Effectiveness of a Post-Acute-Care Rehabilitation Program in Patients with Stroke: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
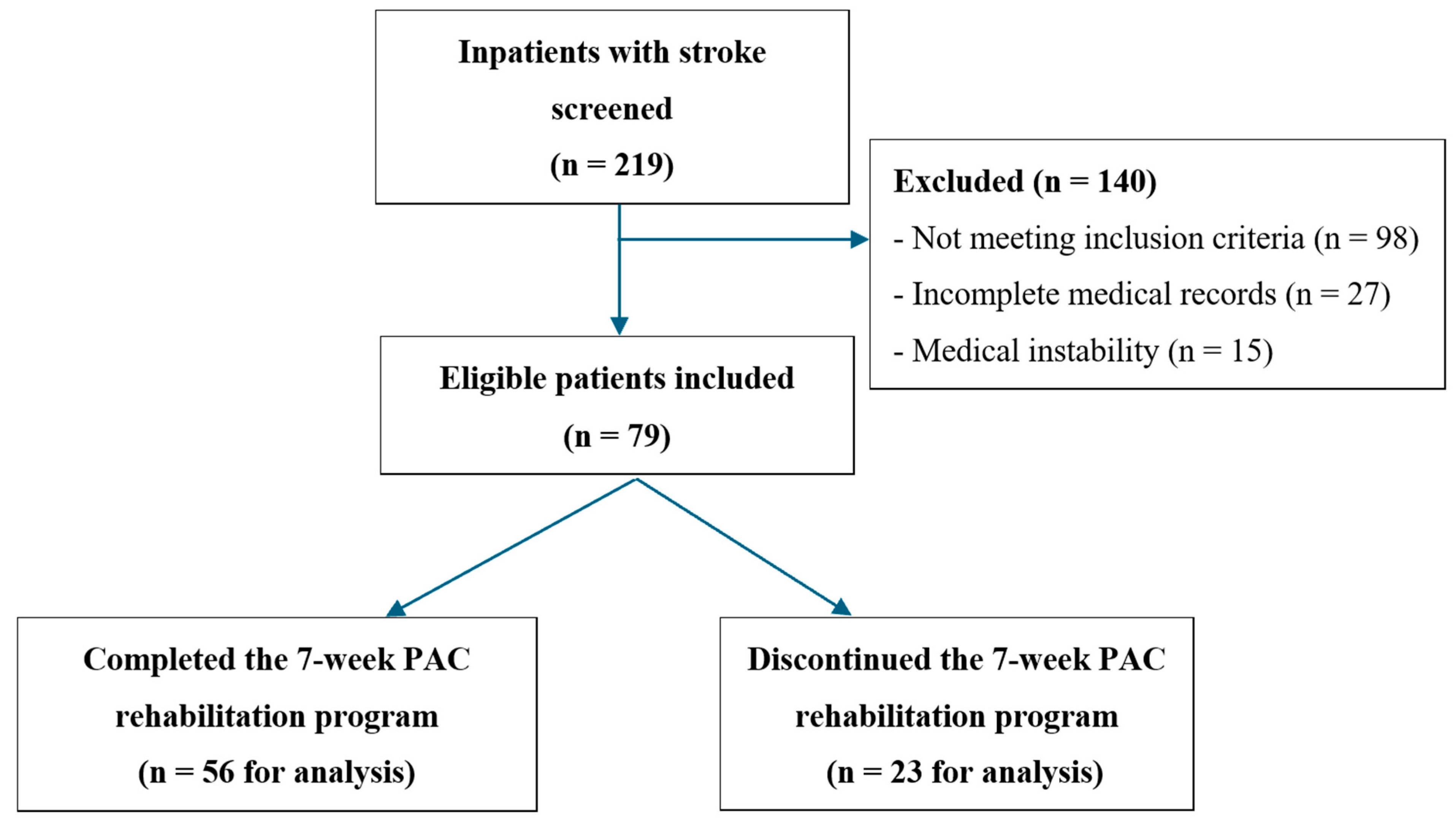
2.2. Participants
2.3. PAC Rehabilitation Program
2.4. Data Collection
2.4.1. Sociodemographic and Lifestyle
2.4.2. Outcome Indicators
2.4.3. Muscle Strength
2.4.4. Physical Performance
2.4.5. Functional Recovery
2.4.6. Nutritional Status
2.4.7. Cognitive Function
2.4.8. Occupational Performance
2.4.9. Language Ability
2.5. Ethical Consideration
2.6. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Comparisons of Sociodemographic and Disease Characteristics Between Patients Who Completed and Those Who Discontinued the Pac Rehabilitation Program
3.3. Comparisons of Muscle Strength, Physical Performance, and Functional Recovery Between and Within Groups
3.4. Effects of the PAC Rehabilitation Program Based on GEE Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; Abd ElHafeez, S.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2020: Life Expectancy and Leading Causes of Death and Disability; World Health Organization: Geneva, Switzerland, 2020; Available online: https://who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 1 June 2025).
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A. Comprehensive Overview of Nursing and Interdisciplinary Rehabilitation Care of the Stroke Patient. Stroke 2010, 41, 2402–2448. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Tsai, M.M.; Luo, J.Y.; Liao, W.C.; Hsu, P.S.; Chen, H.Y. Post-acute care for stroke–a retrospective cohort study in Taiwan. Patient Prefer. Adherence 2017, 11, 1309–1315. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Marshall, I.J.; Wolfe, C.D.; Wang, Y.; O’Connell, M.D. Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Med. 2023, 20, e1004200. [Google Scholar] [CrossRef]
- Chun, H.-Y.Y.; Ford, A.; Kutlubaev, M.A.; Almeida, O.P.; Mead, G.E. Depression, Anxiety, and Suicide After Stroke: A Narrative Review of the Best Available Evidence. Stroke 2022, 53, 1402–1410. [Google Scholar] [CrossRef]
- Wei, X.; Sun, S.; Zhang, M.; Zhao, Z. A systematic review and meta-analysis of clinical efficacy of early and late rehabilitation interventions for ischemic stroke. BMC Neurol. 2024, 24, 91. [Google Scholar] [CrossRef]
- Chao, T.-C.; Lin, C.-H.; Lee, M.-S.; Chang, C.-C.; Lai, C.-Y.; Huang, C.-Y.; Chang, W.-Y.; Chiang, S.-L. The Efficacy of Early Rehabilitation Combined with Virtual Reality Training in Patients with First-Time Acute Stroke: A Randomized Controlled Trial. Life 2024, 14, 847. [Google Scholar] [CrossRef]
- Buntin, M.B. Access to postacute rehabilitation. Arch. Phys. Med. Rehabil. 2007, 88, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Chi, N.F.; Wang, S.J. Postacute care program of stroke: Better functional recovery. J. Chin. Med. Assoc. 2019, 82, 811. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Lee, T.-H.; Chang, K.-C. A Nationwide Plan for Postacute Care of Stroke in Taiwan. Int. J. Stroke 2014, 9, E3. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; O’Brien, M. Aids to the investigation of peripheral nerve injuries. Brain 2010, 133, 2838–2844. [Google Scholar] [CrossRef]
- Gregson, J.M.; Leathley, M.J.; Moore, A.P.; Smith, T.L.; Sharma, A.K.; Watkins, C.L. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 2000, 29, 223–228. [Google Scholar] [CrossRef]
- Maeda, N.; Kato, J.; Shimada, T. Predicting the probability for fall incidence in stroke patients using the Berg Balance Scale. J. Int. Med. Res. 2009, 37, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Won, C.W.; Kim, B.-S.; Choi, H.; Kim, S.; Choi, S.-E.; Hong, S. The Cut-Off Point of Gait Speed as Predictor of 3 Year Mortality in Korean Community-Dwelling Elderly. Korean J. Fam. Pract. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef]
- Macchiavelli, A.; Giffone, A.; Ferrarello, F.; Paci, M. Reliability of the six-minute walk test in individuals with stroke: Systematic review and meta-analysis. Neurol. Sci. 2021, 42, 81–87. [Google Scholar] [CrossRef]
- Banks, J.L.; Marotta, C.A. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Quinn, T.J.; Langhorne, P.; Stott, D.J. Barthel Index for Stroke Trials. Stroke 2011, 42, 1146–1151. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hodgson, C. Clinimetrics: The Lawton-Brody Instrumental Activities of Daily Living Scale. J. Physiother. 2023, 69, 57. [Google Scholar] [CrossRef]
- Vittengl, J.R.; White, C.N.; McGovern, R.J.; Morton, B.J. Comparative validity of seven scoring systems for the instrumental activities of daily living scale in rural elders. Aging Ment. Health 2006, 10, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.; Ng, B.; Chan, D.; Chan, E.; Ma, D.; Au, B.; Chiu, V.; Chang, A.; Wan, K.; Chan, A.; et al. Development of the Hong Kong Version of the Functional Test for the Hemiplegic Upper Extremity (FTHUE-HK). Hong Kong J. Occup. Ther. 2004, 14, 21–29. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilit. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Crary, M.A.; Mann, G.D.; Groher, M.E. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition: The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pezzotti, P.; Scalmana, S.; Mastromattei, A.; Di Lallo, D.; “Progetto Alzheimer” Working Group. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: A prospective observational study. BMC Fam. Pract. 2008, 9, 29. [Google Scholar] [CrossRef]
- van der Lee, J.H.; Beckerman, H.; Knol, D.L.; de Vet, H.C.W.; Bouter, L.M. Clinimetric Properties of the Motor Activity Log for the Assessment of Arm Use in Hemiparetic Patients. Stroke 2004, 35, 1410–1414. [Google Scholar] [CrossRef]
- Chung, Y.M.; Lee, S.E.; Chang, M.H.; Hsu, T.C. The concise Chinese aphasia test and its application. J Speech Lang Hear. Assoc 1998, 13, 119–137. [Google Scholar]
- Ballinger, G.A. Using Generalized Estimating Equations for Longitudinal Data Analysis. Organ. Res. Methods 2004, 7, 127–150. [Google Scholar] [CrossRef]
- Xing, F.; Liu, J.; Mei, C.; Chen, J.; Wen, Y.; Zhou, J.; Xie, S. Adherence to rehabilitation exercise and influencing factors among people with acute stroke: A cross-sectional study. Front. Neurol. 2025, 16, 1554949. [Google Scholar] [CrossRef] [PubMed]
- Brusco, N.; Morris, M.E.; Foster, S.; Woods, J.; McCaskie, D.; Goodman, S.; Barnes, C.; Keren, C.; Frawley, H. Improving stroke clinical guideline adherence in an Australian hospital using a clinician-led implementation process. Top. Stroke Rehabil. 2023, 30, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Gaalema, D.E.; Cutler, A.Y.; Higgins, S.T.; Ades, P.A. Smoking and cardiac rehabilitation participation: Associations with referral, attendance and adherence. Prev. Med. 2015, 80, 67–74. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Du, Y.; Li, J.; Wang, A.; Zhao, X. Gait speed after mild stroke/transient ischemic attack was associated with long-term adverse outcomes: A cohort study. Ann. Clin. Transl. Neurol. 2024, 11, 3163–3174. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, C.; Dong, J.; Xue, Z.; Cai, F.; Li, X.; Hu, Z.; Xue, X. The effect of unaffected side resistance training on upper limb function reconstruction and prevention of sarcopenia in stroke patients: A randomized controlled trial. Sci. Rep. 2024, 14, 25330. [Google Scholar] [CrossRef]
- Kang, D.; Park, J.A. Community-Based Resistance Training Exercise for Post-Stroke Patients with Sarcopenia: Bridging Institutional and Community-Based Rehabilitation in a Multicenter, Randomized Controlled Trial. Life 2025, 15, 748. [Google Scholar] [CrossRef]
- Roelofs, J.M.B.; Zandvliet, S.B.; Schut, I.M.; Huisinga, A.C.M.; Schouten, A.C.; Hendricks, H.T.; de Kam, D.; Aerden, L.A.M.; Bussmann, J.B.J.; Geurts, A.C.H.; et al. Mild Stroke, Serious Problems: Limitations in Balance and Gait Capacity and the Impact on Fall Rate and Physical Activity. Neurorehabilit. Neural Repair 2023, 37, 786–798. [Google Scholar] [CrossRef]
- Chien, S.-H.; Sung, P.-Y.; Liao, W.-L.; Tsai, S.-W. A functional recovery profile for patients with stroke following post-acute rehabilitation care in Taiwan. J. Formos. Med. Assoc. 2020, 119, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.R.; Moudgal, R.; Lang, K.; Hyacinth, H.I.; Awosika, O.O.; Kissela, B.M.; Feng, W. Early Rehabilitation After Stroke: A Narrative Review. Curr. Atheroscler. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Hu, M.H.; Hsu, S.S.; Yip, P.K.; Jeng, J.S.; Wang, Y.H. Early and intensive rehabilitation predicts good functional outcomes in patients admitted to the stroke intensive care unit. Disabil. Rehabil. 2010, 32, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Ibhiedu, J.O.; Egbunu, E.O.; Ndam, A.R.; Ogala, F.; Ologunde, T.; Peterson, J.C.; Boluwatife, A.I.; et al. Stroke and cognitive impairment: Understanding the connection and managing symptoms. Ann. Med. Surg. 2023, 85, 6057–6066. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Li, Y.-C.; Huang, C.-Y.; Lin, C.-J.; Tien, C.-J.; Chen, W.-S.; Chen, C.-L.; Lin, K.-C. Predicting Arm Nonuse in Individuals with Good Arm Motor Function after Stroke Rehabilitation: A Machine Learning Study. J. Environ. Res. Public Health 2023, 20, 4123. [Google Scholar] [CrossRef]
- Stewart, J.C.; Lewthwaite, R.; Rocktashel, J.; Winstein, C.J. Self-efficacy and reach performance in individuals with mild motor impairment due to stroke. Neurorehabilit. Neural Repair 2019, 33, 319–328. [Google Scholar] [CrossRef]
- Brady, M.C.; Kelly, H.; Godwin, J.; Enderby, P.; Campbell, P. Speech and language therapy for aphasia following stroke. Cochrane Database Syst. Rev. 2016, 6, Cd000425. [Google Scholar] [CrossRef] [PubMed]
| Variable | All (n = 79) | Completed (n = 56) | Discontinued (n = 23) | x2/Z | p * |
|---|---|---|---|---|---|
| Mean (SD)/n (%) | Mean (SD)/n (%) | Mean (SD)/n (%) | |||
| Stroke type | 1.81 a | 0.215 | |||
| Hemorrhagic stroke | 14 (17.7) | 12 (21.4) | 2 (8.7) | ||
| Ischemic stroke | 65 (82.3) | 44 (78.6) | 21 (91.3) | ||
| Sociodemographic | |||||
| Age (years) | 67.35 (13.62) | 66.00 (13.25) | 70.65 (14.24) | −1.46 | 0.143 |
| Sex | 0.01 a | 0.910 | |||
| Men | 42 (53.2) | 30 (53.6) | 12 (52.2) | ||
| Women | 37 (46.8) | 26 (46.4) | 11 (47.8) | ||
| Caregiver | 0.01 a | 0.994 | |||
| Family members | 38 (48.1) | 27 (48.2) | 11 (47.8) | ||
| Foreign domestic worker | 21 (26.6) | 15 (26.8) | 6 (26.1) | ||
| Professional caregiver | 20 (25.3) | 14 (25.0) | 6 (26.1) | ||
| Marital status | 3.13 a | 0.373 | |||
| Single/divorced/widowed | 16 (20.3) | 12 (21.4) | 4 (17.4) | ||
| Married | 63 (79.7) | 44 (78.6) | 19 (82.6) | ||
| Religious belief | 41 (51.9) | 31 (55.4) | 10 (43.5) | 2.13 a | 0.831 |
| Currently employed | 26 (32.9) | 20 (35.7) | 6 (26.1) | 2.30 a | 0.890 |
| Operation history | 59 (74.7) | 41 (73.2) | 18 (78.3) | 5.47 a | 0.362 |
| Living status | 0.07 a | 0.787 | |||
| Alone | 8 (10.1) | 6 (10.7) | 2 (8.7) | ||
| Living with families | 71 (89.9) | 50 (89.3) | 21 (91.3) | ||
| Lifestyle | |||||
| Current smoker | 16 (20.3) | 8 (14.3) | 8 (34.8) | 4.24 a | 0.039 |
| Alcohol consumption | 12 (15.2) | 8 (14.3) | 4 (17.4) | 0.12 a | 0.727 |
| Length of stay (days) | 35.00 (9.93) | 40.05 (4.10) | 22.70 (9.19) | −6.73 | <0.001 |
| Comorbidities | |||||
| Hypertension | 55 (69.6) | 40 (71.4) | 15 (65.2) | 0.30 a | 0.586 |
| Heart disease | 21 (26.6) | 15 (26.8) | 6 (26.1) | 0.01 a | 0.949 |
| Type 2 diabetes | 31 (39.2) | 21 (37.5) | 10 (43.5) | 0.24 a | 0.621 |
| Lipidemia | 5 (6.3) | 3 (5.4) | 2 (8.7) | 0.31 a | 0.580 |
| Muscle strength | |||||
| Affected upper limb | 2.71 (1.40) | 2.52 (1.41) | 3.17 (1.27) | −2.00 | 0.046 |
| Affected lower limb | 3.03 (1.30) | 2.86 (1.33) | 3.43 (1.16) | −2.02 | 0.043 |
| Unaffected upper limb | 4.53 (0.71) | 4.52 (0.76) | 4.57 (0.59) | −0.14 | 0.888 |
| Unaffected lower limb | 4.34 (0.83) | 4.38 (0.87) | 4.26 (0.75) | −0.90 | 0.368 |
| Physical performance | |||||
| BBS (range: 0–56) | 23.97 (21.08) | 23.60 (21.08) | 24.83 (21.53) | −0.51 | 0.608 |
| Gait speed (m/s) | 0.26 (0.33) | 0.21 (0.33) | 0.47 (0.26) | −3.16 | 0.002 |
| 6MWT (meters) | 87.17 (110.56) | 68.85 (101.78) | 172.67 (113.90) | −3.42 | <0.001 |
| Functional recovery | |||||
| mRS (range: 0–6) | 3.87 (0.34) | 3.88 (0.33) | 3.87 (0.34) | −0.07 | 0.948 |
| 3 | 10 (12.7) | 7 (12.5) | 3 (13.0) | 0.01 a | 0.947 |
| 4 | 69 (87.3) | 49 (87.5) | 20 (87.0) | ||
| Barthel Index (range: 0–100) | 38.18 (24.01) | 35.80 (21.68) | 45.56 (29.65) | −0.99 | 0.320 |
| IADL | 1.36 (1.44) | 1.37 (1.42) | 1.32 (1.52) | −0.22 | 0.826 |
| Male (range: 0–5) | 1.61 (1.61) | 1.63 (1.56) | 1.55 (1.81) | −0.29 | 0.773 |
| Female (range: 0–8) | 1.06 (1.16) | 1.04 (1.16) | 1.09 (1.22) | −0.13 | 0.896 |
| FMA-Sensory (Range: 0–44) | 23.55 (17.64) | 21.14 (17.81) | 29.95 (15.81) | −2.27 | 0.023 |
| FMA-Upper extremity (Range: 0–66) | 29.00 (21.86) | 26.78 (21.50) | 34.30 (22.29) | −1.54 | 0.125 |
| Nutritional status | |||||
| FOIS (range: 1–7) | 4.92 (2.41) | 5.02 (2.45) | 4.70 (2.36) | −0.42 | 0.675 |
| MNA (range: 0–30) | 11.78 (1.72) | 11.64 (1.86) | 12.13 (1.25) | −0.99 | 0.324 |
| Cognitive function | |||||
| MMSE (range: 0–30) | 20.05 (10.29) | 19.41 (10.93) | 21.85 (8.24) | −0.84 | 0.400 |
| Occupational performance | |||||
| Amount of use (range: 0–5) | 2.87 (6.25) | 3.33 (7.28) | 1.70 (1.56) | −0.74 | 0.462 |
| Quality of movement (range: 0–5) | 1.20 (1.56) | 1.03 (1.53) | 1.64 (1.58) | −1.70 | 0.089 |
| Language ability | |||||
| CCAT (range: 0–12) | 8.25 (3.75) | 8.42 (3.85) | 7.65 (3.42) | −1.36 | 0.174 |
| Completed (n = 56) | Discontinued (n = 23) | Between-Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Baseline | 3-Week | 7-Week | Baseline vs. 3-Week | Baseline vs. 7-Week | Baseline | 3-Week | Baseline vs. 3-Week | Baseline | 3-Week | 3-Week vs. Baseline Mean Differences | ||||||
| t | p | t | p | t | p | Z | p * | Z | p * | Z | p * | ||||||
| Muscle strength | |||||||||||||||||
| Affected side | |||||||||||||||||
| Upper limb | 2.52 (1.41) | 3.00 (1.27) | 3.56 (1.42) | −1.90 | 0.060 | 3.79 | <0.001 | 3.17 (1.27) | 3.15 (1.21) | 0.05 | 0.963 | −2.00 | 0.046 | −0.29 | 0.771 | −0.21 | 0.831 |
| Lower limb | 2.86 (1.33) | 3.21 (1.20) | 3.82 (1.13) | −1.49 | 0.138 | 3.99 | <0.001 | 3.43 (1.16) | 3.23 (1.17) | 0.51 | 0.616 | −2.02 | 0.043 | −0.14 | 0.888 | −1.04 | 0.300 |
| Unaffected side | |||||||||||||||||
| Upper limb | 4.52 (0.76) | 4.77 (0.57) | 4.86 (0.41) | −1.96 | 0.052 | 2.93 | 0.004 | 4.57 (0.59) | 4.54 (0.66) | 0.13 | 0.901 | −0.14 | 0.888 | −1.59 | 0.111 | −0.90 | 0.369 |
| Lower limb | 4.38 (0.87) | 4.54 (0.79) | 4.70 (0.71) | −1.03 | 0.305 | 2.13 | 0.036 | 4.26 (0.75) | 4.08 (1.12) | 0.59 | 0.558 | −0.90 | 0.368 | −1.79 | 0.073 | −0.04 | 0.967 |
| Physical performance | |||||||||||||||||
| BBS (score) | 23.60 (21.08) | 31.37 (21.55) | 33.79 (21.85) | −1.88 | 0.063 | 2.38 | 0.020 | 24.83 (21.52) | 30.42 (24.35) | −0.70 | 0.490 | −0.51 | 0.608 | −0.22 | 0.829 | −0.72 | 0.470 |
| Gait speed (m/s) | 0.21 (0.33) | 0.31 (0.36) | 0.48 (0.78) | −1.48 | 0.143 | 2.15 | 0.035 | 0.47 (0.26) | 0.62 (0.38) | −1.10 | 0.284 | −3.16 | 0.002 | −2.14 | 0.032 | −1.75 | 0.080 |
| 6MWT (meters) | 68.85 (101.78) | 111.46 (131.16) | 148.88 (156.08) | −1.92 | 0.058 | 3.02 | 0.003 | 172.67 (113.90) | 239.25 (154.75) | −1.11 | 0.281 | −3.42 | <0.001 | −2.28 | 0.022 | −1.73 | 0.084 |
| Functional recovery | |||||||||||||||||
| mRS | 3.88 (0.33) | 3.82 (0.43) | 3.75 (0.51) | 0.74 | 0.464 | −1.53 | 0.130 | 3.87 (0.34) | 3.74 (0.54) | 0.98 | 0.335 | −0.07 | 0.948 | −0.63 | 0.531 | −1.16 | 0.244 |
| Barthel Index | 35.80 (21.68) | 49.04 (26.76) | 57.10 (28.98) | −2.88 | 0.005 | 4.24 | <0.001 | 45.56 (29.65) | 50.56 (33.68) | −0.40 | 0.696 | −0.99 | 0.320 | −0.01 | 0.992 | −0.47 | 0.638 |
| IADL | 1.37 (1.42) | 2.49 (1.96) | 2.98 (2.18) | −3.33 | 0.001 | 4.32 | <0.001 | 1.32 (1.52) | 2.36 (2.06) | −1.65 | 0.109 | −0.22 | 0.826 | −0.25 | 0.799 | −0.53 | 0.596 |
| FMA-Sensory | 21.14 (17.81) | 28.88 (17.87) | 33.65 (15.28) | −2.29 | 0.024 | 3.87 | <0.001 | 29.95 (15.81) | 31.18 (17.08) | −0.20 | 0.840 | −2.27 | 0.023 | −0.27 | 0.786 | −0.99 | 0.324 |
| FMA-Upper extremity | 26.78 (21.50) | 36.55 (22.06) | 39.98 (22.32) | −2.36 | 0.020 | 3.09 | 0.003 | 34.30 (22.29) | 41.83 (25.00) | −0.91 | 0.369 | −1.54 | 0.125 | −1.08 | 0.281 | −1.17 | 0.241 |
| Nutritional status | |||||||||||||||||
| FOIS | 5.02 (2.45) | 5.68 (2.42) | 5.80 (1.90) | −1.44 | 0.154 | 1.85 | 0.067 | 4.70 (2.36) | 5.33 (2.15) | −0.78 | 0.440 | −0.42 | 0.675 | −0.42 | 0.676 | −0.04 | 0.971 |
| MNA | 11.64 (1.86) | 11.70 (2.24) | 12.08 (1.66) | −0.14 | 0.891 | 1.27 | 0.208 | 12.13 (1.25) | 12.25 (1.14) | −0.28 | 0.784 | −0.99 | 0.324 | −0.47 | 0.638 | −0.28 | 0.778 |
| Cognitive function | |||||||||||||||||
| MMSE | 19.41 (10.93) | 22.25 (9.97) | 24.00 (6.56) | −1.44 | 0.154 | 2.63 | 0.010 | 21.85 (8.24) | 22.55 (7.45) | −0.23 | 0.818 | −0.84 | 0.400 | −0.09 | 0.932 | −0.25 | 0.806 |
| Occupational performance | |||||||||||||||||
| Amount of use | 3.33 (7.28) | 3.08 (6.03) | 2.03 (2.03) | 0.20 | 0.844 | −1.28 | 0.204 | 1.70 (1.56) | 2.08 (2.10) | −0.56 | 0.584 | −0.74 | 0.462 | −0.06 | 0.950 | −0.42 | 0.678 |
| Quality of movement | 1.03 (1.53) | 1.63 (1.82) | 2.07 (2.03) | −1.91 | 0.059 | 2.95 | 0.004 | 1.64 (1.58) | 2.08 (2.10) | −0.66 | 0.520 | −1.70 | 0.089 | −0.42 | 0.673 | −0.52 | 0.605 |
| Language ability | |||||||||||||||||
| CCAT | 8.42 (3.85) | 8.96 (3.47) | 9.34 (3.35) | −0.78 | 0.438 | 1.30 | 0.196 | 7.65 (3.42) | 7.64 (3.60) | 0.00 | 0.999 | −1.36 | 0.174 | −1.42 | 0.157 | −0.489 | 0.625 |
| Parameter | B | SE | 95% CI | p | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Muscle strength | ||||||
| Affected side | ||||||
| Upper limb | ||||||
| 3-week | 0.31 | 0.20 | −0.08 | 0.70 | 0.115 | |
| 7-week | 0.93 | 0.22 | 0.49 | 1.36 | <0.001 | |
| Lower limb | ||||||
| 3-week | 0.19 | 0.18 | −0.16 | 0.54 | 0.28 | |
| 7-week | 0.88 | 0.18 | 0.52 | 1.24 | <0.001 | |
| Unaffected side | ||||||
| Upper limb | ||||||
| 3-week | 0.18 | 0.10 | −0.01 | 0.37 | 0.069 | |
| 7-week | 0.32 | 0.09 | 0.15 | 0.50 | <0.001 | |
| Lower limb | ||||||
| 3-week | 0.09 | 0.12 | −0.16 | 0.33 | 0.489 | |
| 7-week | 0.33 | 0.12 | 0.09 | 0.57 | 0.006 | |
| Physical performance | ||||||
| BBS | ||||||
| 3-week | 6.95 | 2.88 | 1.32 | 12.6 | 0.016 | |
| 7-week | 9.70 | 3.26 | 3.32 | 16.1 | 0.003 | |
| Gait speed (m/s) | ||||||
| 3-week | 0.10 | 0.05 | 0.00 | 0.20 | 0.044 | |
| 7-week | 0.23 | 0.10 | 0.03 | 0.44 | 0.024 | |
| 6MWT (meters) | ||||||
| 3-week | 43.6 | 17.20 | 9.88 | 77.3 | 0.011 | |
| 7-week | 67.6 | 20.5 | 27.4 | 108 | 0.001 | |
| Functional recovery | ||||||
| mRS | ||||||
| 3-week | −0.08 | 0.05 | −0.18 | 0.03 | 0.159 | |
| 7-week | −0.13 | 0.06 | −0.24 | −0.01 | 0.028 | |
| Barthel Index | ||||||
| 3-week | 10.8 | 3.14 | 4.62 | 16.9 | 0.001 | |
| 7-week | 19.5 | 3.66 | 12.3 | 26.7 | <0.001 | |
| IADL | ||||||
| 3-week | 1.02 | 0.23 | 0.56 | 1.48 | <0.001 | |
| 7-week | 1.48 | 0.28 | 0.93 | 2.03 | <0.001 | |
| FMA-Sensory | ||||||
| 3-week | 5.96 | 2.52 | 1.03 | 10.9 | 0.018 | |
| 7-week | 10.2 | 2.65 | 5.03 | 15.4 | <0.001 | |
| FMA-Upper extremity | ||||||
| 3-week | 8.06 | 3.06 | 2.07 | 14.0 | 0.008 | |
| 7-week | 11.8 | 3.37 | 5.21 | 18.4 | <0.001 | |
| Nutritional status | ||||||
| FOIS | ||||||
| 3-week | 0.65 | 0.35 | −0.03 | 1.34 | 0.061 | |
| 7-week | 0.85 | 0.34 | 0.19 | 1.51 | 0.012 | |
| MNA | ||||||
| 3-week | −0.02 | 0.27 | −0.55 | 0.51 | 0.934 | |
| 7-week | 0.24 | 0.25 | −0.26 | 0.73 | 0.355 | |
| Cognitive function | ||||||
| MMSE | ||||||
| 3-week | 2.13 | 1.47 | −0.75 | 5.00 | 0.147 | |
| 7-week | 3.36 | 1.45 | 0.52 | 6.19 | 0.02 | |
| Occupational performance | ||||||
| Amount of use (AOU) | ||||||
| 3-week | −0.07 | 0.89 | −1.81 | 1.66 | 0.933 | |
| 7-week | −0.96 | 0.77 | −2.48 | 0.56 | 0.216 | |
| Quality of movement (QOM) | ||||||
| 3-week | 0.48 | 0.21 | 0.06 | 0.90 | 0.026 | |
| 7-week | 0.93 | 0.25 | 0.44 | 1.42 | <0.001 | |
| Language ability | ||||||
| CCAT | ||||||
| 3-week | 0.50 | 0.57 | −0.61 | 1.61 | 0.380 | |
| 7-week | 0.98 | 0.60 | −0.20 | 2.15 | 0.105 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, Y.-P.; Wang, M.-C.; Chen, Y.-H.; Chiang, S.-L.; Lin, C.-H. Effectiveness of a Post-Acute-Care Rehabilitation Program in Patients with Stroke: A Retrospective Cohort Study. Life 2025, 15, 1216. https://doi.org/10.3390/life15081216
Lo Y-P, Wang M-C, Chen Y-H, Chiang S-L, Lin C-H. Effectiveness of a Post-Acute-Care Rehabilitation Program in Patients with Stroke: A Retrospective Cohort Study. Life. 2025; 15(8):1216. https://doi.org/10.3390/life15081216
Chicago/Turabian StyleLo, Yi-Pang, Mei-Chen Wang, Yao-Hsiang Chen, Shang-Lin Chiang, and Chia-Huei Lin. 2025. "Effectiveness of a Post-Acute-Care Rehabilitation Program in Patients with Stroke: A Retrospective Cohort Study" Life 15, no. 8: 1216. https://doi.org/10.3390/life15081216
APA StyleLo, Y.-P., Wang, M.-C., Chen, Y.-H., Chiang, S.-L., & Lin, C.-H. (2025). Effectiveness of a Post-Acute-Care Rehabilitation Program in Patients with Stroke: A Retrospective Cohort Study. Life, 15(8), 1216. https://doi.org/10.3390/life15081216






