Hepatic T1 Mapping in Takotsubo Syndrome: A Preliminary Imaging Insight into the Cardiohepatic Axis
Abstract
1. Introduction
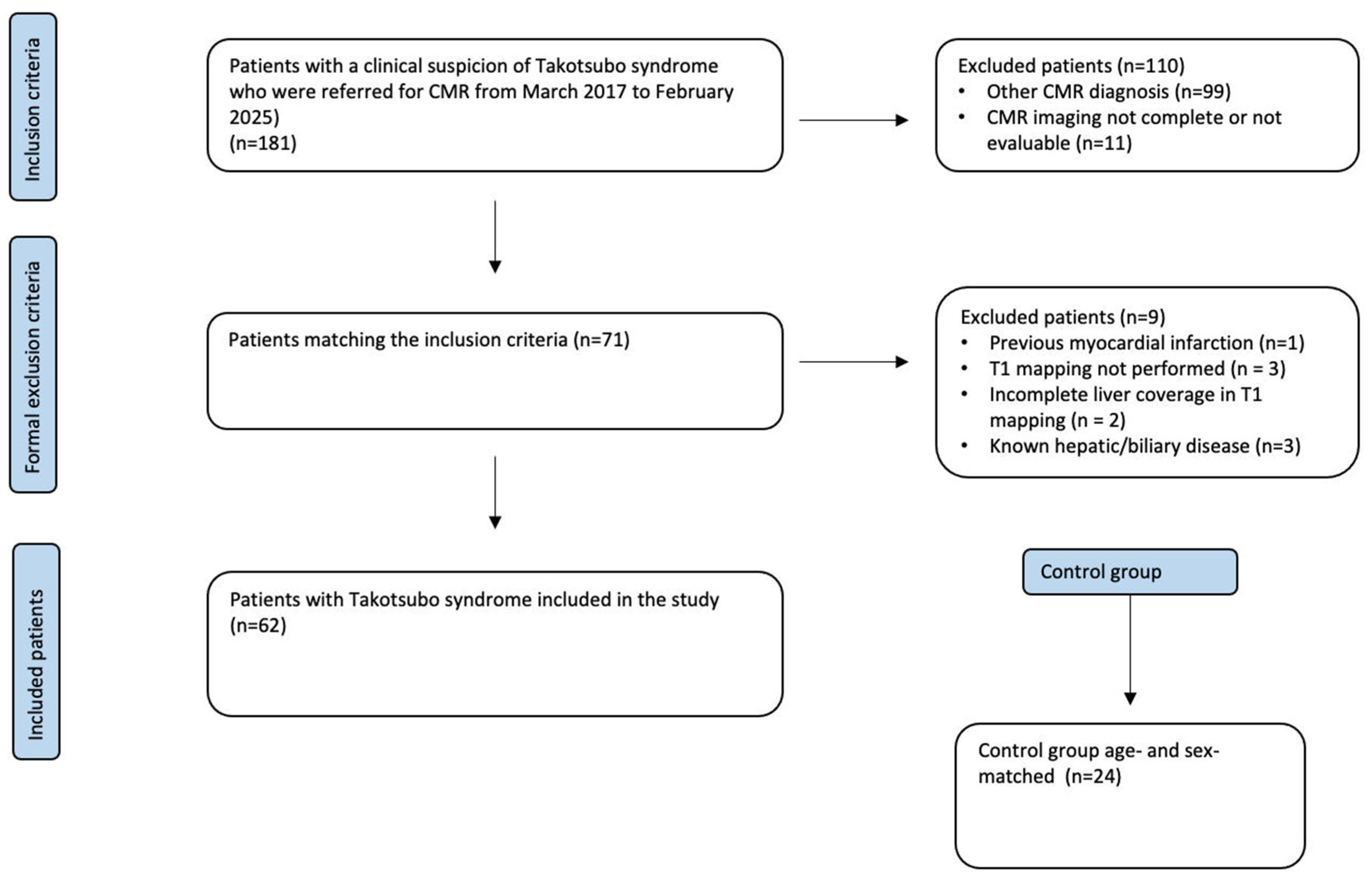
2. Materials and Methods
2.1. Study Population
2.2. CMR Acquisition
2.3. CMR Image Post-Processing
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics, Clinical, and CMR Parameters in TS
3.2. Hepatic T1 Mapping and Its Determinants in TS Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Cau, R.; Masala, S.; Manelli, L.; Porcu, M.; Scaglione, M.; D’ANgelo, T.; Salgado, R.; Saba, L. Cardiovascular Magnetic Resonance Imaging of Takotsubo Syndrome: Evolving Diagnostic and Prognostic Perspectives. Echocardiography 2024, 41, e15949. [Google Scholar] [CrossRef]
- Cau, R.; Loewe, C.; Cherchi, V.; Porcu, M.; Ciet, P.; Suri, J.S.; Saba, L. Atrial Impairment as a Marker in Discriminating Between Takotsubo and Acute Myocarditis Using Cardiac Magnetic Resonance. J. Thorac. Imaging 2022, 37, W78–W84. [Google Scholar] [CrossRef] [PubMed]
- Madias, J.E. Cardiac takotsubo syndrome in association with cerebral, renal, gastrointestinal, vascular, and perhaps total body, “takotsubo” syndrome? J. Neurol. Sci. 2017, 378, 238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, J.; Hu, X. The Brain-Heart Connection in Takotsubo Syndrome: The Central Nervous System, Sympathetic Nervous System, and Catecholamine Overload. Cardiol. Res. Pract. 2020, 2020, 4150291. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stollberger, C. Is the Takotsubo syndrome a brain-heart or multiorgan disorder? J. Neurol. Sci. 2017, 378, 239–240. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Mascherbauer, K.; Donà, C.; Koschutnik, M.; Dannenberg, V.; Nitsche, C.; Duca, F.; Heitzinger, G.; Halavina, K.; Steinacher, E.; Kronberger, C.; et al. Hepatic T1-Time Predicts Cardiovascular Risk in All-Comers Referred for Cardiovascular Magnetic Resonance: A Post-Hoc Analysis. Circ. Cardiovasc. Imaging 2022, 15, 721–732. [Google Scholar] [CrossRef]
- Reis Santos, D.R.; Sousa Paiva, D.M.; Freitas, D.P.; Maltes, S.; Carvalho, R.; Pereira, J.C.; Domingues, M.; Santos, A.C.; Silva, C.; Guerreiro, S.; et al. Hepatic t1 mapping: A new easily obtained biomarker for heart failure patients undergoing cardiac magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2023, 24 (Suppl. 1), jead119-064. [Google Scholar] [CrossRef]
- Capone, F.; Vacca, A.; Bidault, G.; Sarver, D.; Kaminska, D.; Strocchi, S.; Vidal-Puig, A.; Greco, C.M.; Lusis, A.J.; Schiattarella, G.G. Decoding the Liver-Heart Axis in Cardiometabolic Diseases. Circ. Res. 2025, 136, 1335–1362. [Google Scholar] [CrossRef]
- Jackson, E.; Dennis, A.; Alkhouri, N.; Samala, N.; Vuppalanchi, R.; Sanyal, A.J.; Muthiah, M.; Banerjee, R.; Banerjee, A. Cardiac and liver impairment on multiorgan MRI and risk of major adverse cardiovascular and liver events. Nat. Med. 2025, 31, 2289–2296. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Arangalage, D.; Maurizi, N.; Pizzi, C.; Valgimigli, M.; Iglesias, J.F.; Landi, A.; Leo, L.A.; Eeckhout, E.; Schwitter, J.; et al. Hepatic T1 mapping as a novel cardio-hepatic axis imaging biomarker early after ST-elevation myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2024, 26, 229–238. [Google Scholar] [CrossRef]
- Cau, R.; Palmisano, A.; Suri, J.S.; Pisu, F.; Esposito, A.; Saba, L. Prognostic role of cardiovascular magnetic resonance in Takotsubo syndrome: A systematic review. Eur. J. Radiol. 2024, 177, 111576. [Google Scholar] [CrossRef] [PubMed]
- Jensch, P.J.; Stiermaier, T.; Eitel, I. Takotsubo Syndrome—Is There a Need for CMR? Curr. Heart Fail. Rep. 2021, 18, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Busch, K.; Lange, T.; Pätz, T.; Meusel, M.; Backhaus, S.J.; Frydrychowicz, A.; Barkhausen, J.; Gutberlet, M.; Thiele, H.; et al. Prognostic Value of Different CMR-Based Techniques to Assess Left Ventricular Myocardial Strain in Takotsubo Syndrome. J. Clin. Med. 2020, 9, 3882. [Google Scholar] [CrossRef]
- Arcari, L.; Camastra, G.; Ciolina, F.; Limite, L.R.; Danti, M.; Sclafani, M.; Ansalone, G.; Musumeci, M.B.; Nagel, E.; Puntmann, V.; et al. Myocardial oedema contributes to interstitial expansion and associates with mechanical and electrocardiographic changes in takotsubo syndrome: A CMR T1 and T2 mapping study. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Bratis, K. Cardiac Magnetic Resonance in Takotsubo Syndrome. Eur. Cardiol. Rev. 2017, 12, 58–62. [Google Scholar] [CrossRef]
- Bossone, E.; Lyon, A.; Citro, R.; Athanasiadis, A.; Meimoun, P.; Parodi, G.; Cimarelli, S.; Omerovic, E.; Ferrara, F.; Limongelli, G.; et al. Takotsubo cardiomyopathy: An integrated multi-imaging approach. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 366–377. [Google Scholar] [CrossRef]
- Eitel, I.; Lücke, C.; Grothoff, M.; Sareban, M.; Schuler, G.; Thiele, H.; Gutberlet, M. Inflammation in takotsubo cardiomyopathy: Insights from cardiovascular magnetic resonance imaging. Eur. Radiol. 2010, 20, 422–431. [Google Scholar] [CrossRef]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Dabir, D.; Luetkens, J.; Kuetting, D.L.R.; Feisst, A.; Isaak, A.; Schild, H.H.; Thomas, D. Cardiac magnetic resonance including parametric mapping in acute Takotsubo syndrome: Preliminary findings. Eur. J. Radiol. 2019, 113, 217–224. [Google Scholar] [CrossRef]
- Aikawa, Y.; Noguchi, T.; Morita, Y.; Tateishi, E.; Kono, A.; Miura, H.; Komori, Y.; Asaumi, Y.; Fukuda, T.; Yasuda, S. Clinical impact of native T1 mapping for detecting myocardial impairment in takotsubo cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1147–1155. [Google Scholar] [CrossRef]
- Arcari, L.; Camastra, G.; Ciolina, F.; Belmonte, E.; De Santis, D.; Danti, M.; Caruso, D.; Maestrini, V.; Santoro, F.; Brunetti, N.D.; et al. Cardiac magnetic resonance in patients with Takotsubo syndrome: Clinical correlates of T2 mapping. Int. J. Cardiol. 2025, 419, 132716. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, J.; Symons, R.; Rafouli-Stergiou, P.; Droogné, W.; Dresselaers, T.; Masci, P.G. Assessment of Right-Sided Heart Failure in Patients with Dilated Cardiomyopathy using Magnetic Resonance Relaxometry of the Liver. Am. J. Cardiol. 2021, 149, 103–111. [Google Scholar] [CrossRef]
- Bogaert, J.; Claessen, G.; Dresselaers, T.; Masci, P.G.; Belge, C.; Delcroix, M.; Symons, R. Magnetic resonance relaxometry of the liver—A new imaging biomarker to assess right heart failure in pulmonary hypertension. J. Heart Lung Transplant. 2022, 41, 86–94. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Guglielmo, M.; Serra, A.; Gatti, M.; Volpato, V.; Schoepf, U.J.; Saba, L.; Cau, R.; Faletti, R.; McGill, L.J.; et al. Multimodality Imaging in Ischemic Chronic Cardiomyopathy. J. Imaging 2022, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- World Health Organization Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia; WHO: Geneva, Switzerland, 2006.
- Flegal, K.M.; Carroll, M.D.; Kuczmarski, R.J.; Johnson, C.L. Overweight and obesity in the United States: Prevalence and trends, 1960–1994. Int. J. Obes. 1998, 22, 39–47. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef]
- Cau, R.; Bassareo, P.; Cademartiri, F.; Cadeddu, C.; Balestrieri, A.; Mannelli, L.; Suri, J.S.; Saba, L. Epicardial fat volume assessed with cardiac magnetic resonance imaging in patients with Takotsubo cardiomyopathy. Eur. J. Radiol. 2023, 160, 110706. [Google Scholar] [CrossRef]
- Ostovaneh, M.R.; Ambale-Venkatesh, B.; Fuji, T.; Bakhshi, H.; Shah, R.; Murthy, V.L.; Tracy, R.P.; Guallar, E.; Wu, C.O.; Bluemke, D.A.; et al. Association of Liver Fibrosis With Cardiovascular Diseases in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ. Cardiovasc. Imaging 2018, 11, e007241. [Google Scholar] [CrossRef]
- Huber, A.T.; Razakamanantsoa, L.; Lamy, J.; Giron, A.; Cluzel, P.; Kachenoura, N.; Redheuil, A. Multiparametric Differentiation of Idiopathic Dilated Cardiomyopathy With and Without Congestive Heart Failure by Means of Cardiac and Hepatic T1-Weighted MRI Mapping. Am. J. Roentgenol. 2020, 215, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kazour, I.; Serai, S.D.; Xanthakos, S.A.; Fleck, R.J. Using T1 mapping in cardiovascular magnetic resonance to assess congestive hepatopathy. Abdom. Radiol. 2018, 43, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Mihailovici, A.R.; Donoiu, I.; Gheonea, D.I.; Mirea, O.; Târtea, G.C.; Buşe, M.; Calborean, V.; Obleagă, C.; Pădureanu, V.; Istrătoaie, O. NT-proBNP and Echocardiographic Parameters in Liver Cirrhosis: Correlations with Disease Severity. Med. Princ. Pract. 2019, 28, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Haghi, D.; Athanasiadis, A.; Papavassiliu, T.; Suselbeck, T.; Fluechter, S.; Mahrholdt, H.; Borggrefe, M.; Sechtem, U. Right ventricular involvement in Takotsubo cardiomyopathy. Eur. Heart J. 2006, 27, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Kagiyama, N.; Okura, H.; Tamada, T.; Imai, K.; Yamada, R.; Kume, T.; Hayashida, A.; Neishi, Y.; Kawamoto, T.; Yoshida, K. Impact of right ventricular involvement on the prognosis of takotsubo cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 210–216. [Google Scholar] [CrossRef]
- Ong, G.J.; Nguyen, T.H.; Stansborough, J.; Surikow, S.; Mahadavan, G.; Worthley, M.; Horowitz, J. The N-AcetylCysteine and RAMipril in Takotsubo Syndrome Trial (NACRAM): Rationale and design of a randomised controlled trial of sequential N-Acetylcysteine and ramipril for the management of Takotsubo Syndrome. Contemp. Clin. Trials 2020, 90, 105894. [Google Scholar] [CrossRef]
- Ford, R.M.; Book, W.; Spivey, J.R. Liver disease related to the heart. Transplant. Rev. 2015, 29, 33–37. [Google Scholar] [CrossRef]
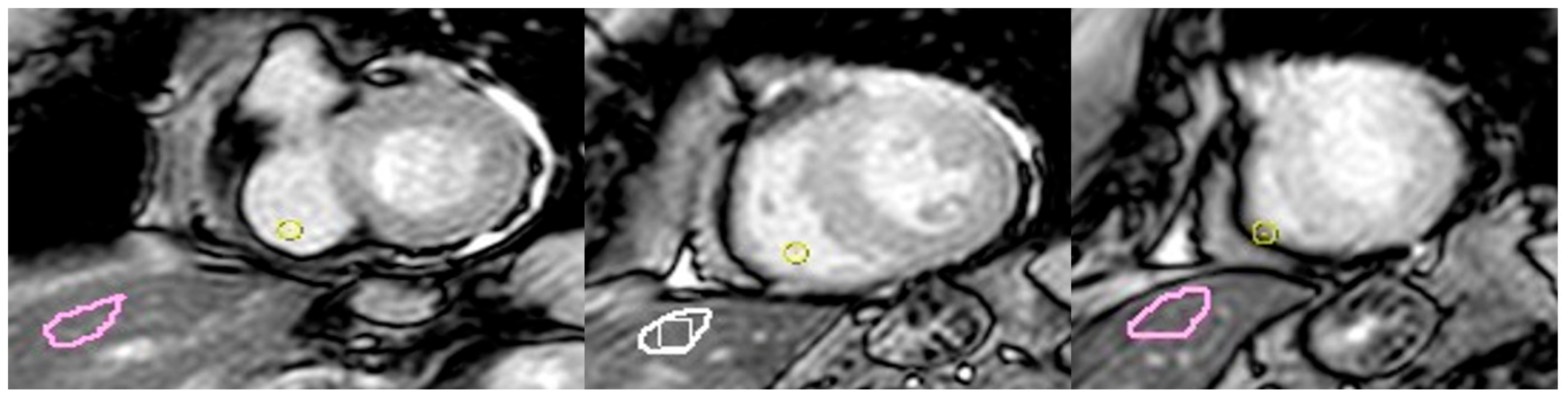
| Variables | Takotsubo | Control | p-Value |
|---|---|---|---|
| Sex (female), n (%) | 54 (87%) | 20 (83%) | 0.656 |
| Age (years) | 73.47 ± 9.88 | 69.67 ± 6.88 | 0.129 |
| Hypertension, n (%) | 33 (53%) | 15 (62%) | 0.063 |
| Dyslipidemia, n (%) | 18 (27%) | 7 (29%) | 0.060 |
| Smoke, n (%) | 9 (14%) | 4 (17%) | 0.634 |
| Obesity, n (%) | 6 (9%) | 2 (8%) | 0.800 |
| Diabetes, n (%) | 7 (11%) | 4 (16%) | 0.584 |
| Family History of CAD, n (%) | 10 (16%) | 3 (12%) | 0.122 |
| Triggers | |||
| Emotional Trigger, n (%) | 33 (53%) | / | / |
| Physical Trigger, n (%) | 22 (35%) | / | / |
| Lab data | |||
| Troponine | 3643.36 ± 2275.5 | / | / |
| NT-proBNP | 6631.55 ± 7025 | / | / |
| Variables | Takotsubo | Control | p-Value |
|---|---|---|---|
| LVEF, % | 49.49 ± 11.01 | 61.28 ± 6.42 | 0.001 |
| LVEDV/BSA, mL/m2 | 84.54 ± 40.17 | 84.63 ± 20 | 0.322 |
| LVESV/BSA, mL/m2 | 40.58 ± 24.60 | 33.63 ± 11.24 | 0.206 |
| LVSV/BSA, mL/m2 | 43.80 ± 19.37 | 50.99 ± 10.78 | 0.103 |
| RVEF, % | 58.23 ± 8.09 | 58.60 ± 7.09 | 0.860 |
| RVEDV/BSA, mL/m2 | 63.54 ± 25.43 | 73.67 ± 22.99 | 0.151 |
| RVESV/BSA, mL/m2 | 26.43 ± 11.45 | 33.45 ± 14.18 | 0.226 |
| RVSV/BSA, mL/m2 | 37.12 ± 15.67 | 41.16 ± 10.49 | 0.058 |
| GLS, % | −11.41 ± 4.33 | −15.5 ± 2.52 | 0.001 |
| GCS, % | −14.07 ± 6.11 | −16.85 ± 2.99 | 0.027 |
| GRS, % | 24,21 ± 9.84 | 28.10 ± 7.53 | 0.096 |
| RV GLS, % | −16.67 ± 6.33 | −19.97 ± 4.99 | 0.026 |
| Reservoir, % | 23.03 ± 8.69 | 32.24 ± 8.99 | 0.001 |
| Conduit, % | 11.43 ± 6.05 | 18.87 ± 6.46 | 0.001 |
| Booster, % | 11.74 ± 8.19 | 13.36 ± 4.82 | 0.599 |
| RA Reservoir, % | 28.76 ± 14.18 | 36.67 ± 11.90 | 0.020 |
| RA Conduit, % | 17.20 ± 10.23 | 21.06 ± 8.16 | 0.047 |
| RA Booster, % | 10.96 ± 9.99 | 15.54 ± 7.40 | 0.051 |
| T2 STIR, number of segments (n) | 7.45 ± 2.80 | / | / |
| LGE present, n (%) | 19 (30%) | / | / |
| T2 mapping, ms | 66.96 ± 5.83 | 53.18 ± 3.62 | 0.001 |
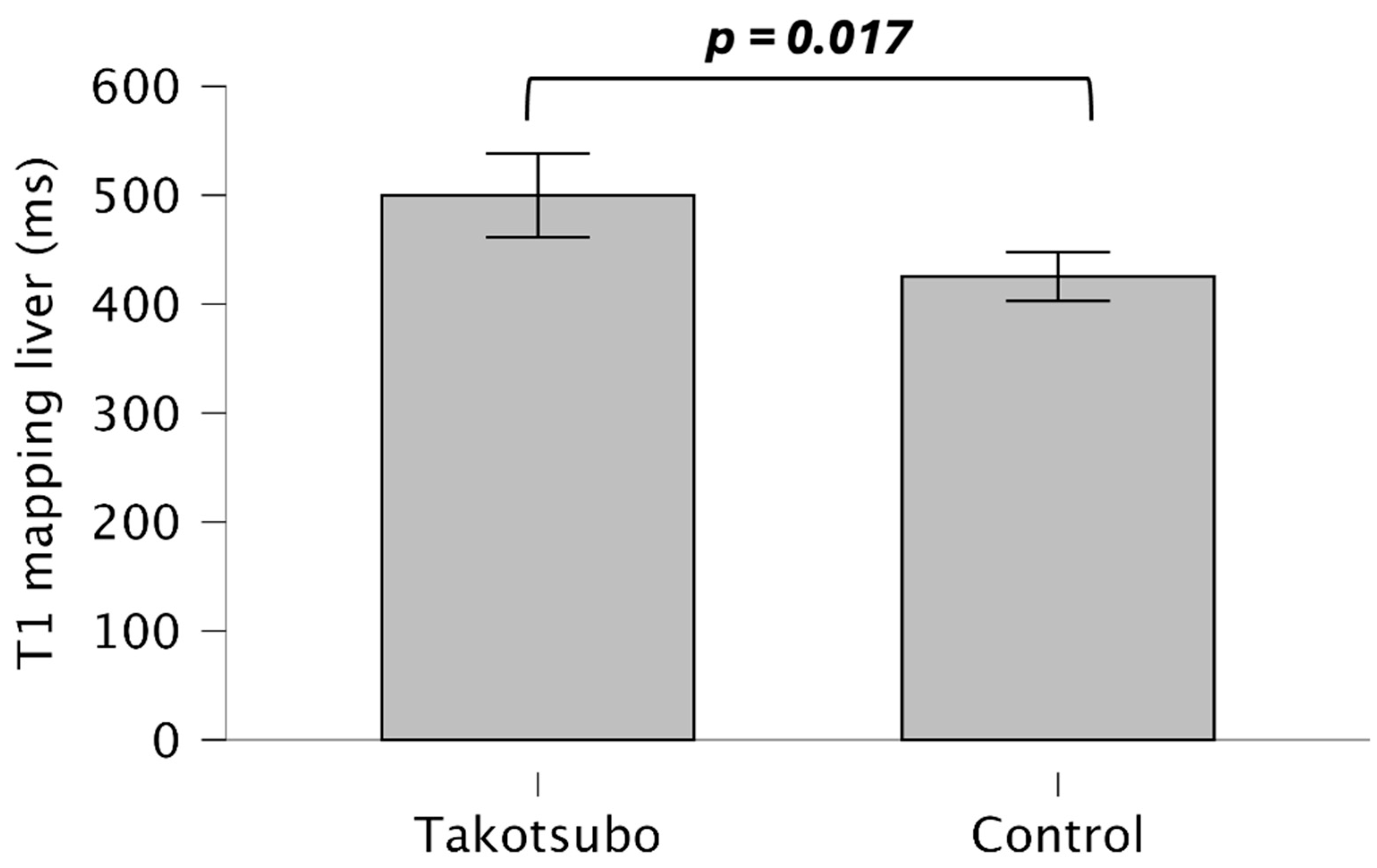
| T1 liver | 499.80 ± 141.86 | 425.26 ± 51.91 | 0.017 |
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| β Coefficient | p Values | β Coefficient | p Values | |
| Sex | 1.332 | 0.189 | ||
| Age | 1.006 | 0.319 | ||
| Hypertension | −0.252 | 0.802 | ||
| Dyslipidemia | 1.275 | 0.208 | ||
| Smoke | −1.240 | 0.221 | ||
| Obesity | −2.591 | 0.013 | −1.914 | 0.064 |
| Diabetes | −0.383 | 0.703 | ||
| Family History of CAD | 1.553 | 0.127 | ||
| Emotional Trigger | −0.255 | 0.823 | ||
| Physical Trigger | 0.428 | 0.672 | ||
| Troponine | −0.554 | 0.584 | ||
| NT-proBNP | 2.578 | 0.015 | 2.395 | 0.024 |
| LVEF, % | 0.621 | 0.537 | ||
| LVEDV/BSA, mL/m2 | 2.551 | 0.014 | 0.004 | 0.997 |
| LVESV/BSA, mL/m2 | 1.861 | 0.068 | ||
| LVSV/BSA, mL/m2 | 2.984 | 0.004 | 0.446 | 0.660 |
| RVEF, % | −0.760 | 0.450 | ||
| RVEDV/BSA, mL/m2 | 3.525 | 0.001 | 0.387 | 0.702 |
| RVESV/BSA, mL/m2 | 3.446 | 0.001 | 0.403 | 0.676 |
| RVSV/BSA, mL/m2 | 3.182 | 0.002 | 0.375 | 0.711 |
| GLS, % | −1.221 | 0.228 | ||
| GCS, % | −0.498 | 0.620 | ||
| GRS, % | 0.554 | 0.582 | ||
| RV GLS, % | 4.314 | 0.001 | 2.936 | 0.007 |
| Reservoir, % | −0.247 | 0.806 | ||
| Conduit, % | 1.235 | 0.222 | ||
| Booster, % | −1.040 | 0.303 | ||
| RA Reservoir, % | 1.410 | 0.164 | ||
| RA Conduit, % | 1.992 | 0.04 | 1.794 | 0.085 |
| RA Booster, % | −0.288 | 0.775 | ||
| T2 STIR segments | 0.572 | 0.570 | ||
| LGE | −0.315 | 0.730 | ||
| T2 mapping, ms | −0.284 | 0.807 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cau, R.; Pinna, A.; Marchetti, M.F.; Suri, J.S.; Montisci, R.; Saba, L. Hepatic T1 Mapping in Takotsubo Syndrome: A Preliminary Imaging Insight into the Cardiohepatic Axis. Life 2025, 15, 1335. https://doi.org/10.3390/life15091335
Cau R, Pinna A, Marchetti MF, Suri JS, Montisci R, Saba L. Hepatic T1 Mapping in Takotsubo Syndrome: A Preliminary Imaging Insight into the Cardiohepatic Axis. Life. 2025; 15(9):1335. https://doi.org/10.3390/life15091335
Chicago/Turabian StyleCau, Riccardo, Alessandro Pinna, Maria Francesca Marchetti, Jasjit S. Suri, Roberta Montisci, and Luca Saba. 2025. "Hepatic T1 Mapping in Takotsubo Syndrome: A Preliminary Imaging Insight into the Cardiohepatic Axis" Life 15, no. 9: 1335. https://doi.org/10.3390/life15091335
APA StyleCau, R., Pinna, A., Marchetti, M. F., Suri, J. S., Montisci, R., & Saba, L. (2025). Hepatic T1 Mapping in Takotsubo Syndrome: A Preliminary Imaging Insight into the Cardiohepatic Axis. Life, 15(9), 1335. https://doi.org/10.3390/life15091335








