Complete Revascularization in NSTE-ACS and Multivessel Disease: Clinical Outcomes and Prognostic Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population Under Study
2.3. Statistical Evaluations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| CR | Complete revascularization |
| IR | Incomplete revascularization |
| MACE | Major adverse cardiovascular events |
| MVD | Multivessel disease |
| NSTE-ACS | Non-ST-segment elevation acute coronary syndrome |
| NSTEMI | Non-ST-elevation myocardial infarction |
| PCI | Percutaneous coronary intervention |
| STEMI | ST-segment elevation myocardial infarction |
| GRACE | Global Registry of Acute Coronary Events |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| LVEF | Left ventricular ejection fraction |
| LM | Left main coronary artery |
| LAD | Left anterior descending coronary artery |
| LCX | Left circumflex coronary artery |
| RCA | Right coronary artery |
| HRs | Hazard ratios |
| CIs | Confidence intervals |
References
- Ghaffari, S. Non-ST-Elevation Acute Coronary Syndromes. In Practical Cardiology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 413–428. ISBN 978-0-323-80915-3. [Google Scholar]
- Babes, E.E.; Zaha, D.C.; Tit, D.M.; Nechifor, A.C.; Bungau, S.; Andronie-Cioara, F.L.; Behl, T.; Stoicescu, M.; Munteanu, M.A.; Rus, M.; et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics 2021, 11, 850. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Balbi, M.M.; Scarparo, P.; Tovar, M.N.; Masdjedi, K.; Daemen, J.; Den Dekker, W.; Ligthart, J.; Witberg, K.; Cummins, P.; Wilschut, J.; et al. Culprit Lesion Detection in Patients Presenting with Non-ST Elevation Acute Coronary Syndrome and Multivessel Disease. Cardiovasc. Revascularization Med. 2022, 35, 110–118. [Google Scholar] [CrossRef]
- Pandit, N.; Rahatekar, P.; Rekwal, L.; Kuber, D.; Nath, R.K.; Aggarwal, P. Target Vessel Versus Complete Revascularization in Non-ST Elevation Myocardial Infarction Without Cardiogenic Shock. Cureus 2022, 14, e23139. [Google Scholar] [CrossRef] [PubMed]
- Elscot, J.J.; Kakar, H.; Scarparo, P.; den Dekker, W.K.; Bennett, J.; Schotborgh, C.E.; van der Schaaf, R.; Sabaté, M.; Moreno, R.; Ameloot, K.; et al. Timing of Complete Multivessel Revascularization in Patients Presenting with Non-ST-Segment Elevation Acute Coronary Syndrome. JACC Cardiovasc. Interv. 2024, 17, 771–782. [Google Scholar] [CrossRef]
- Kite, T.A.; Ladwiniec, A.; Greenwood, J.P.; Gale, C.P.; Anantharam, B.; More, R.; Hetherington, S.L.; Khan, S.Q.; O’Kane, P.; Rakhit, R.; et al. Very Early Invasive Strategy in Higher Risk Non-ST-Elevation Acute Coronary Syndrome: The RAPID NSTEMI Trial. Heart 2024, 110, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Siebert, V.R.; Borgaonkar, S.; Jia, X.; Nguyen, H.L.; Birnbaum, Y.; Lakkis, N.M.; Alam, M. Meta-Analysis Comparing Multivessel Versus Culprit Coronary Arterial Revascularization for Patients with Non-ST-Segment Elevation Acute Coronary Syndromes. Am. J. Cardiol. 2019, 124, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Koganti, S.; Jain, A.K.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Kalra, S.S.; Dalby, M.C.; O’Mahony, C.; Malik, I.S.; et al. Complete Versus Culprit-Only Lesion Intervention in Patients with Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 72, 1989–1999. [Google Scholar] [CrossRef]
- Pavasini, R.; Biscaglia, S.; Barbato, E.; Tebaldi, M.; Dudek, D.; Escaned, J.; Casella, G.; Santarelli, A.; Guiducci, V.; Gutierrez-Ibanes, E.; et al. Complete Revascularization Reduces Cardiovascular Death in Patients with ST-Segment Elevation Myocardial Infarction and Multivessel Disease: Systematic Review and Meta-Analysis of Randomized Clinical Trials. Eur. Heart J. 2020, 41, 4103–4110. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; López-Sendón, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef]
- Diletti, R.; den Dekker, W.K.; Bennett, J.; Schotborgh, C.E.; van der Schaaf, R.; Sabaté, M.; Moreno, R.; Ameloot, K.; van Bommel, R.; Forlani, D.; et al. Immediate versus Staged Complete Revascularisation in Patients Presenting with Acute Coronary Syndrome and Multivessel Coronary Disease (BIOVASC): A Prospective, Open-Label, Non-Inferiority, Randomised Trial. Lancet 2023, 401, 1172–1182. [Google Scholar] [CrossRef]
- Sardella, G.; Lucisano, L.; Garbo, R.; Pennacchi, M.; Cavallo, E.; Stio, R.E.; Calcagno, S.; Ugo, F.; Boccuzzi, G.; Fedele, F.; et al. Single-Staged Compared with Multi-Staged PCI in Multivessel NSTEMI Patients: The SMILE Trial. J. Am. Coll. Cardiol. 2016, 67, 264–272. [Google Scholar] [CrossRef]
- Farooq, V.; Serruys, P.W.; Bourantas, C.V.; Zhang, Y.; Muramatsu, T.; Feldman, T.; Holmes, D.R.; Mack, M.; Morice, M.C.; Ståhle, E.; et al. Quantification of Incomplete Revascularization and Its Association with Five-Year Mortality in the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) Trial Validation of the Residual SYNTAX Score. Circulation 2013, 128, 141–151. [Google Scholar] [CrossRef]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Messenger, J.C.; Rumsfeld, J.S.; et al. Contemporary Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Interventions: Insights from the NCDR Cath-PCI Registry. JACC Cardiovasc. Interv. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary Microvascular Obstruction in Acute Myocardial Infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Pantea-Roșan, L.R.; Bungau, S.G.; Radu, A.-F.; Pantea, V.A.; Moisi, M.I.; Vesa, C.M.; Behl, T.; Nechifor, A.C.; Babes, E.E.; Stoicescu, M.; et al. A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon. Diagnostics 2022, 12, 932. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.-A.; Steg, P.G.; Morel, M.; Mauri, L.; Vranckx, P.; et al. Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Malkin, C.J.; George, V.; Ghobrial, M.S.A.; Krishnan, A.; Siotia, A.; Raina, T.; Morton, A.C.; Gunn, J. Residual SYNTAX Score after PCI for Triple Vessel Coronary Artery Disease: Quantifying the Adverse Effect of Incomplete Revascularisation. EuroIntervention 2013, 8, 1286–1295. [Google Scholar] [CrossRef]
- IBM SPSS Statistics 25. Available online: https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-25 (accessed on 18 July 2025).
- Desperak, P.; Hawranek, M.; Gąsior, P.; Desperak, A.; Lekston, A.; Gąsior, M. Long-Term Outcomes of Patients with Multivessel Coronary Artery Disease Presenting Non-ST-Segment Elevation Acute Coronary Syndromes. Cardiol. J. 2019, 26, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Faro, D.C.; Laudani, C.; Agnello, F.G.; Ammirabile, N.; Finocchiaro, S.; Legnazzi, M.; Mauro, M.S.; Mazzone, P.M.; Occhipinti, G.; Rochira, C.; et al. Complete Percutaneous Coronary Revascularization in Acute Coronary Syndromes with Multivessel Coronary Disease: A Systematic Review. JACC Cardiovasc. Interv. 2023, 16, 2347–2364. [Google Scholar] [CrossRef]
- Bianchini, E.; Basile, M.; Bianchini, F.; Zito, A.; Romagnoli, E.; Aurigemma, C.; Paraggio, L.; Lunardi, M.; Laborante, R.; Fracassi, F.; et al. Multivessel Revascularization in Non-ST Segment Elevation Acute Coronary Syndromes: A Systematic Review and Meta-Analysis of 182,798 Patients. Int. J. Cardiol. 2024, 413, 132392. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Díaz, V.A.; Routledge, H.; Malik, F.-T.-N.; Hildick-Smith, D.; Guédès, A.; Baello, P.; Kuramitsu, S.; Das, R.; Dewilde, W.; Portales, J.F.; et al. Complete versus Incomplete Revascularization in Patients with a Non-ST-Elevation Myocardial Infarction: Analysis from the e-ULTIMASTER Registry. Cardiovasc. Revascularization Med. 2025, 75, 25–30. [Google Scholar] [CrossRef]
- Manuca, R.-D.; Covic, A.M.; Brinza, C.; Floria, M.; Statescu, C.; Covic, A.; Burlacu, A. Updated Strategies in Non-Culprit Stenosis Management of Multivessel Coronary Disease—A Contemporary Review. Medicina 2024, 60, 263. [Google Scholar] [CrossRef]
- Baumann, A.A.W.; Tavella, R.; Air, T.M.; Mishra, A.; Montarello, N.J.; Arstall, M.; Zeitz, C.; Worthley, M.I.; Beltrame, J.F.; Psaltis, P.J. Prevalence and Real-World Management of NSTEMI with Multivessel Disease. Cardiovasc. Diagn. Ther. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Howard, J.P.; Arnold, A.; Prasad, M.; Seligman, H.; Cook, C.M.; Warisawa, T.; Shun-Shun, M.; Ali, Z.; Parikh, M.A.; et al. Complete Revascularization by Percutaneous Coronary Intervention for Patients with ST-Segment-Elevation Myocardial Infarction and Multivessel Coronary Artery Disease: An Updated Meta-Analysis of Randomized Trials. J. Am. Heart Assoc. 2020, 9, e015263. [Google Scholar] [CrossRef]
- Bax, J.J.; Poldermans, D.; Elhendy, A.; Cornel, J.H.; Boersma, E.; Rambaldi, R.; Roelandt, J.R.T.C.; Fioretti, P.M. Improvement of Left Ventricular Ejection Fraction, Heart Failure Symptoms and Prognosis after Revascularization in Patients with Chronic Coronary Artery Disease and Viable Myocardium Detected by Dobutamine Stress Echocardiography. J. Am. Coll. Cardiol. 1999, 34, 163–169. [Google Scholar] [CrossRef]
- Dahroug, M.S.A.; Mustafa, S.A.; Farag, S.I.; Ateya, W.M. Short Term Effect of Total Revascularization on Left Ventricular Recovery in NSTEMI Patients: Speckle Tracking Study. Benha J. Appl. Sci. 2022, 7, 113–116. [Google Scholar] [CrossRef]
- Fagel, N.D.; van Nooijen, F.C.; Maarse, M.; Slagboom, T.; Herrman, J.P.; van der Schaaf, R.J.; Amoroso, G.; Patterson, M.S.; Laarman, G.J.; Suttorp, M.J.; et al. Five-Year Results of the Complete versus Culprit Vessel Percutaneous Coronary Intervention in Multivessel Disease Using Drug-Eluting Stents II (CORRECT II) Study: A Prospective, Randomised Controlled Trial. Netherlands Heart J. 2019, 27, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Marengo, G.; De Filippo, O.; Wanha, W.; Leonardi, S.; Raposeiras Roubin, S.; Fabris, E.; Popovic, M.; Giannino, G.; Truffa, A.; et al. Impact of Complete Revascularization on Development of Heart Failure in Patients with Acute Coronary Syndrome and Multivessel Disease: A Subanalysis of the CORALYS Registry. J. Am. Heart Assoc. 2023, 12, e028475. [Google Scholar] [CrossRef]
- Palmerini, T.; Dangas, G.; Mehran, R.; Caixeta, A.; Généreux, P.; Fahy, M.P.; Xu, K.; Cristea, E.; Lansky, A.J.; Stone, G.W. Predictors and Implications of Stent Thrombosis in Non-ST-Segment Elevation Acute Coronary Syndromes the ACUITY Trial. Circ. Cardiovasc. Interv. 2011, 4, 577–584. [Google Scholar] [CrossRef]
- Cuculi, F.; Banning, A.P.; Abizaid, A.; Bartorelli, A.L.; Baux, A.C.; Dzavík, V.; Ellis, S.; Gao, R.; Holmes, D.; Jeong, M.H.; et al. Outcomes in Patients Undergoing Multivessel Percutaneous Coronary Intervention Using Sirolimus-Eluting Stents: A Report from the e-SELECT Registry. EuroIntervention 2011, 7, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Hannan, E.L.; Samadashvili, Z.; Walford, G.; Holmes, D.R.J.; Jacobs, A.K.; Stamato, N.J.; Venditti, F.J.; Sharma, S.; King, S.B., 3rd. Culprit Vessel Percutaneous Coronary Intervention versus Multivessel and Staged Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction Patients with Multivessel Disease. JACC Cardiovasc. Interv. 2010, 3, 22–31. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years | Cardiogenic shock or hemodynamic instability |
| Diagnosis of NSTE-ACS, defined according to contemporary guidelines (including both unstable angina and non-ST-elevation myocardial infarction) [3] | Prior coronary artery bypass grafting |
| Presence of multivessel coronary artery disease (≥2 major epicardial vessels with ≥70% stenosis) | Presentation with STEMI |
| Hemodynamic stability at the time of PCI (systolic blood pressure > 90 mmHg without inotropes or mechanical support) | Severe valvular heart disease or other structural heart disease requiring intervention |
| Underwent PCI during the index hospitalization | Missing data on key clinical outcomes or procedural details |
| Variable Type | Data Presentation | Statistical Test/Method |
|---|---|---|
| Continuous variables | Mean ± standard deviation or median (interquartile range), depending on distribution | Student’s t-test or Mann–Whitney U test |
| Categorical variables | Frequencies and percentages | Chi-square test or Fisher’s exact test |
| Primary composite outcome | Time-to-event data | Kaplan–Meier survival analysis and log-rank test |
| Hazard estimation | HRs with 95% CIs | Cox proportional-hazards regression, unadjusted and adjusted for covariates |
| Software | — | SPSS version 25 (IBM Corp., Armonk, NY, USA) [20] |
| Statistical significance | p-value < 0.05 |
| Parameter | CR Group (n = 218) | IR Group (n = 64) | p Value |
|---|---|---|---|
| Age, years | 66.17 ± 9.59 | 67.16 ± 9.75 | 0.89 |
| Sex (male) n (%) | 144 (66.05) | 34 (53.12) | 0.01 |
| Hypertension n (%) | 148 (67.89) | 53 (82.81) | 0.02 |
| Diabetes n (%) | 108 (49.54) | 44 (68.75) | 0.004 |
| Hypercholesterolemia n (%) | 150 (68.81) | 37 (57.81) | 0.11 |
| Current smoker n (%) | 148 (67.89) | 53 (82.81) | 0.02 |
| CKD n (%) | 34 (15.60) | 20 (31.25) | 0.01 |
| Atrial fibrillation n (%) | 46 (21.10) | 16 (25) | 0.51 |
| Serum creatinine mg/dL | 0.89 (0.79–1.07) | 0.92 (0.8–1.1) | 0.57 |
| Troponin, ng/L | 1386 (405–2412) | 1059.5 (472.5–2366.5) | 0.27 |
| GRACE risk score | 114 ± 26 | 113 ± 23 | 0.19 |
| SBP, mmHg | 144 ± 19 | 149 ± 24 | 0.10 |
| DBP, mmHg | 85 ± 11 | 86 ± 12 | 0.63 |
| Heart rate, beats/min | 78 ± 17 | 84 ± 16 | 0.83 |
| LVEF at admission, % | 41 ± 8 | 43 ± 8 | 0.40 |
| Parameter | CR Group (n = 218) | IR Group (n = 64) | p Value | |
|---|---|---|---|---|
| Radial access n (%) | 210 (96.33) | 62 (96.88) | 0.83 | |
| Femoral access n (%) | 8 (3.67) | 2 (3.12) | 0.30 | |
| Culprit lesion | LM n (%) | 26 (11.97) | 7 (10.94) | 0.66 |
| LAD n (%) | 178 (81.65) | 52 (81.25) | 0.88 | |
| LCX n (%) | 164 (75.23) | 44 (68.75) | 0.30 | |
| RCA n (%) | 132 (60.55) | 52 (81.25) | 0.001 | |
| Clopidogrel n (%) | 88 (40.37) | 40 (62.50) | 0.002 | |
| Ticagrelor n (%) | 130 (59.63) | 24 (37.50) | 0.002 | |
| Parameter | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| CR Group (n = 218) | IR Group (n = 64) | p Value | CR Group (n = 218) | IR Group (n = 64) | p Value | |
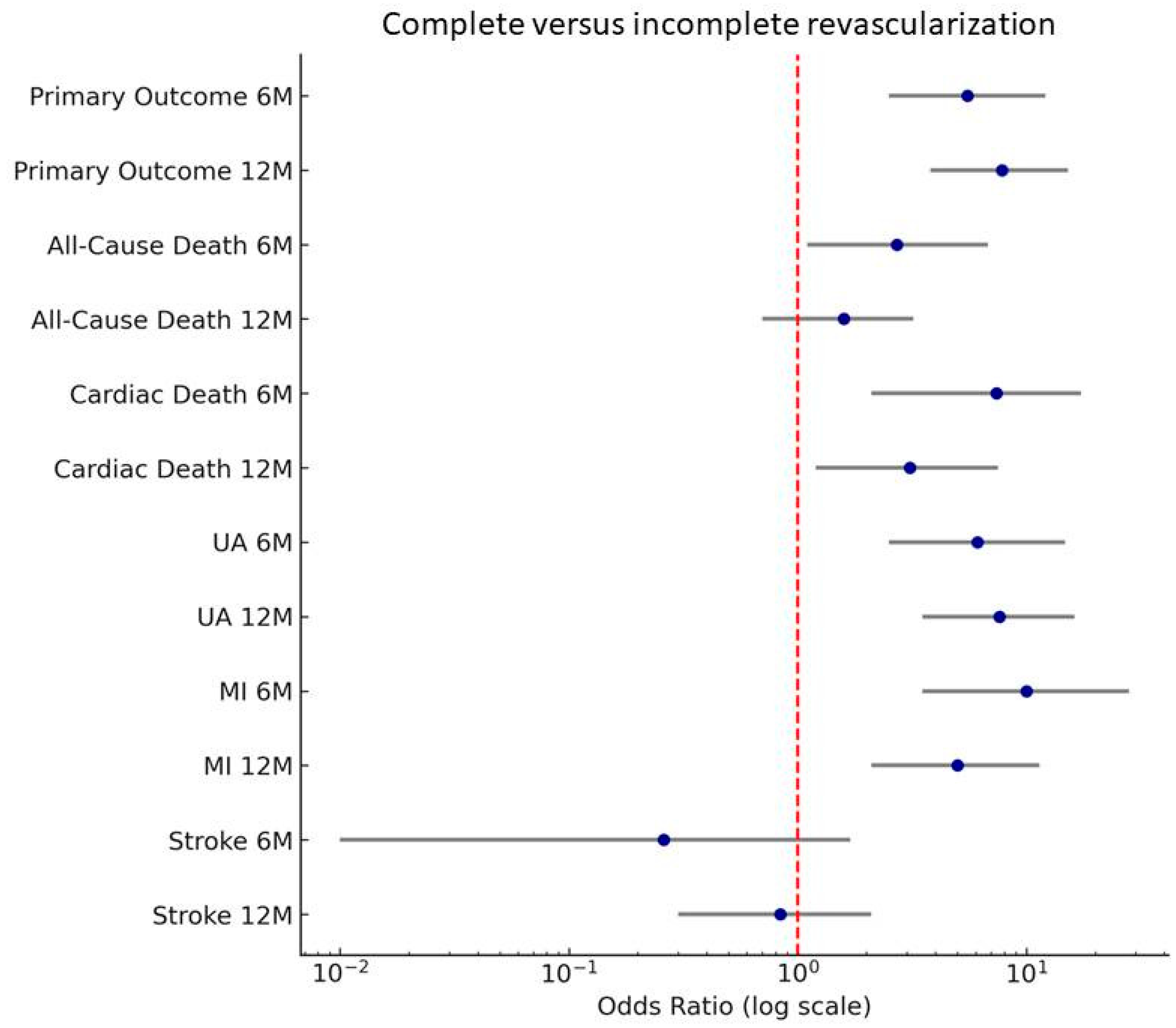
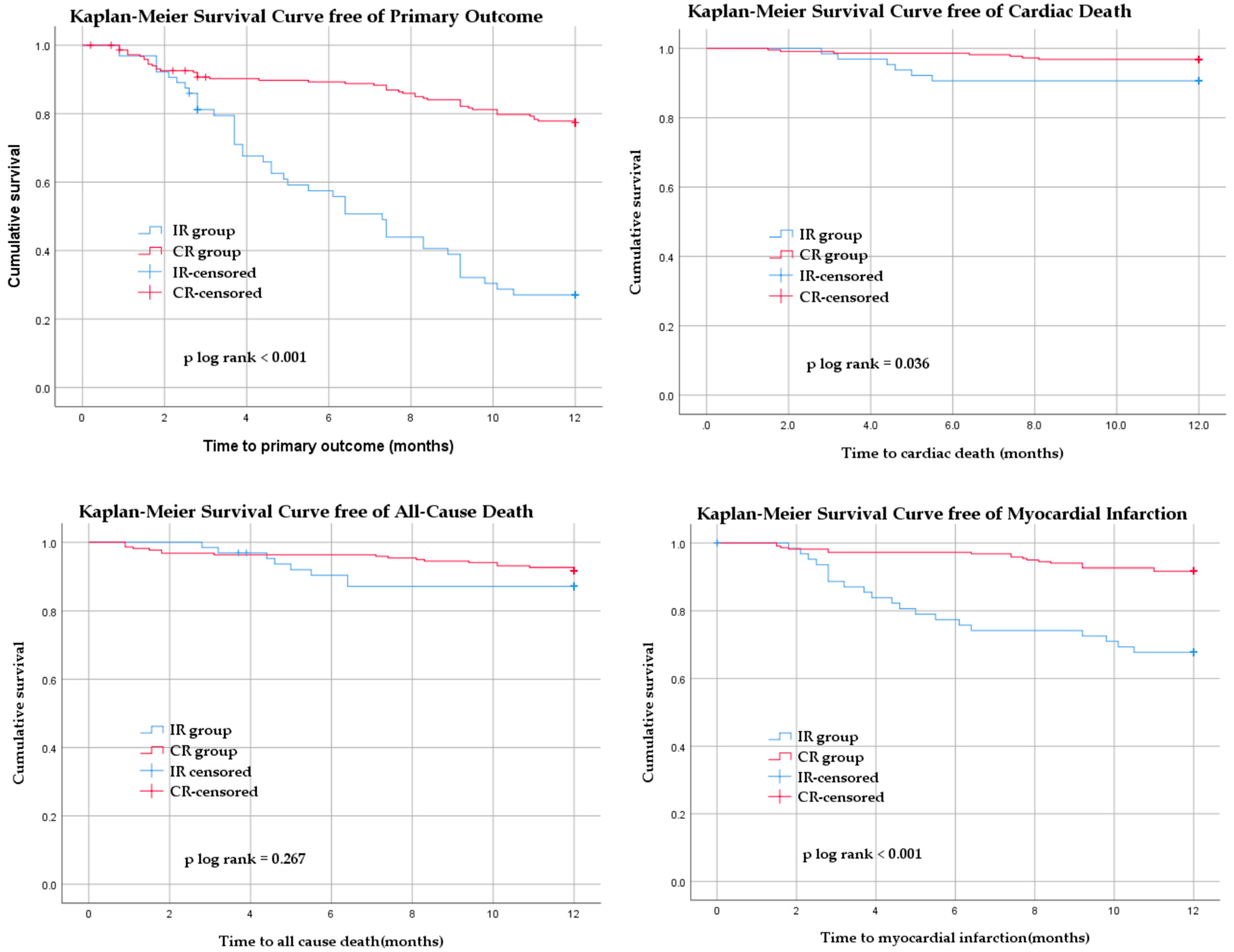
| Primary outcome n (%) | 24 (11) | 26 (40.63) | <0.001 | 48 (22.02) | 44 (68.75) | <0.001 |
| All-cause death n (%) | 8 (3.67) | 6 (9.38) | 18 (8.26) | 8 (12.5) | 0.04 | |
| Cardiac death n (%) | 3 (1.38) | 6 (9.38) | 7 (3.21) | 6 (9.38) | <0.001 | |
| Unstable angina n (%) | 8 (3.67) | 12 (18.75) | 14 (6.42) | 22 (34.38) | ||
| Myocardial infarction n (%) | 6 (2.75) | 14 (21.88) | 0.001 | 18 (8.26) | 20 (31.25) | |
| Stroke n (%) | 6 (2.75) | 0 | 0.18 | 8 (3.67) | 2 (3.13) | 0.67 |
| Parameter Mean ± SD | CR Group (n = 218) | IR Group (n = 64) | p Value |
|---|---|---|---|
| LVEF at discharge, % | 45 ± 6 | 43 ± 8 | 0.059 |
| LVEF improvement, % | 5 ± 3.8 | 0 ± 2.7 | <0.001 |
| Length of hospital stay, days | 5.3 ± 2.9 | 5.8 ± 1.9 | 0.88 |
| Parameter | Primary Outcome | p Value | ||
|---|---|---|---|---|
| Present n = 92 (32.6%) | Absent n = 190 (67.4%) | |||
| Age, years | 66.77 ± 9.12 | 66.22 ± 9.87 | 0.48 | |
| Sex (male) | 50 (54.35) | 128 (67.37) | 0.03 * | |
| Current smoker | 38 (41.30) | 80 (42.10) | 0.79 | |
| Hypertension | 73 (79.35) | 128 (67.37) | 0.03 * | |
| Diabetes mellitus | 60 (65.22) | 90 (47.37) | 0.005 * | |
| Hypercholesterolemia | 64 (69.57) | 123 (64.74) | 0.42 | |
| Atrial fibrillation | 24 (26.08) | 38 (20) | 0.24 | |
| CKD | 20 (21.74) | 34 (17.89) | 0.13 | |
| SBP, mmHg | 148 ± 21 | 144 ± 20 | 0.65 | |
| DBP, mmHg | 85 ± 12 | 86 ± 11 | 0.23 | |
| Heart rate | 80 ± 12 | 79 ± 19 | 0.49 | |
| Troponins ng/L | 964 (320.5–2416.5) | 918.5 (369.5–2349) | 0.976 | |
| GRACE risk score | 112.5 ± 22 | 114.2 ± 27 | 0.52 | |
| LVEF admission, % | 41 ± 10 | 43 ± 8 | 0.04 * | |
| LVEF discharge, % | 43 ± 8 | 46 ± 6 | 0.01 * | |
| LVEF improvement, % | 2.4 ± 2 | 3.9 ± 3 | 0.20 | |
| Culprit lesion | LM | 7 (7.61) | 26 (13.68) | 0.002 * |
| LAD | 76 (82.61) | 154 (81.05) | 0.40 | |
| ACX | 71 (77.17) | 137 (72.11) | 0.06 | |
| ACD | 62 (67.39) | 122 (64.21) | 0.27 | |
| Clopidogrel | 50 (54.35) | 78 (41.05) | 0.03 * | |
| Ticagrelor | 42 (45.65) | 112 (58.95) | 0.03 * | |
| CR | 48 (52.17) | 170 (89.47) | <0.001 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muste, S.R.; Bustea, C.; Babes, E.E.; Muste, F.A.; Bungau, G.S.; Tit, D.M.; Tarce, A.G.; Radu, A.-F. Complete Revascularization in NSTE-ACS and Multivessel Disease: Clinical Outcomes and Prognostic Implications. Life 2025, 15, 1299. https://doi.org/10.3390/life15081299
Muste SR, Bustea C, Babes EE, Muste FA, Bungau GS, Tit DM, Tarce AG, Radu A-F. Complete Revascularization in NSTE-ACS and Multivessel Disease: Clinical Outcomes and Prognostic Implications. Life. 2025; 15(8):1299. https://doi.org/10.3390/life15081299
Chicago/Turabian StyleMuste, Silviu Raul, Cristiana Bustea, Elena Emilia Babes, Francesca Andreea Muste, Gabriela S. Bungau, Delia Mirela Tit, Alexandra Georgiana Tarce, and Andrei-Flavius Radu. 2025. "Complete Revascularization in NSTE-ACS and Multivessel Disease: Clinical Outcomes and Prognostic Implications" Life 15, no. 8: 1299. https://doi.org/10.3390/life15081299
APA StyleMuste, S. R., Bustea, C., Babes, E. E., Muste, F. A., Bungau, G. S., Tit, D. M., Tarce, A. G., & Radu, A.-F. (2025). Complete Revascularization in NSTE-ACS and Multivessel Disease: Clinical Outcomes and Prognostic Implications. Life, 15(8), 1299. https://doi.org/10.3390/life15081299








