Ocular and Neurological Sequelae in Long COVID: Dry Eye, Asthenopia, Sleep Disorders, Asthenia, and Restless Legs Syndrome—A Case Report with Literature Review
Abstract
1. Introduction
2. Case Description
3. Discussion
- Neuroinflammation: SARS-CoV-2 virus can directly affect the central nervous system, leading to neuroinflammation. Several studies have shown that SARS-CoV-2 may cross the Blood–Brain Barrier (BBB) via the ACE2 receptor, leading to the activation of glial cells and the release of pro-inflammatory cytokines [55]. This process may explain many of the neuropsychiatric symptoms seen in LC, including fatigue, sleep disturbances, and RLS. Furthermore, the inflammatory response may contribute to ocular symptoms, as immune dysregulation can affect the ocular surface, tear film, and neuromotor balance, causing dry mouth and visual asthenopia.
- Autonomic Nervous System Dysfunction: There is growing evidence suggesting that LC may involve dysautonomia, a condition characterized by the dysfunction of the autonomic nervous system [56]. Dysautonomia can lead to altered tear production and increased ocular dryness, as well as disturbances in sleep patterns, which could explain the patient’s insomnia and RLS. Furthermore, autonomic dysfunction could exacerbate fatigue and asthenopia, as the body’s response to stress and physical activity is impaired.
- Immune Dysregulation and Post-Viral Fatigue: Post-viral fatigue is a hallmark of LC and may be mediated by ongoing immune activation. This includes increased levels of cytokines such as interleukins (IL-6, IL-1β), which have been linked to fatigue, mood disturbances, and cognitive dysfunction in LC patients [57]. Immune dysregulation could contribute to the persistence of symptoms like DED and visual asthenopia, as the inflammation in peripheral tissues, including the ocular surface, may be sustained even after the initial infection resolves.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Christie, B. COVID-19: Early studies give hope omicron is milder than other variants. BMJ 2021, 375, 3144. [Google Scholar] [CrossRef]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Troisi, M.; Zannella, C.; Troisi, S.; De Bernardo, M.; Galdiero, M.; Franci, G.; Rosa, N. Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results. Microorganisms 2022, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bartolomé, F.; Sánchez-Quirós, J. Ocular manifestations of SARS-CoV-2: Literature review. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2021, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, M.; Brambati, M.; Starace, V.; Capone, L.; Nadin, F.; Pederzolli, M.; Gorgoni, F.; Di Biase, C.; Corbelli, E.; Battista, M.; et al. Ocular Features and Associated Systemic Findings in SARS-CoV-2 Infection. Ocul. Immunol. Inflamm. 2020, 28, 916–921. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Chen, L.; Deng, C.; Zou, X.; Liu, W.; Yu, H.; Chen, B.; Sun, X. The evidence of SARS-CoV-2 infection on ocular surface. Ocul. Surf. 2020, 18, 360–362. [Google Scholar] [CrossRef]
- Seah, I.Y.J.; Anderson, D.E.; Kang, A.E.Z.; Wang, L.; Rao, P.; Young, B.E.; Lye, D.C.; Agrawal, R. Assessing Viral Shedding and Infectivity of Tears in Coronavirus Disease 2019 (COVID-19) Patients. Ophthalmology 2020, 127, 977–979. [Google Scholar] [CrossRef]
- Petronio, G.; Di Marco, R.; Costagliola, C. Do Ocular Fluids Represent a Transmission Route of SARS-CoV-2 Infection? Front. Med. 2021, 7, 620412. [Google Scholar] [CrossRef]
- Bertoli, F.; Veritti, D.; Danese, C.; Samassa, F.; Sarao, V.; Rassu, N.; Gambato, T.; Lanzetta, P. Ocular Findings in COVID-19 Patients: A Review of Direct Manifestations and Indirect Effects on the Eye. J. Ophthalmol. 2020, 2020, 4827304. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharif, E.; Strianese, D.; AlMadhi, N.H.; D’Aponte, A.; dell’Omo, R.; Di Benedetto, R.; Costagliola, C. Ocular tropism of coronavirus (CoVs): A comparison of the interaction between the animal-to-human transmitted coronaviruses (SARS-CoV-1, SARS-CoV-2, MERS-CoV, CoV-229E, NL63, OC43, HKU1) and the eye. Int. Ophthalmol. 2021, 41, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, K.; Agarwal, A.; Jaiswal, N.; Dahiya, N.; Ahuja, A.; Mahajan, S.; Tong, L.; Duggal, M.; Singh, M.; Agrawal, R.; et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241661. [Google Scholar] [CrossRef] [PubMed]
- Gambini, G.; Savastano, M.C.; Savastano, A.; De Vico, U.; Crincoli, E.; Cozzupoli, G.M.; Culiersi, C.; Rizzo, S. Ocular Surface Impairment After Coronavirus Disease 2019: A Cohort Study. Cornea 2021, 40, 477–483. [Google Scholar] [CrossRef]
- Wan, K.H.; Lui, G.C.Y.; Poon, K.C.F.; Ng, S.S.S.; Young, A.L.; Hui, D.S.C.; Tham, C.C.Y.; Chan, P.K.S.; Pang, C.P.; Chong, K.K.L. Ocular surface disturbance in patients after acute COVID-19. Clin. Exp. Ophthalmol. 2022, 50, 398–406. [Google Scholar] [CrossRef]
- Lin, Y.N.; Liu, Z.R.; Li, S.Q.; Li, C.X.; Zhang, L.; Li, N.; Sun, X.W.; Li, H.P.; Zhou, J.P.; Li, Q.Y. Burden of Sleep Disturbance During COVID-19 Pandemic: A Systematic Review. Nat. Sci. Sleep 2021, 2813, 933–966. [Google Scholar] [CrossRef]
- Singh, A.; Acharya, M.; Sangwan, V.S. Commentary: Impact of COVID-19 on ocular surface health. Indian. J. Ophthalmol. 2021, 69, 1066–1067. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, K.; Zhu, Y.; Lyu, D.; Yu, Y.; Li, S.; Yao, K. Ocular manifestations in COVID-19 patients: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2021, 44, 102191. [Google Scholar] [CrossRef]
- Savastano, M.C.; Gambini, G.; Savastano, A.; Falsini, B.; De Vico, U.; Sanguinetti, M.; Cattani, P.; Marchetti, S.; Larici, A.R.; Franceschi, F.; et al. Evidence-based of conjunctival COVID-19 positivity: An Italian experience: Gemelli Against COVID Group. Eur. J. Ophthalmol. 2021, 31, 2886–2893. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Marinkovic, A.; Prakash, S.; Zhao, A.; Balendra, V.; Haider, N.; Jain, I.; Simic, T.; Okorie, C. Post-acute Sequelae in COVID-19 Survivors: An Overview. SN Compr. Clin. Med. 2022, 4, 91. [Google Scholar] [CrossRef]
- Abdelmassih, Y.; Azar, G.; Bonnin, S.; Scemama Timsit, C.; Vasseur, V.; Spaide, R.F.; Behar-Cohen, F.; Mauget-Faysse, M. COVID-19 Associated Choroidopathy. J. Clin. Med. 2021, 10, 4686. [Google Scholar] [CrossRef]
- Layikh, H.A.; Hashim, Z.A.; Kadum, A.A. Conjunctivitis and other ocular findings in patients with COVID-19 infection. Ann. Saudi Med. 2021, 41, 280–284. [Google Scholar] [CrossRef]
- Okamatsu, H.; Koyama, J.; Sakai, Y.; Negishi, K.; Hayashi, K.; Tsurugi, T.; Tanaka, Y.; Nakao, K.; Sakamoto, T.; Okumura, K. High-power application is associated with shorter procedure time and higher rate of first-pass pulmonary vein isolation in ablation index-guided atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2019, 30, 2751–2758. [Google Scholar] [CrossRef]
- Wong, N.S.Q.; Liu, C.; Lin, M.T.-Y.; Lee, I.X.Y.; Tong, L.; Liu, Y.-C. Neuropathic Corneal Pain after Coronavirus Disease 2019 (COVID-19) Infection. Diseases 2024, 12, 37. [Google Scholar] [CrossRef]
- Mirza, E.; Belviranli, S.; Gundogan, A.O.; Adam, M.; Oltulu, R. Quantitative assessment of the effect of SARS-CoV-2 on the corneal sub-basal nerve plexus of post-COVID-19 patients using in vivo confocal microscopy. Eye 2023, 37, 660–664. [Google Scholar] [CrossRef]
- Natoli, S.; Oliveira, V.; Calabresi, P.; Maia, L.F.; Pisani, A. Does SARS-CoV-2 invade the brain? Translational lessons from animal models. Eur. J. Neurol. 2020, 27, 1764–1773. [Google Scholar] [CrossRef]
- Roehrich, H.; Yuan, C.; Hou, J.H. Immunohistochemical Study of SARS-CoV-2 Viral Entry Factors in the Cornea and Ocular Surface. Cornea 2020, 39, 1556–1562. [Google Scholar] [CrossRef]
- Barros, A.; Queiruga-Piñeiro, J.; Lozano-Sanroma, J.; Alcalde, I.; Gallar, J.; Fernández-Vega Cueto, L.; Alfonso, J.F.; Quirós, L.M.; Merayo-Lloves, J. Small fiber neuropathy in the cornea of COVID-19 patients associated with the generation of ocular surface disease. Ocul. Surf. 2022, 23, 40–48. [Google Scholar] [CrossRef]
- Shiers, S.; Ray, P.R.; Wangzhou, A.; Sankaranarayanan, I.; Tatsui, C.E.; Rhines, L.D.; Li, Y.; Uhelski, M.L.; Dougherty, P.M.; Price, T.J. ACE2 and SCARF expression in human dorsal root ganglion nociceptors: Implications for SARS-CoV-2 virus neurological effects. Pain 2020, 161, 2494–2501. [Google Scholar] [CrossRef]
- Yang, L.W.Y.; Mehta, J.S.; Liu, Y.C. Corneal neuromediator profiles following laser refractive surgery. Neural Regen. Res. 2021, 16, 2177–2183. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Troisi, M.; Troisi, S.; Strianese, D.; Rinaldi, M.; Turco, M.V.; Costagliola, C. Clinical and Biological Evaluation of NAAGA Versus Azelastine Eye Drops in Patients with Allergic Conjunctivitis and Tear Film Dysfunction: A Randomized Controlled Trial. Ophthalmol. Ther. 2025, 14, 1581–1595. [Google Scholar] [CrossRef]
- Troisi, M.; Del Prete, S.; Troisi, S.; Marasco, D.; Rinaldi, M.; Costagliola, C. Scanning Electron Microscopy (SEM) Evaluation of the Ultrastructural Effects on Conjunctival Epithelial Cells of a New Multiple-Action Artificial Tear Containing Cross-Linked Hyaluronic Acid, Cationic Liposomes and Trehalose. Biomedicines 2024, 12, 1945. [Google Scholar] [CrossRef]
- Troisi, M.; Del Prete, S.; Troisi, S.; Del Prete, A.; Bellucci, C.; Marasco, D.; Costagliola, C. The Role of Scanning Electron Microscopy in the Evaluation of Conjunctival Microvilli as an Early Biomarker of Ocular Surface Health: A Literature Review. J. Clin. Med. 2024, 13, 7569. [Google Scholar] [CrossRef]
- Troisi, M.; Caruso, C.; D’Andrea, L.; Rinaldi, M.; Piscopo, R.; Troisi, S.; Costagliola, C. Compatibility of a New Ocular Surface Dye with Disposable and Bi-Weekly Soft Contact Lenses: An Experimental Study. Life 2024, 14, 653. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Lin, N.; Chen, X.; Wu, X.T.; Tian, F.Y.; Yang, M.Y.; Liu, Y.S.; Lyu, F.; Deng, R.Z. Sleep and mental status as key factors to asthenopia in Chinese adults. Int. J. Ophthalmol. 2025, 18, 716–722. [Google Scholar] [CrossRef]
- Lin, N.; Zhu, Y.; Wu, X.; Yang, M.; Lu, F.; Deng, R. Prevalence and determinants of asthenopia among ophthalmologists in China: A national cross-sectional survey. Front. Public Health 2023, 11, 1290811. [Google Scholar] [CrossRef]
- Bitirgen, G.; Korkmaz, C.; Zamani, A.; Ozkagnici, A.; Zengin, N.; Ponirakis, G.; Malik, R.A. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br. J. Ophthalmol. 2022, 106, 1635–1641. [Google Scholar] [CrossRef]
- Mehraeen, E.; Behnezhad, F.; Salehi, M.A.; Noori, T.; Harandi, H.; SeyedAlinaghi, S. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): A review of current evidence. Eur. Arch. Otorhinolaryngol. 2021, 278, 307–312. [Google Scholar] [CrossRef]
- Dinkin, M.; Gao, V.; Kahan, J.; Bobker, S.; Simonetto, M.; Wechsler, P.; Harpe, J.; Greer, C.; Mints, G.; Salama, G.; et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology 2020, 95, 221–223. [Google Scholar] [CrossRef]
- O’Neill, F.; De Stefano, G.; Pridgeon, M.; Bhargava, D.; Marshall, A.; Marshall, A.; Frank, B. Trigeminal neuropathy presenting secondary to SARS-CoV-2 infection. Pain Rep. 2023, 8, e1103. [Google Scholar] [CrossRef]
- Nakamura, I.; Itoi, T.; Inoue, T. Case report of restless anal syndrome as restless legs syndrome variant after COVID-19. BMC Infect. Dis. 2021, 21, 993. [Google Scholar] [CrossRef]
- Tony, A.A.; Tony, E.A.; Ali, S.B.; Ezzeldin, A.M.; Mahmoud, A.A. COVID-19-associated sleep disorders: A case report. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100057. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Restless legs syndrome is associated with long-COVID in women. J. Clin. Sleep Med. 2022, 18, 1413–1418. [Google Scholar] [CrossRef]
- Westman, M.; Liinamaa, M.J. Relief of asthenopic symptoms with orthoptic exercises in convergence insufficiency is achieved in both adults and children. J. Optom. 2012, 5, 62–67. [Google Scholar] [CrossRef]
- Spiteri, R.; Barakat, S.; Vukicevic, M. COVID-19 and sudden-onset ocular neurogenic palsy in prior healthy patients: A systematic review. Strabismus 2023, 31, 145–151. [Google Scholar] [CrossRef]
- Altpeter, E.K.; Marx, T.; Nguyen, N.X.; Naumann, A.; Trauzettel-Klosinski, S. Measurement of reading speed with standardized texts: A comparison of single sentences and paragraphs. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1369–1375. [Google Scholar] [CrossRef]
- Pelli, D.G.; Chung, S.T.; Legge, G.E. Theories of reading should predict reading speed. Behav. Brain Sci. 2012, 35, 297–298. [Google Scholar] [CrossRef]
- Franco, B.; Morais, M.A.; Holanda, A.S.S.; Manconi, M.; de Mello, M.T.; Esteves, A.M. Impact of COVID-19 on the restless legs syndrome. Sleep Sci. 2020, 13, 186–190. [Google Scholar]
- Mohiuddin, O.; Khan, A.A.; Shah, S.M.I.; Malick, M.D.Z.; Memon, S.F.; Jalees, S.; Yasmin, F. New-onset restless leg syndrome in a COVID-19 patient: A case report with literature review. Pan. Afr. Med. J. 2021, 38, 318. [Google Scholar] [CrossRef]
- Borm, C.D.J.M.; Smilowska, K.; de Vries, N.M.; Bloem, B.R.; Theelen, T. How I do it: The Neuro-Ophthalmological Assessment in Parkinson’s Disease. J. Park. Dis. 2019, 9, 427–435. [Google Scholar]
- Armstrong, R.A. Oculo-Visual Dysfunction in Parkinson’s Disease. J. Park. Dis. 2015, 5, 715–726. [Google Scholar] [CrossRef]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Davitt, E.; Davitt, C.; Mazer, M.B.; Areti, S.S.; Hotchkiss, R.S.; Remy, K.E. COVID-19 disease and immune dysregulation. Best Pract. Res. Clin. Haematol. 2022, 35, 101401. [Google Scholar] [CrossRef]
- Dartt, D.A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef]
- Mauduit, P.; Jammes, H.; Rossignol, B. M3 muscarinic acetylcholine receptor coupling to PLC in rat exorbital lacrimal acinar cells. Am. J. Physiol. 1993, 264, C1550–C1560. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Tan, D.T.H.; Beuerman, R.W. Expression and Function of Muscarinic Receptor Subtypes on Human Cornea and Conjunctiva. Invest. Ophthalmol. Vis. Sci. 2007, 48, 2987–2996. [Google Scholar] [CrossRef]
- Kam, W.R.; Sullivan, D.A. Neurotransmitter Influence on Human Meibomian Gland Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2011, 52, 8543–8548. [Google Scholar] [CrossRef]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Invest. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative damage: The biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef]
- Hwang, J.S.; Shin, Y.J. Role of Choline in Ocular Diseases. Int. J. Mol. Sci. 2021, 22, 4733. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Singh, B.P.; Arora, N.; Gaur, S.N. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology 2010, 215, 527–534. [Google Scholar] [CrossRef]
- Hoover, D.B. Cholinergic modulation of the immune system presents new approaches for treating inflammation. Pharmacol. Ther. 2017, 179, 1–16. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, S.; Xia, G.; Wu, J.; Shen, Y.; Wang, Y.; Ostrom, R.S.; Du, A.; Shen, C.; Xu, C. Anti-inflammatory effects of α7-nicotinic ACh receptors are exerted through interactions with adenylyl cyclase-6. Br. J. Pharmacol. 2021, 178, 2324–2338. [Google Scholar] [CrossRef]
- Keever, K.R.; Cui, K.; Casteel, J.L.; Singh, S.; Hoover, D.B.; Williams, D.L.; Pavlov, V.A.; Yakubenko, V.P. Cholinergic signaling via the α7 nicotinic acetylcholine receptor regulates the migration of monocyte-derived macrophages during acute inflammation. J. Neuroinflamm. 2024, 21, 3. [Google Scholar] [CrossRef]
- Xu, X.M.; Ruan, J.H.; Tao, T.; Xiang, S.L.; Meng, R.L.; Chen, X. Role of vitamins in the pathogenesis and treatment of restless leg syndrome: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0313571. [Google Scholar] [CrossRef]
- González-Parejo, P.; Martín-Núñez, J.; Cabrera-Martos, I.; Valenza, M.C. Effects of Dietary Supplementation in Patients with Restless Legs Syndrome: A Systematic Review. Nutrients 2024, 16, 2315. [Google Scholar] [CrossRef]
- Lem, D.W.; Gierhart, D.L.; Davey, P.G. Can Nutrition Play a Role in Ameliorating Digital Eye Strain? Nutrients 2022, 14, 4005. [Google Scholar] [CrossRef] [PubMed]
- Barnish, M.; Sheikh, M.; Scholey, A. Nutrient Therapy for the Improvement of Fatigue Symptoms. Nutrients 2023, 15, 2154. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Y.; Cui, L.; Huang, L.; Guo, Q.; Huang, G. Study of Diet Habits and Cognitive Function in the Chinese Middle-Aged and Elderly Population: The Association between Folic Acid, B Vitamins, Vitamin D, Coenzyme Q10 Supplementation and Cognitive Ability. Nutrients 2023, 15, 1243. [Google Scholar] [CrossRef]
- Maggini, S.; Óvári, V.; Ferreres Giménez, I.; Pueyo Alamán, M.G. Benefits of micronutrient supplementation on nutritional status, energy metabolism, and subjective wellbeing. Nutr. Hosp. 2021, 38, 3–8. [Google Scholar] [PubMed]
- Monjotin, N.; Amiot, M.J.; Fleurentin, J.; Morel, J.M.; Raynal, S. Clinical Evidence of the Benefits of Phytonutrients in Human Healthcare. Nutrients 2022, 14, 1712. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline transport for phospholipid synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- Shaik-Dasthagirisaheb, Y.B.; Varvara, G.; Murmura, G.; Saggini, A.; Caraffa, A.; Antinolfi, P.; Tete’, S.; Tripodi, D.; Conti, F.; Cianchetti, E.; et al. Role of vitamins D, E and C in immunity and inflammation. J. Biol. Regul. Homeost Agents 2013, 27, 291–295. [Google Scholar]
- Shoeibi, A.; Olfati, N.; Soltani Sabi, M.; Salehi, M.; Mali, S.; Akbari Oryani, M. Effectiveness of coenzyme Q10 in prophylactic treatment of migraine headache: An open-label, add-on, controlled trial. Acta Neurol. Belg. 2017, 117, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Hargreaves, I.P.; Domingo, J.C.; Castro-Marrero, J. Mitochondrial Dysfunction and Coenzyme Q10 Supplementation in Post-Viral Fatigue Syndrome: An Overview. Int. J. Mol. Sci. 2024, 25, 574. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef]
- Ikemoto, K.; Mohamad Ishak, N.S.; Akagawa, M. The effects of pyrroloquinoline quinone disodium salt on brain function and physiological processes. J. Med. Investig. 2024, 71, 23–28. [Google Scholar] [CrossRef]
- Cruz, K.J.; Morais, J.B.; de Oliveira, A.R.; Severo, J.S.; Marreiro, D.D. The Effect of Zinc Supplementation on Insulin Resistance in Obese Subjects: A Systematic Review. Biol. Trace Elem. Res. 2017, 176, 239–243. [Google Scholar] [CrossRef]
- Khorshidi, M.; Zarezadeh, M.; Sadeghi, A.; Teymouri, A.; Emami, M.R.; Kord-Varkaneh, H.; Aryaeian, N.; Rahmani, J.; Mousavi, S.M. The Effect of Zinc Supplementation on Serum Leptin Levels: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2019, 51, 503–510. [Google Scholar] [CrossRef]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef] [PubMed]
- Bediz, C.S.; Baltaci, A.K.; Mogulkoc, R. Both zinc deficiency and supplementation affect plasma melatonin levels in rats. Acta Physiol. Hung. 2003, 90, 335–339. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B.; Beck, F.W.; Kucuk, O.; Sarkar, F.H. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004, 37, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Al-Bassam, L.; Shearman, G.C.; Brocchini, S.; Alany, R.G.; Williams, G.R. The Potential of Selenium-Based Therapies for Ocular Oxidative Stress. Pharmaceutics 2024, 16, 631. [Google Scholar] [CrossRef]
- Tutan, D.; Ulfberg, J.; Aydemir, N.; Eser, B.; Doğan, İ. The Relationship between Serum Selenium Levels and Restless Leg Syndrome in Chronic Kidney Disease Patients. Medicina 2023, 59, 1795. [Google Scholar] [CrossRef]
- Rahmanto, A.S.; Davies, M.J. Selenium-containing amino acids as direct and indirect antioxidants. IUBMB Life 2012, 64, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, G.; Sajjad, M.; Niloufar, R.; Neda, S.; Leila, S.; Khadijeh, M. Effect of melatonin supplementation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2022, 269, 205–216. [Google Scholar] [CrossRef] [PubMed]
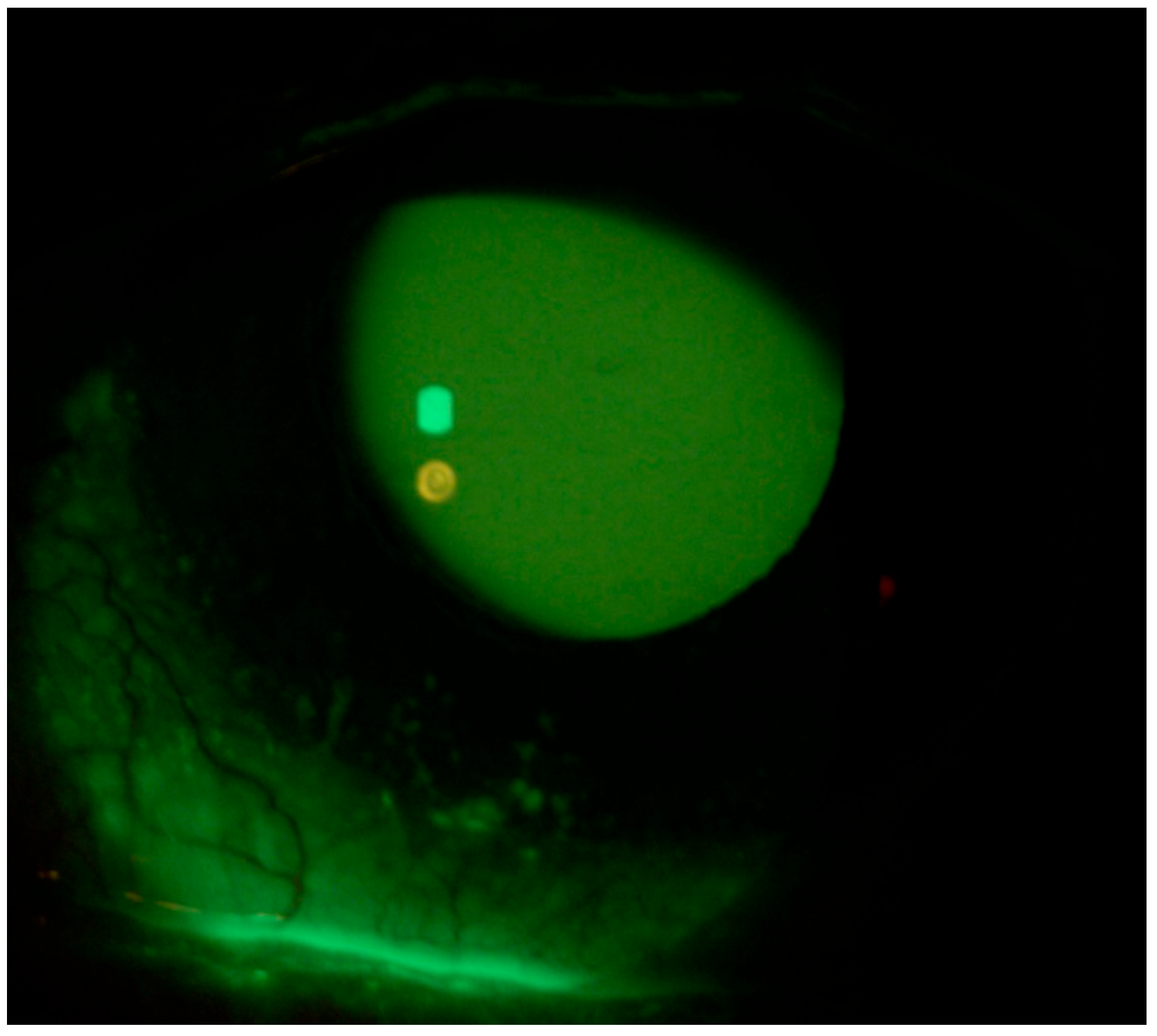

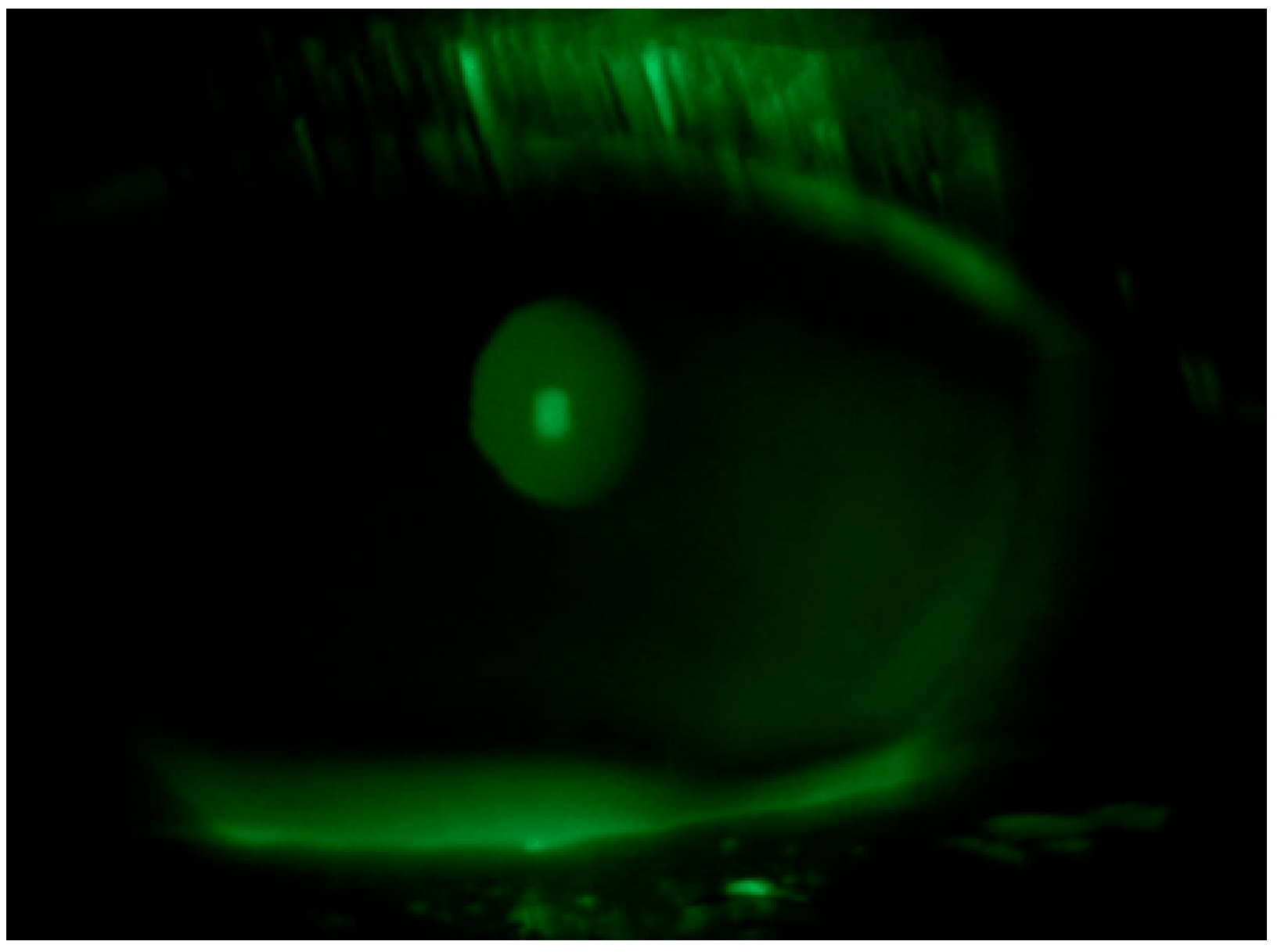

| Fluotest (NEI Score) | TBUT | Schirmer Test I (mm) | OSDI Score | Reading Speed (wpm) | Proximal Point of Convergence (PPC) (cm) | Neurological Disorders | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |||||
| Time 0 | 2 | 3 | 3” | 2” | 8 | 6 | 36 | 95 | 38 | Sleep Disorders, Asthenia, and Restless Legs Syndrome |
| 10 Days | 0 | 0 | 6” | 5” | 10 | 9 | 25 | 128 | 36 | Sleep Disorders, Asthenia, and Restless Legs Syndrome |
| 42 Days | 0 | 0 | 9” | 8” | 10 | 9 | 11 | 222 | 19 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troisi, M.; Troisi, S.; Vitiello, L.; Strianese, D.; Bellucci, C.; Rinaldi, M.; D’Andrea, L.; Costagliola, C. Ocular and Neurological Sequelae in Long COVID: Dry Eye, Asthenopia, Sleep Disorders, Asthenia, and Restless Legs Syndrome—A Case Report with Literature Review. Life 2025, 15, 1289. https://doi.org/10.3390/life15081289
Troisi M, Troisi S, Vitiello L, Strianese D, Bellucci C, Rinaldi M, D’Andrea L, Costagliola C. Ocular and Neurological Sequelae in Long COVID: Dry Eye, Asthenopia, Sleep Disorders, Asthenia, and Restless Legs Syndrome—A Case Report with Literature Review. Life. 2025; 15(8):1289. https://doi.org/10.3390/life15081289
Chicago/Turabian StyleTroisi, Mario, Salvatore Troisi, Livio Vitiello, Diego Strianese, Carlo Bellucci, Michele Rinaldi, Luca D’Andrea, and Ciro Costagliola. 2025. "Ocular and Neurological Sequelae in Long COVID: Dry Eye, Asthenopia, Sleep Disorders, Asthenia, and Restless Legs Syndrome—A Case Report with Literature Review" Life 15, no. 8: 1289. https://doi.org/10.3390/life15081289
APA StyleTroisi, M., Troisi, S., Vitiello, L., Strianese, D., Bellucci, C., Rinaldi, M., D’Andrea, L., & Costagliola, C. (2025). Ocular and Neurological Sequelae in Long COVID: Dry Eye, Asthenopia, Sleep Disorders, Asthenia, and Restless Legs Syndrome—A Case Report with Literature Review. Life, 15(8), 1289. https://doi.org/10.3390/life15081289







