Resmetirom in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis
Abstract
1. Introduction
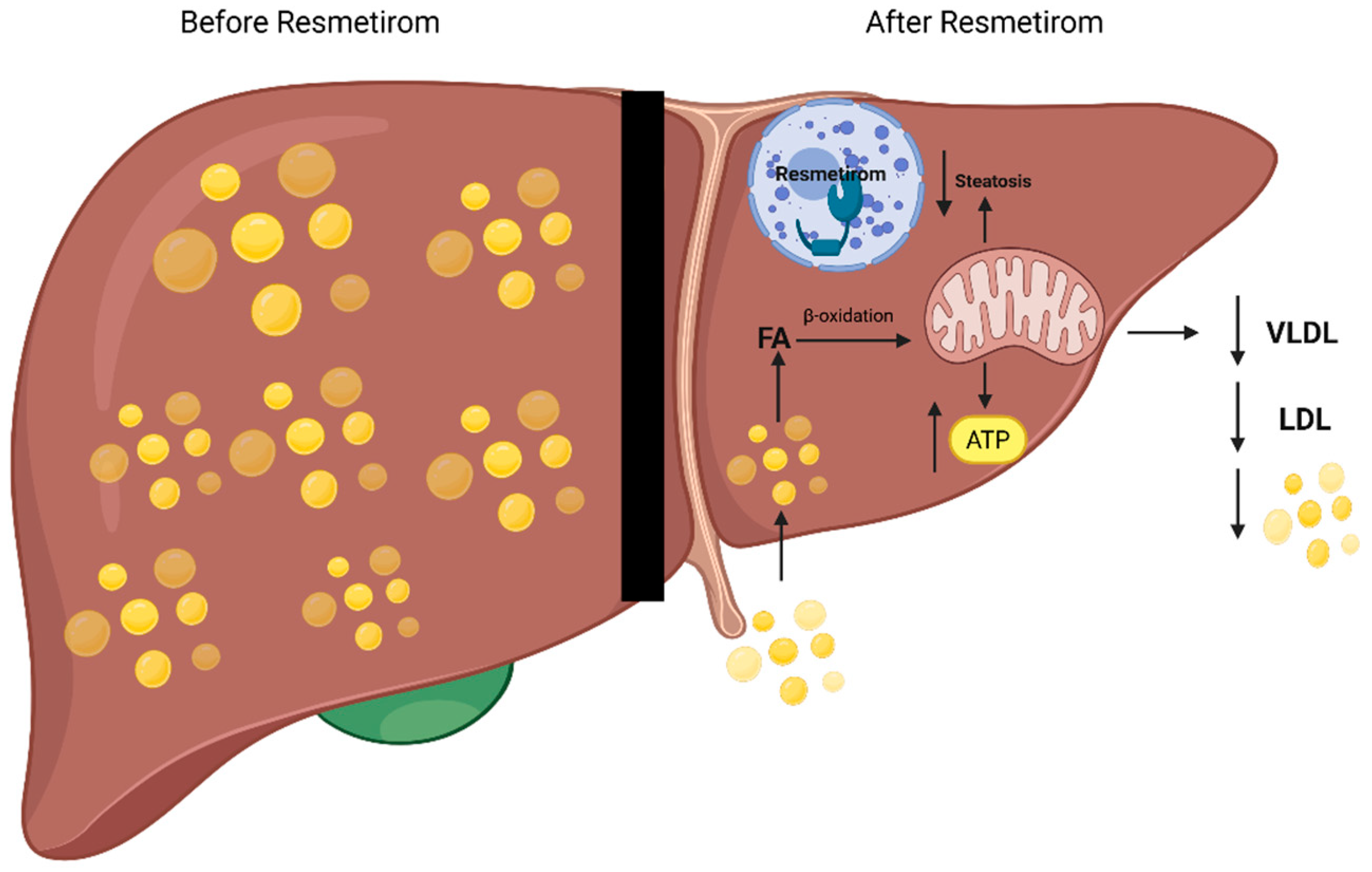
2. Pathobiology and Mechanism of Action
3. Preclinical Trials
4. Clinical Trials
5. Guidelines
6. Future Implications and Research Gaps
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.-Y.; Zheng, M.-H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Ochoa-Allemant, P.; Hubbard, R.A.; Kaplan, D.E.; Serper, M. Adverse Liver Outcomes, Cardiovascular Events, and Mortality in Steatotic Liver Disease. JAMA Intern. Med. 2025, 185, 986–995. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction–Associated Steatotic Liver Disease (MASLD) in People With Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients With Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2024, 22, 1999–2010.e8. [Google Scholar] [CrossRef]
- Fan, W.; Bradford, T.M.; Török, N.J. Metabolic dysfunction−associated liver disease and diabetes: Matrix remodeling, fibrosis, and therapeutic implications. Ann. N. Y. Acad. Sci. 2024, 1538, 21–33. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef]
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.-E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.-S.; et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J Med. Chem. 2014, 57, 3912–3923. [Google Scholar] [CrossRef]
- Petta, S.; Targher, G.; Romeo, S.; Pajvani, U.B.; Zheng, M.; Aghemo, A.; Valenti, L.V.C. The first MASH drug therapy on the horizon: Current perspectives of resmetirom. Liver Int. 2024, 44, 1526–1536. [Google Scholar] [CrossRef]
- Chen, V.L.; Morgan, T.R.; Rotman, Y.; Patton, H.M.; Cusi, K.; Kanwal, F.; Kim, W.R. Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance. Hepatology 2025, 81, 312–320. [Google Scholar] [CrossRef]
- Peiseler, M.; Schwabe, R.; Hampe, J.; Kubes, P.; Heikenwälder, M.; Tacke, F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease—novel insights into cellular communication circuits. J. Hepatol. 2022, 77, 1136–1160. [Google Scholar] [CrossRef]
- Puri, M.; Sonawane, S. Liver Sinusoidal Endothelial Cells in the Regulation of Immune Responses and Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease. Int. J. Mol. Sci. 2025, 26, 3988. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Karim, G.; Bansal, M.B. Resmetirom: An Orally Administered, Small-molecule, Liver-directed, beta-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. Eur. Endocrinol. 2023, 19, 60–70. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.-H.; Zhou, J.; Siddique, M.M.; Bay, B.-H.; Zhu, X.; Privalsky, M.L.; Cheng, S.-Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef]
- Krause, C.; Grohs, M.; El Gammal, A.T.; Wolter, S.; Lehnert, H.; Mann, O.; Mittag, J.; Kirchner, H. Reduced expression of thyroid hormone receptor β in human nonalcoholic steatohepatitis. Endocr. Connect. 2018, 7, 1448–1456. [Google Scholar] [CrossRef]
- Bohinc, B.N.; Michelotti, G.; Xie, G.; Pang, H.; Suzuki, A.; Guy, C.D.; Piercy, D.; Kruger, L.; Swiderska-Syn, M.; Machado, M.; et al. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology 2014, 155, 4591–4601. [Google Scholar] [CrossRef]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173–1191. [Google Scholar] [CrossRef]
- A Harrison, S.; Bashir, M.R.; Guy, C.D.; Zhou, R.; A Moylan, C.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; A Neuschwander-Tetri, B.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Isaacs, S.; Misra, A. Intrahepatic hypothyroidism in MASLD: Role of liver-specific thyromimetics including resmetirom. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 103034. [Google Scholar] [CrossRef]
- Freund, M.E.T.; van der Most, F.; Groeneweg, S.; van Geest, F.S.; Visser, W.E. Thyroid Hormone Analogs: Recent Developments. Thyroid 2025, ahead of print. [Google Scholar] [CrossRef]
- Knezović, E.; Hefer, M.; Blažanović, S.; Petrović, A.; Tomičić, V.; Srb, N.; Kirner, D.; Smolić, R.; Smolić, M. Drug Pipeline for MASLD: What Can Be Learned from the Successful Story of Resmetirom. Curr. Issues Mol. Biol. 2025, 47, 154. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Wohlfart, P.; Madsen, A.N.; Veidal, S.S.; Feigh, M.; Schmoll, D. Activation of thyroid hormone receptor-beta improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br. J. Pharmacol. 2021, 178, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-M.; Song, Y.; Shi, Y.-Q.; Sun, L.-L. Thyroid Hormone Receptor-beta Agonists in NAFLD Therapy: Possibilities and Challenges. J. Clin. Endocrinol. Metab. 2023, 108, 1602–1613. [Google Scholar] [CrossRef]
- Marino, L.; Kim, A.; Ni, B.; Celi, F.S. Thyroid hormone action and liver disease, a complex interplay. Hepatology 2025, 81, 651–669. [Google Scholar] [CrossRef]
- Wu, D.; Yang, Y.; Hou, Y.; Zhao, Z.; Liang, N.; Yuan, P.; Yang, T.; Xing, J.; Li, J. Increased mitochondrial fission drives the reprogramming of fatty acid metabolism in hepatocellular carcinoma cells through suppression of Sirtuin 1. Cancer Commun. 2022, 42, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yin, G.; Kohlhepp, M.S.; Schumacher, F.; Hundertmark, J.; Hassan, M.I.A.; Heymann, F.; Puengel, T.; Kleuser, B.; Mosig, A.S.; et al. Dissecting Acute Drug-Induced Hepatotoxicity and Therapeutic Responses of Steatotic Liver Disease Using Primary Mouse Liver and Blood Cells in a Liver-On-A-Chip Model. Adv. Sci. 2024, 11, e2403516. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Racila, A.; Henry, L.; Labriola, D.; Taub, R.; Nader, F. Health-related quality of life (HRQL) assessments in a 52-week, double-blind, randomized, placebo-controlled phase III study of resmetirom (MGL-3196) in patients with metabolic dysfunction–associated steatohepatitis (MASH) and fibrosis. Hepatology 2025, 81, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Administration USFaD. FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (accessed on 13 August 2025).
| Trial Name | Phase | Population | Sample Size | Design | Primary Endpoint | Key Findings |
|---|---|---|---|---|---|---|
| MAESTRO-NASH (NCT03900429) [30] | III | Biopsy-confirmed MASH and liver fibrosis stages: F1B, F2, or F31 | 966 | Randomized, double-blind, placebo-controlled | MASH resolution and fibrosis improvement at week 52 | Significant MASH resolution and fibrosis improvement vs. placebo |
| MAESTRO-NAFLD-1 (NCT04197479) [8] | III | MASLD/presumed MASH via non-invasive testing | 1143 | Randomized, double-blind, placebo-controlled | TEAEs at 52 weeks; metabolic and imaging endpoints | Improvements in LDL-C, apoB, TGs, and hepatic fat; good safety profile |
| HRQL Study (NCT02912260) [31] | II | Biopsy-proven MASH and a hepatic fat fraction of ≥10% as measured by MRI-PDFF | 125 | Randomized, double-blind, placebo-controlled | HRQL change (SF-36); hepatic fat reduction | Improvements in HRQL and liver fat in responders; histological benefit tied to QOL |
| Category | Criteria |
|---|---|
| Indication | Adults with biopsy-confirmed MASH and moderate-to-advanced fibrosis (F2–F3) |
| Resmetirom Dosing |
|
| Statin Dosing |
|
| Contraindications |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, F.; Elshaer, A.; Odeh, N.B.; Alatout, M.H.; Shahin, T.; Worden, A.R.; Albunni, H.N.; Lizaola-Mayo, B.C.; Jayasekera, C.R.; Chascsa, D.M.H.; et al. Resmetirom in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Life 2025, 15, 1306. https://doi.org/10.3390/life15081306
Jamal F, Elshaer A, Odeh NB, Alatout MH, Shahin T, Worden AR, Albunni HN, Lizaola-Mayo BC, Jayasekera CR, Chascsa DMH, et al. Resmetirom in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Life. 2025; 15(8):1306. https://doi.org/10.3390/life15081306
Chicago/Turabian StyleJamal, Fares, Amani Elshaer, Nour B. Odeh, Mayar H. Alatout, Tala Shahin, Astin R. Worden, Hashem N. Albunni, Blanca C. Lizaola-Mayo, Channa R. Jayasekera, David M. H. Chascsa, and et al. 2025. "Resmetirom in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis" Life 15, no. 8: 1306. https://doi.org/10.3390/life15081306
APA StyleJamal, F., Elshaer, A., Odeh, N. B., Alatout, M. H., Shahin, T., Worden, A. R., Albunni, H. N., Lizaola-Mayo, B. C., Jayasekera, C. R., Chascsa, D. M. H., Vargas, H. E., & Aqel, B. A. (2025). Resmetirom in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis. Life, 15(8), 1306. https://doi.org/10.3390/life15081306






