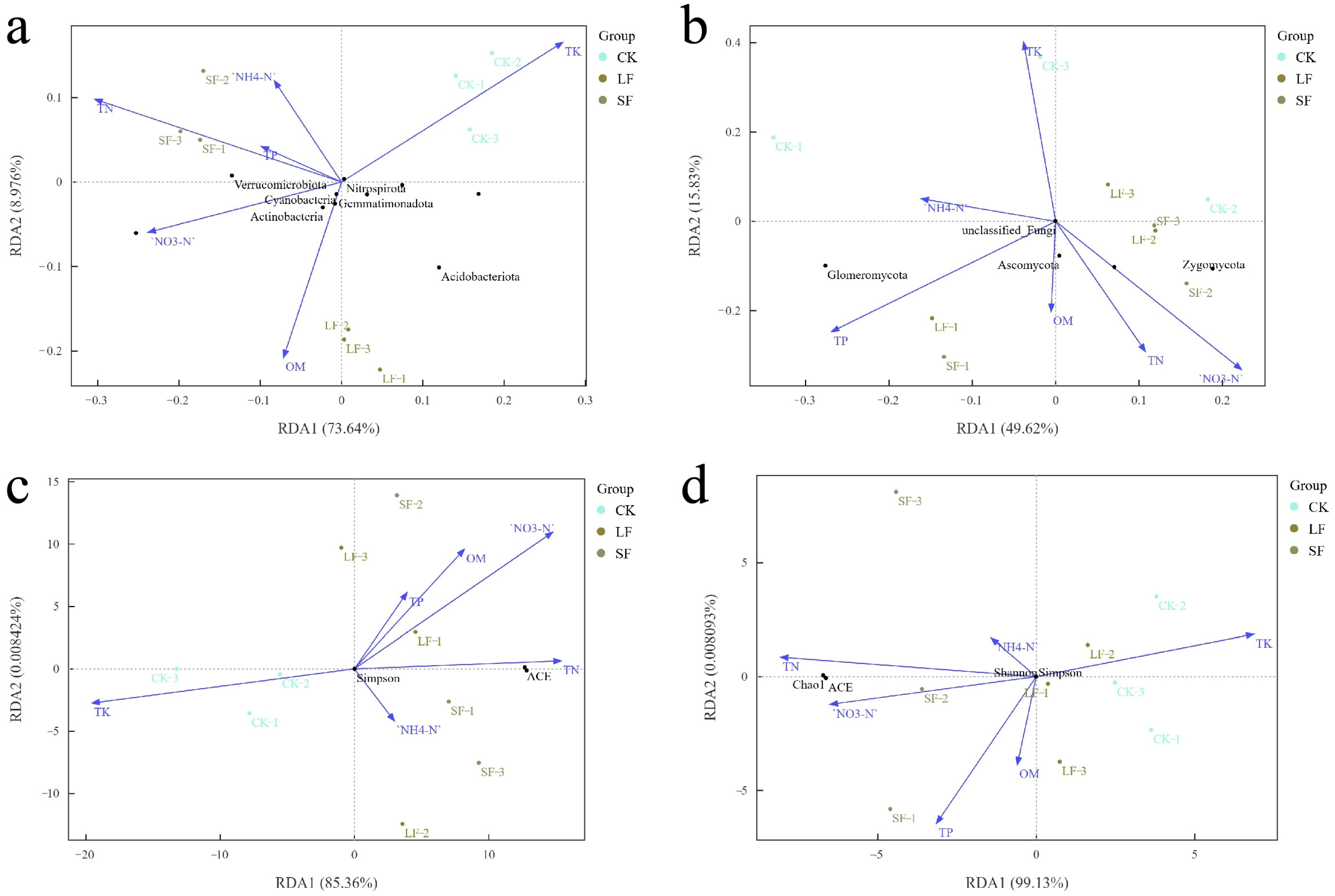
3.3.1. Effects of Growth-Promoting Fungi on Soil Nutrients and pH
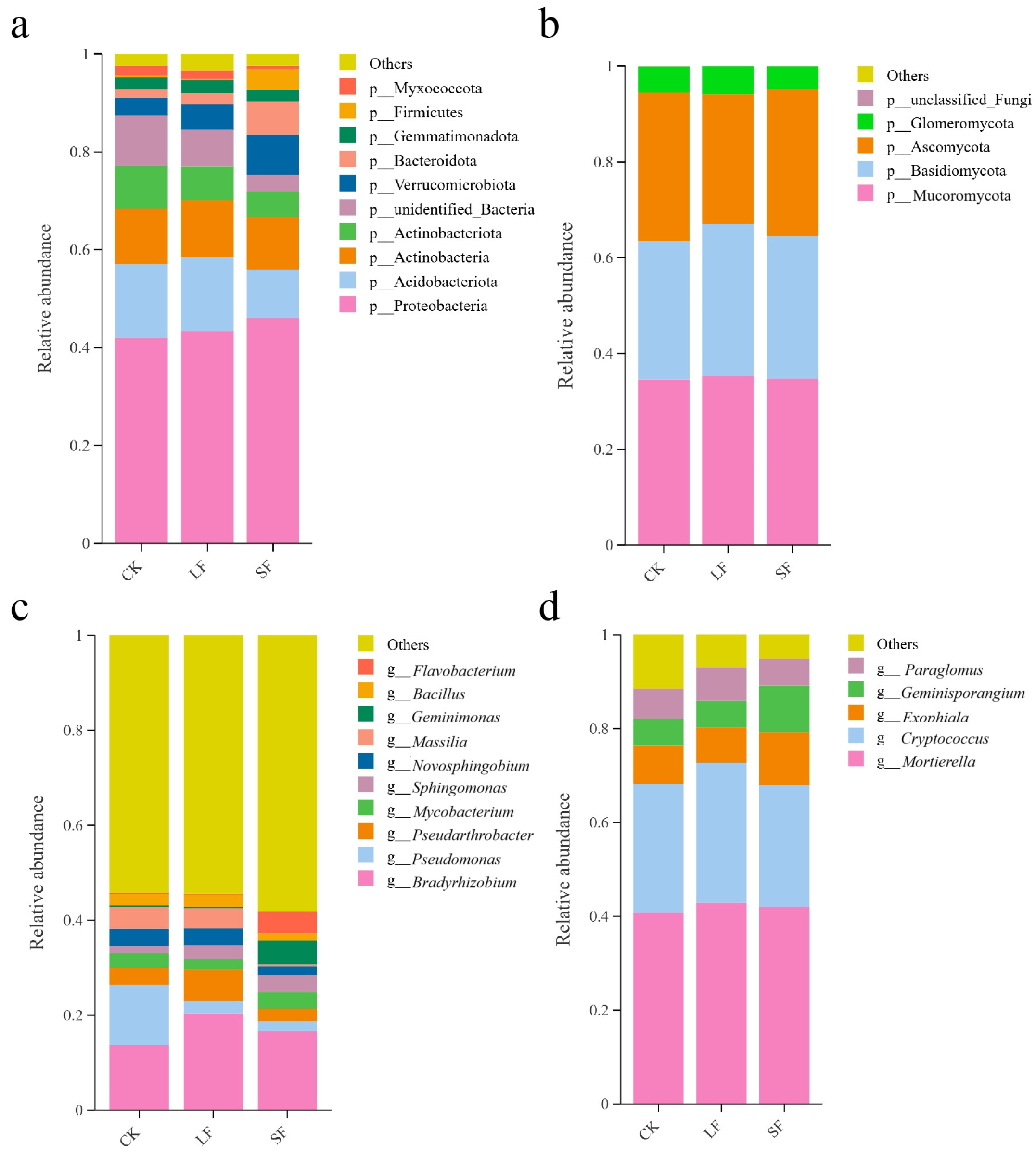
3.3.2. Effects of Growth-Promoting Fungi on Taxonomic Composition of Rhizosphere Soil Microorganisms at the Phylum Level in Understory Panax notoginseng
3.3.3. Effects of Growth-Promoting Fungi on Taxonomic Composition of Rhizosphere Soil Microorganisms at the Genus Level in Understory Panax notoginseng
3.3.4. α Diversity Analysis of Bacteria and Fungi
3.3.5. β Diversity Analysis of Rhizosphere Soil Microorganisms
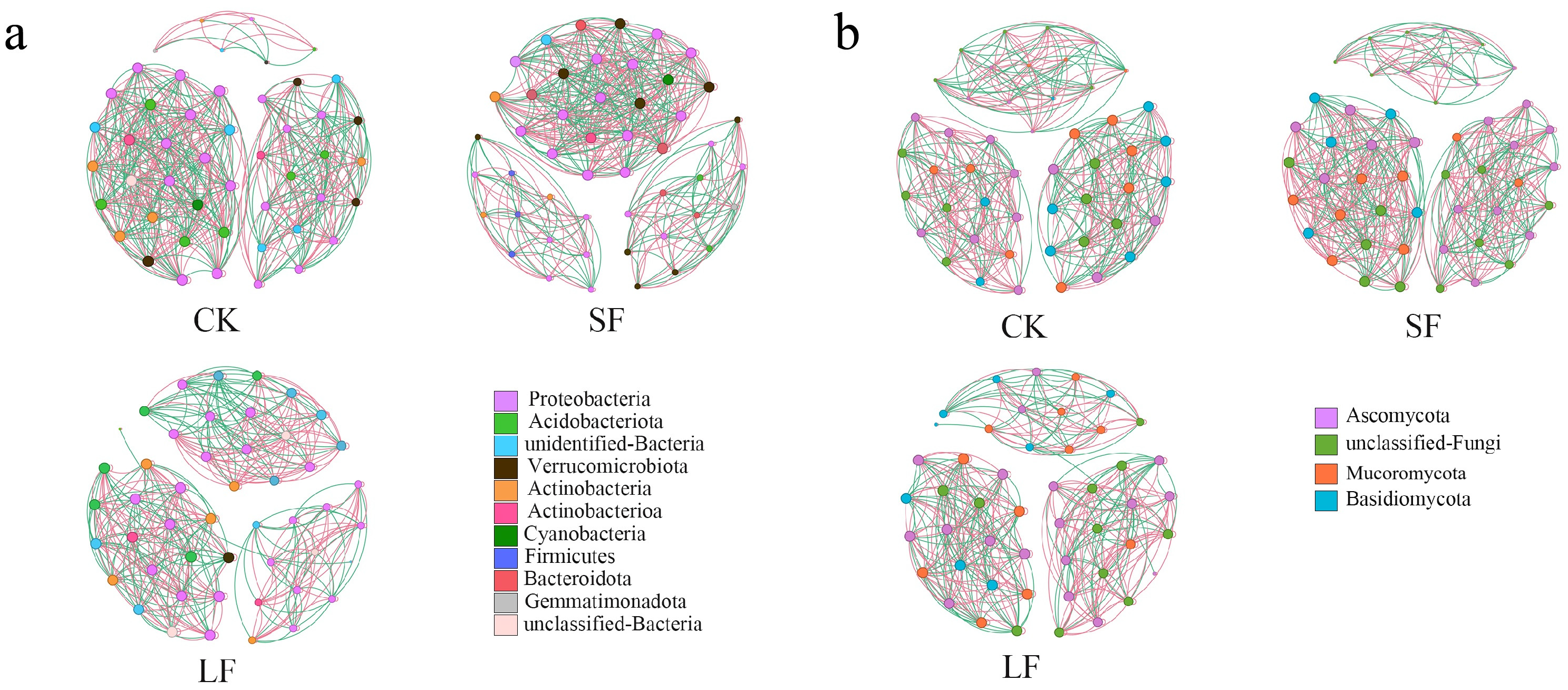
3.3.6. Network Stability Analysis
3.3.7. Analysis of Environmental Factors Affecting Microbial Communities
4. Discussion
4.1. Effects of Growth-Promoting Fungi on Root Exudates
4.2. Effects of Growth-Promoting Fungi on Soil Chemical Properties
4.3. Effects of Growth-Promoting Fungi on Rhizosphere Soil Microbial Community Structure
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mancuso, C. Panax notoginseng: Pharmacological Aspects and Toxicological Issues. Nutrients 2024, 16, 2120. [Google Scholar] [CrossRef]
- Pu, R.; Cheng, Y.; Zeng, J.; Wang, H.; Li, N.; Gao, M.; Ma, J.; Cui, X. Metabolomic and transcriptomic analysis of Panax Notoginseng root: Mechanistic insights into the effects of flower bud removal on yield and phytochemical composition. Ind. Crops Prod. 2025, 223, 120138. [Google Scholar] [CrossRef]
- Li, Y.; Dai, S.; Wang, B.; Jiang, Y.; Ma, Y.; Pan, L.; Wu, K.; Huang, X.; Zhang, J.; Cai, Z.; et al. Autotoxic Ginsenoside Disrupts Soil Fungal Microbiomes by Stimulating Potentially Pathogenic Microbes. Appl. Environ. Microbiol. 2020, 86, e00130-20. [Google Scholar] [CrossRef]
- Salvi, P.; Mahawar, H.; Agarrwal, R.; Kajal; Gautam, V.; Deshmukh, R. Advancement in the molecular perspective of plant-endophytic interaction to mitigate drought stress in plants. Front. Microbiol. 2022, 13, 981355. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-G.; Zhang, F.-M.; Yang, T.; Chen, Y.; Li, X.-G.; Dai, C.-C. Endophytic Fungus Drives Nodulation and N2 Fixation Attributable to Specific Root Exudates. mBio 2019, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef]
- Jha, P.; Kaur, T.; Chhabra, I.; Panja, A.; Paul, S.; Kumar, V.; Malik, T. Endophytic fungi: Hidden treasure chest of antimicrobial metabolites interrelationship of endophytes and metabolites. Front. Microbiol. 2023, 14, 1227830. [Google Scholar] [CrossRef]
- Pellissier, L.; Gaudry, A.; Vilette, S.; Lecoultre, N.; Rutz, A.; Allard, P.-M.; Marcourt, L.; Ferreira Queiroz, E.; Chave, J.; Eparvier, V.; et al. Comparative metabolomic study of fungal foliar endophytes and their long-lived host Astrocaryum sciophilum: A model for exploring the chemodiversity of host-microbe interactions. Front. Plant Sci. 2023, 14, 1278745. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.M.; Sun, Y.B.; Zhang, Z.S.; Jin, C.X. Isolation of Endophytic Bacteria from Panax notoginseng and Optimization of Fermentation Conditions for Saponin Production. J. Dalian Polytech. Univ. 2016, 35, 15–18. [Google Scholar]
- Huang, Y.; Dong, L.; Wei, G.; Zhang, X. Development and Evaluation of a Biocontrol Agent against Panax Root Rot. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 182–190. [Google Scholar]
- Zhang, J.L.; Mao, C.P. Isolation of Endophytic Actinomycetes from Panax notoginseng and Their Antagonistic Activity against Root Rot Pathogens of Panax notoginseng. J. Microbiol. 2023, 43, 85–91. [Google Scholar]
- Xu, Z.X.; Zhu, X.M.; Yin, H.; Li, B.; Chen, X.J.; Fan, X.L.; Li, N.Q.; Selosse, M.A.; Gao, J.Y.; Han, J.J. Symbiosis between Dendrobium catenatum protocorms and Serendipita indica involves the plant hypoxia response pathway. Plant Physiol. 2023, 192, 2554–2568. [Google Scholar] [CrossRef]
- Pu, L.F.; Du, W.B.; Wu, L.Y. Preliminary Studies on the Bioprophylactic and Growth-Promoting Effects of an Endophytic Fungus of Lycium barbarum. J. Org. Chem. Res. 2024, 12, 582–588. [Google Scholar] [CrossRef]
- Sanneboyina, N.; Audipudi, A.V. Fungal Antagonism and Plant Growth Promoting Efficacy of Aspergillus oryzaeAVNF4 isolated from the Rhizome of Curcuma longa on Lycopersicum esculantum L. (tomato). Curr. Agri. Res. 2024, 12, 1153. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Fadiji, A.E.; Ayilara, M.S.; Igiehon, O.N.; Nwachukwu, B.C.; Babalola, O.O. Strategies to Enhance the Use of Endophytes as Bioinoculants in Agriculture. Horticulturae 2022, 8, 498. [Google Scholar] [CrossRef]
- Asiloglu, R.; Bodur, S.O.; Samuel, S.O.; Aycan, M.; Murase, J.; Harada, N. Trophic modulation of endophytes by rhizosphere protists. ISME J. 2024, 18, wrae235. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Kailoo, S.; Khan, R.T.; Khan, S.S.; Rasool, S. Harnessing fungal endophytes for natural management: A biocontrol perspective. Front. Microbiol. 2023, 14, 1280258. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Z.; White, J.F.; Malik, K.; Li, C. Interactions between Epichloë endophyte and the plant microbiome impact nitrogen responses in host Achnatherum inebrians plants. Microbiol. Spectr. 2024, 12, e0257423. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, C.; Wang, X.; Zhang, J.; Wang, A.; Yang, Y.; Jia, X.; Zhang, W.; Zhao, M. The control effect of endophytic fungus Fusarium equiseti FUS-8 on cotton Verticillium wilt and its effects on soil microbial communities. Plant Soil 2024, 512, 1221–1241. [Google Scholar] [CrossRef]
- Seema, N.; Hamayun, M.; Ara, H.; Khan, R.S. Inoculation of Sunflower with Endophytic Fungi Alters Soil Physio-Chemical Properties to Nullify Drought Stress. Sarhad J. Agric. 2019, 35, 109–115. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ra, C.H. Comparison of Liquid and Solid-State Fermentation Processes for the Production of Enzymes and Beta-Glucan from Hulled Barley. J. Microbiol. Biotechnol. 2022, 32, 317–323. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, M.Z.; Ling, H.R.; Wang, Z.T.; Gai, J.Y. A Hyperspectral Image-Based Method for Estimating Water and Chlorophyll Contents in Maize Leaves Under Drought Stress. Smart Agric. 2023, 5, 142–153. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Yin, G.; Wang, L.; Jin, Y.; Huang, Y.; Bi, K.; Lu, Y.; Wang, T. The Study of Steaming Durations and Temperatures on the Chemical Characterization, Neuroprotective, and Antioxidant Activities of Panax notoginseng. Evid.-Based Complement. Altern. Med. 2022, 2022, 3698518. [Google Scholar] [CrossRef]
- Baker, N.R.; Zhalnina, K.; Yuan, M.; Herman, D.; Ceja-Navarro, J.A.; Sasse, J.; Jordan, J.S.; Bowen, B.P.; Wu, L.; Fossum, C.; et al. Nutrient and moisture limitations reveal keystone metabolites linking rhizosphere metabolomes and microbiomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2303439121. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Wu, Y.; Long, X.-N.; You, X.-X.; Chen, D.; Bi, Y.; He, S.; Cao, G.-H. Widely Targeted Metabolomic Analysis Reveals the Improvement in Panax notoginseng Triterpenoids Triggered by Arbuscular Mycorrhizal Fungi via UPLC–ESI–MS/MS. Molecules 2024, 29, 3235. [Google Scholar] [CrossRef]
- Hossen, M.A.; Diwakar, P.K.; Ragi, S. Total nitrogen estimation in agricultural soils via aerial multispectral imaging and LIBS. Sci. Rep. 2021, 11, 12693. [Google Scholar] [CrossRef]
- Bao, S.D. Oil Agricultural Chemistry Analysis (M); China Agriculture Press: Beijing, China, 2000; pp. 34–36, 45–50, 55–59, 80–81, 106. [Google Scholar]
- Liu, J.; Dong, Z.; Xia, J.; Wang, H.; Meng, T.; Zhang, R.; Han, J.; Wang, N.; Xie, J. Estimation of soil organic matter content based on CARS algorithm coupled with random forest. Spectrochim. Acta A 2021, 258, 119823. [Google Scholar] [CrossRef]
- Faria, M.; Bertocco, T.; Barroso, A.; Carvalho, M.; Fonseca, F.; Delerue Matos, C.; Figueiredo, T.; Sequeira Braga, A.; Valente, T.; Jiménez-Ballesta, R. A Comparison of Analytical Methods for the Determination of Soil pH: Case Study on Burned Soils in Northern Portugal. Fire 2023, 6, 227. [Google Scholar] [CrossRef]
- Wang, B.; Geng, Y.; Lin, Y.; Xia, Q.; Wei, F.; Yang, S.; Huang, X.; Zhang, J.; Cai, Z.; Zhao, J. Root rot destabilizes the Sanqi rhizosphere core fungal microbiome by reducing the negative connectivity of beneficial microbes. Appl. Environ. Microbiol. 2024, 90, e0223723. [Google Scholar] [CrossRef]
- Fu, X.; Fu, Q.; Zhu, X.; Yang, X.; Chen, H.; Li, S. Microdiversity sustains the distribution of rhizosphere-associated bacterial species from the root surface to the bulk soil region in maize crop fields. Front. Plant Sci. 2023, 14, 1266218. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Keren, G.; Yehezkel, G.; Satish, L.; Adamov, Z.; Barak, Z.; Ben-Shabat, S.; Kagan-Zur, V.; Sitrit, Y. Root-secreted nucleosides: Signaling chemoattractants of rhizosphere bacteria. Front. Plant Sci. 2024, 15, 1388384. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, D.; Zhao, P.; Dou, M.; Yang, X.; Liu, S.; Nian, F.; Tong, W.; Li, J.; Xu, Z.; et al. Intercropping of tobacco and maize at seedling stage promotes crop growth through manipulating rhizosphere microenvironment. Front. Plant Sci. 2024, 15, 1470229. [Google Scholar] [CrossRef]
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Mechanisms and Impact of Rhizosphere Microbial Metabolites on Crop Health, Traits, Functional Components: A Comprehensive Review. Molecules 2024, 29, 5922. [Google Scholar] [CrossRef]
- Maitra, P.; Hrynkiewicz, K.; Szuba, A.; Jagodziński, A.M.; Al-Rashid, J.; Mandal, D.; Mucha, J. Metabolic niches in the rhizosphere microbiome: Dependence on soil horizons, root traits and climate variables in forest ecosystems. Front. Plant Sci. 2024, 15, 1344205. [Google Scholar] [CrossRef]
- Fite, T.; Kebede, E.; Tefera, T.; Bekeko, Z. Endophytic fungi: Versatile partners for pest biocontrol, growth promotion, and climate change resilience in plants. Front. Sustain. Food Syst. 2023, 7, 1322861. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- Liswadiratanakul, S.; Yamamoto, K.; Matsutani, M.; Wattanadatsaree, V.; Kihara, S.; Shiwa, Y.; Shiwachi, H. Replacement of water yam (Dioscorea alata L.) indigenous root endophytes and rhizosphere bacterial communities via inoculation with a synthetic bacterial community of dominant nitrogen-fixing bacteria. Front. Microbiol. 2023, 14, 1060239. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, K.; Wen, W.; Li, L.; Xu, D. Functional Endophytes Regulating Plant Secondary Metabolism: Current Status, Prospects and Applications. Int. J. Mol. Sci. 2023, 24, 1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, L.; Sun, J.; Yang, Y.; Qu, Y.; Wang, C.; Liu, D.; Huang, L.; Cui, X.; Liu, Y. Structure and Function of Rhizosphere Soil and Root Endophytic Microbial Communities Associated with Root Rot of Panax notoginseng. Front. Plant Sci. 2021, 12, 752683. [Google Scholar] [CrossRef]
- Souza, A.A.; Leitão, V.O.; Ramada, M.H.; Mehdad, A.; Georg Rde, C.; Ulhôa, C.J.; de Freitas, S.M. Trichoderma harzianum Produces a New Thermally Stable Acid Phosphatase, with Potential for Biotechnological Application. PLoS ONE 2016, 11, e0150455. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Wei, J.; Qi, S.; Li, J.; Jin, Y.; Luan, X.; Li, P.; Yan, J. Effect of Purpureocillium lilacinum on inter-root soil microbial community and metabolism of tobacco. Ann. Microbiol. 2023, 73, 30. [Google Scholar] [CrossRef]
- Mousumi Das, M.; Haridas, M.; Sabu, A. Process development for the enhanced production of bio-nematicide Purpureocillium lilacinum KU8 under solid-state fermentation. Bioresour. Technol. 2020, 308, 123328. [Google Scholar] [CrossRef]
- Liu, H.; Su, Y.; Ye, C.; Zuo, D.; Wang, L.; Mei, X.; Deng, W.; Liu, Y.; Huang, H.; Hao, J.; et al. Nucleotides enriched under heat stress recruit beneficial rhizomicrobes to protect plants from heat and root-rot stresses. Microbiome 2025, 13, 160. [Google Scholar] [CrossRef]
- Sun, M.; Dai, P.; Cao, Z.; Dong, J. Purine metabolism in plant pathogenic fungi. Front. Microbiol. 2024, 15, 1352354. [Google Scholar] [CrossRef]
- Lee, D.; Park, T.-H.; Lim, K.; Jeong, M.; Nam, G.; Kim, W.-C.; Shin, J.-H. Biofumigation-Derived Soil Microbiome Modification and Its Effects on Tomato (Solanum lycopersicum L.) Health under Drought. Agronomy 2024, 14, 2225. [Google Scholar] [CrossRef]
- Wang, S.; Huo, J.; Wu, D.; Li, J.; Chen, X.; Hu, F.; Liu, M. Earthworms increase soil carbon dioxide emissions through changing microbial community structure and activity under high nitrogen addition. Appl. Soil Ecol. 2024, 196, 105297. [Google Scholar] [CrossRef]
- Elmaghraby, M.M.K.; Abdellatif, A.A.M.; Amer, M.N.; Sahu, P.K. Enhancing Productivity Through Multiple Microbial Inoculants. In Metabolomics, Proteomes and Gene Editing Approaches in Biofertilizer Industry; Kaur, S., Dwibedi, V., Sahu, P.K., Kocher, G.S., Eds.; Springer Nature: Singapore, 2023; pp. 117–137. [Google Scholar]
- Kiprotich, K.; Muoma, J.; Omayio, D.O.; Ndombi, T.S.; Wekesa, C. Molecular Characterization and Mineralizing Potential of Phosphorus Solubilizing Bacteria Colonizing Common Bean (Phaseolus vulgaris L.) Rhizosphere in Western Kenya. Int. J. Microbiol. 2023, 2023, 6668097. [Google Scholar] [CrossRef]
- Shi, H.; Lu, L.; Ye, J.; Shi, L. Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana. Int. J. Mol. Sci. 2022, 23, 13639. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, L.; Chou, Z.; Deng, S.; Tang, J.; Xiang, W.; Chen, X.; Li, Y. Efficient cadmium-resistant plant growth-promoting bacteria loaded on pig bone biochar has higher efficiency in reducing cadmium phytoavailability and improving maize performance than on rice husk biochar. J. Hazard. Mater. 2024, 479, 135609. [Google Scholar] [CrossRef]
- Shao, D.; He, Y.; Zhai, Y.; Yang, X.; Guo, Z.; Tan, J.; Wei, M. Mechanisms of tomato growth promotion in three soils after applying Bacillus combinations. Soil Tillage Res. 2025, 249, 106477. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, E.; Yang, X.; Yang, J. Shifts in Microbial Community Structure and Co-occurrence Network along a Wide Soil Salinity Gradient. Microorganisms 2024, 12, 1268. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Q.; Zhu, M.; Zhang, J.; Yang, L.; Li, R. The Impact of Artificial Restoration of Alpine Grasslands in the Qilian Mountains on Vegetation, Soil Bacteria, and Soil Fungal Community Diversity. Microorganisms 2024, 12, 854. [Google Scholar] [CrossRef]
- Kong, J.; He, Z.; Chen, L.; Yang, R.; Du, J. Efficiency of biochar, nitrogen addition, and microbial agent amendments in remediation of soil properties and microbial community in Qilian Mountains mine soils. Ecol. Evol. 2021, 11, 9318–9331. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Q.; Guo, Y.; Zhang, Y.; Wang, C.; Zhou, Q.; Wu, Z. PGPR Promotes the Recovery of Submerged Macrophytes via Indigenous Microbiome Modulations under Combined Abiotic Stress. Water 2023, 15, 590. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen Biocontrol Using Plant Growth-Promoting Bacteria (PGPR): Role of Bacterial Diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | Total Potassium (g/kg) | Ammonium Nitrogen (mg/kg) | Nitrate Nitrogen (mg/kg) | Organic Matter (g/kg) | pH |
|---|---|---|---|---|---|---|---|
| LF | 1.118 ± 0.05 b | 1.388 ± 0.01 a | 12.124 ± 0.03 a | 8.048 ± 0.34 a | 57.174 ± 7.8 ab | 16.09 ± 0.44 a | 6.647 ± 0.12 a |
| SF | 1.32 ± 0.02 a | 1.395 ± 0.01 a | 12.104 ± 0.03 a | 9.102 ± 0.98 a | 63.9 ± 3.82 a | 13.702 ± 1.35 ab | 6.413 ± 0.18 a |
| CK | 1.09 ± 0.02 b | 1.388 ± 0.01 a | 12.298 ± 0.03 a | 8.69 ± 2.05 a | 45.316 ± 9.59 b | 13.691 ± 0.9 b | 6.01 ± 0.19 b |
| Microbe | Treatment | Richness Index | Diversity Index | Degree of Coverage | ||
|---|---|---|---|---|---|---|
| Chao1 | ACE | Shannon | Simpson | |||
| bacterial | LF | 1684.1727 ± 38.7319 a | 1689.1887 ± 36.5441 a | 6.2883 ± 0.0015 a | 0.993 ± 0 a | 0.9967 ± 0.0006 a |
| SF | 1736.6377 ± 40.9896 a | 1741.3617 ± 37.73 a | 6.3973 ± 0.087 a | 0.9933 ± 0.0012 a | 0.9967 ± 0.0006 a | |
| CK | 1540.1837 ± 50.6641 b | 1546.4637 ± 49.9319 b | 6.1697 ± 0.0424 b | 0.991 ± 0 b | 0.9967 ± 0.0006 a | |
| fungi | LF | 340.985 ± 4.2508 b | 341.9047 ± 4.3635 b | 3.7527 ± 0.0533 b | 0.9483 ± 0.0012 b | 1 |
| SF | 375.525 ± 3.5996 a | 375.8447 ± 3.5807 a | 3.9627 ± 0.0482 a | 0.956 ± 0.003 a | 1 | |
| CK | 324.9853 ± 4.7512 c | 325.9927 ± 4.7811 c | 3.8853 ± 0.052 a | 0.9583 ± 0.0025 a | 1 | |
| Topological Properties | CK | LF | SF | Topological Properties | CK | LF | SF |
|---|---|---|---|---|---|---|---|
| Node | 48 | 48 | 49 | Network diameter | 1 | 1 | 1 |
| Edge | 388 | 492 | 469 | Component | 6 | 5 | 3 |
| Negative correlations | 40.5% | 45.5% | 44% | Modularity | 3.047 | 7.251 | 2.853 |
| Average degree | 15.6 | 20.5 | 19.14 | Average clustering coefficient | 0.973 | 0.981 | 0.979 |
| Graph density | 0.318 | 0.436 | 0.399 | Average path | 1 | 1 | 1 |
| Topological Properties | CK | LF | SF | Topological Properties | CK | LF | SF |
|---|---|---|---|---|---|---|---|
| Node | 48 | 49 | 50 | Network diameter | 1 | 1 | 1 |
| Edge | 388 | 429 | 468 | Component | 6 | 4 | 3 |
| Negative correlations | 40.5% | 43% | 40.6% | Modularity | 3.155 | 6.115 | 2.026 |
| Average degree | 15.6 | 17.2 | 18.7 | Average clustering coefficient | 0.973 | 0.974 | 0.977 |
| Graph density | 0.318 | 0.351 | 0.382 | Average path | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, J.; Sun, Y.; Wang, M.; Liu, S.; Ma, Y.; Lu, J. Effects of Endophytic Fungus Setophoma terrestris on Growth of Panax notoginseng and Its Rhizosphere Soil Microorganisms. Life 2025, 15, 1353. https://doi.org/10.3390/life15091353
Li H, Liu J, Sun Y, Wang M, Liu S, Ma Y, Lu J. Effects of Endophytic Fungus Setophoma terrestris on Growth of Panax notoginseng and Its Rhizosphere Soil Microorganisms. Life. 2025; 15(9):1353. https://doi.org/10.3390/life15091353
Chicago/Turabian StyleLi, Huali, Jian Liu, Yajiao Sun, Mengyao Wang, Shuwen Liu, Yunqiang Ma, and Junjia Lu. 2025. "Effects of Endophytic Fungus Setophoma terrestris on Growth of Panax notoginseng and Its Rhizosphere Soil Microorganisms" Life 15, no. 9: 1353. https://doi.org/10.3390/life15091353
APA StyleLi, H., Liu, J., Sun, Y., Wang, M., Liu, S., Ma, Y., & Lu, J. (2025). Effects of Endophytic Fungus Setophoma terrestris on Growth of Panax notoginseng and Its Rhizosphere Soil Microorganisms. Life, 15(9), 1353. https://doi.org/10.3390/life15091353






