Epigenetic Modifications in Osteosarcoma: Mechanisms and Therapeutic Strategies
Abstract
1. Introduction
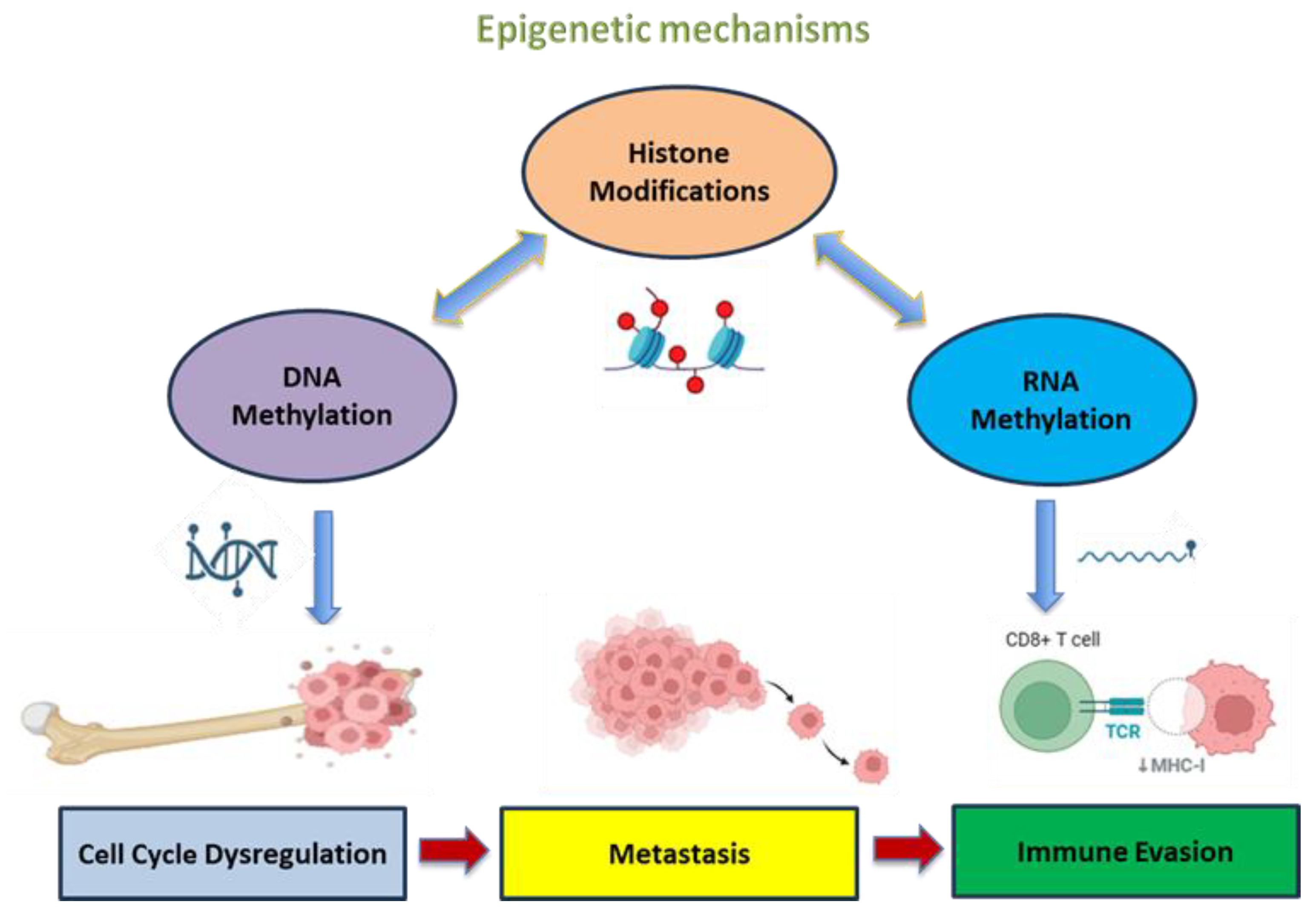
2. Epigenetic Mechanisms in Cancer
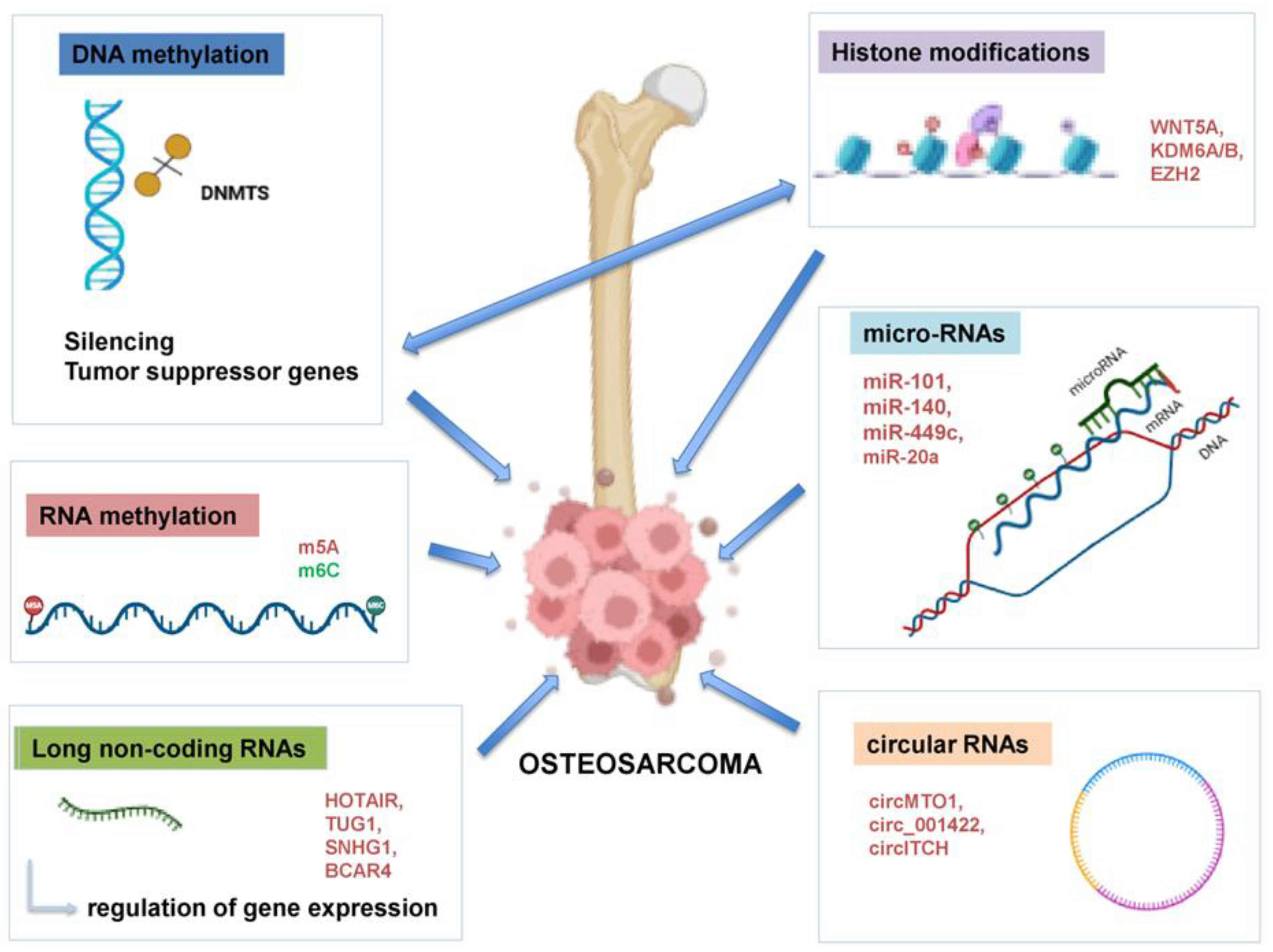
3. Implication of Epigenetic Mechanisms in OS
3.1. Tumor Suppressor Pathways Affected by Methylation
3.2. DNA Methyltransferases (DNMTs)
3.3. Additional Methylation Targets
3.4. RNA Methylation
3.5. Histone Modification in OS
3.6. MicroRNAs (miRNAs)
| miRNA | Expression | Target | Effect in OS | Mechanism | Reference |
|---|---|---|---|---|---|
| miR-9 | Elevated | E-cadherin | Loss of epithelial characteristics | Induces EMT and lung metastasis | [82] |
| miR-21 | Elevated | PTEN, PDCD4 | Activates Akt pathway | Apoptosis resistance and proliferation | [74,83] |
| miR-27a | Elevated | FOXO1 | Promotes cell migration | Induces invasion | [73] |
| miR-221/222 | Elevated | p27, TIMP3 | Promotes survival and cell cycle progression | Resistance to cisplatin and methotrexate | [75,84] |
| miR-29 family | Reduced | MCL-1, COL1A1/2 | Enhances ECM and anti-apoptotic proteins | Broad resistance to chemotherapy | [85] |
| miR-34a | Reduced | c-MET, CDK6, BCL2 | c-MET promotes motility and EMT, inhibits apoptosis when downregulated | Induces migration and lung metastasis. Resistance to doxorubicin and cisplatin | [86] |
| miR-133b | Reduced | MDR1/P-gp | Increases efflux pump expression | Multidrug resistance | [87] |
| miR-140 | Reduced | HDAC4 | HDAC4 promotes survival signaling | Cisplatin resistance | [77,78] |
| miR-143 | Reduced | MMP-13 | Promotes ECM degradation | Induces invasion and metastasis | [77] |
| miR-192 | Reduced | TGFβ1 | TGFβ pathway upregulated | Promotes EMT and metastasis | [88] |
| miR-200 family | Reduced | ZEB1/ZEB2 | Promotes EMT via E-cadherin suppression | Induces metastasis | [89] |
3.7. Long Non-Coding RNAs (lncRNAs)
3.8. Circular RNAs (circRNAs)
| lncRNA/circRNA | Role | Mechanism/Targeted miRNA | OS Phenotype | Reference |
|---|---|---|---|---|
| HOXD-AS1 | Oncogenic | EZH2 recruitment, p53 suppression | Promotes metastasis, induces drug resistance | [92,103] |
| HOTAIR | Oncogenic | EZH2 recruitment, miR-126 sponge | Promotes EMT, increases resistance | [91] |
| TUG1 | Oncogenic | miR-144/miR-132 sponge | Promotes metastasis and drug resistance | [93] |
| MALAT1 | Oncogenic | miR-129 sponge leads to increased RET | Promotes migration, invasion | [90,104] |
| BCAR4 | Oncogenic | miR-1260a | Promotes migration, invasion | [95,105] |
| MEG3 | Tumor suppressor | p53 activation | Reduces proliferation, increases drug sensitivity | [106] |
| Loc285194 | Tumor suppressor | miR-211 | Reduces proliferation | [90,107] |
| circTADA2A | Oncogenic | miR-203a-3p | Promotes EMT and invasion | [100] |
| circPVT1 | Oncogenic | miR-152-3p, miR-205-5p | Promotes metastasis and drug resistance | [98] |
| circ_001569 | Oncogenic | miR-145 | Increases MMP-9, AKT and drug resistance | [99] |
| circLARP4 | Tumor suppressor | miR-424 | Increases apoptosis, increases cisplatin sensitivity | [101] |
| circMTO1 | Tumor suppressor | miR-630 | Inhibits proliferation, migration and metastasis | [108] |
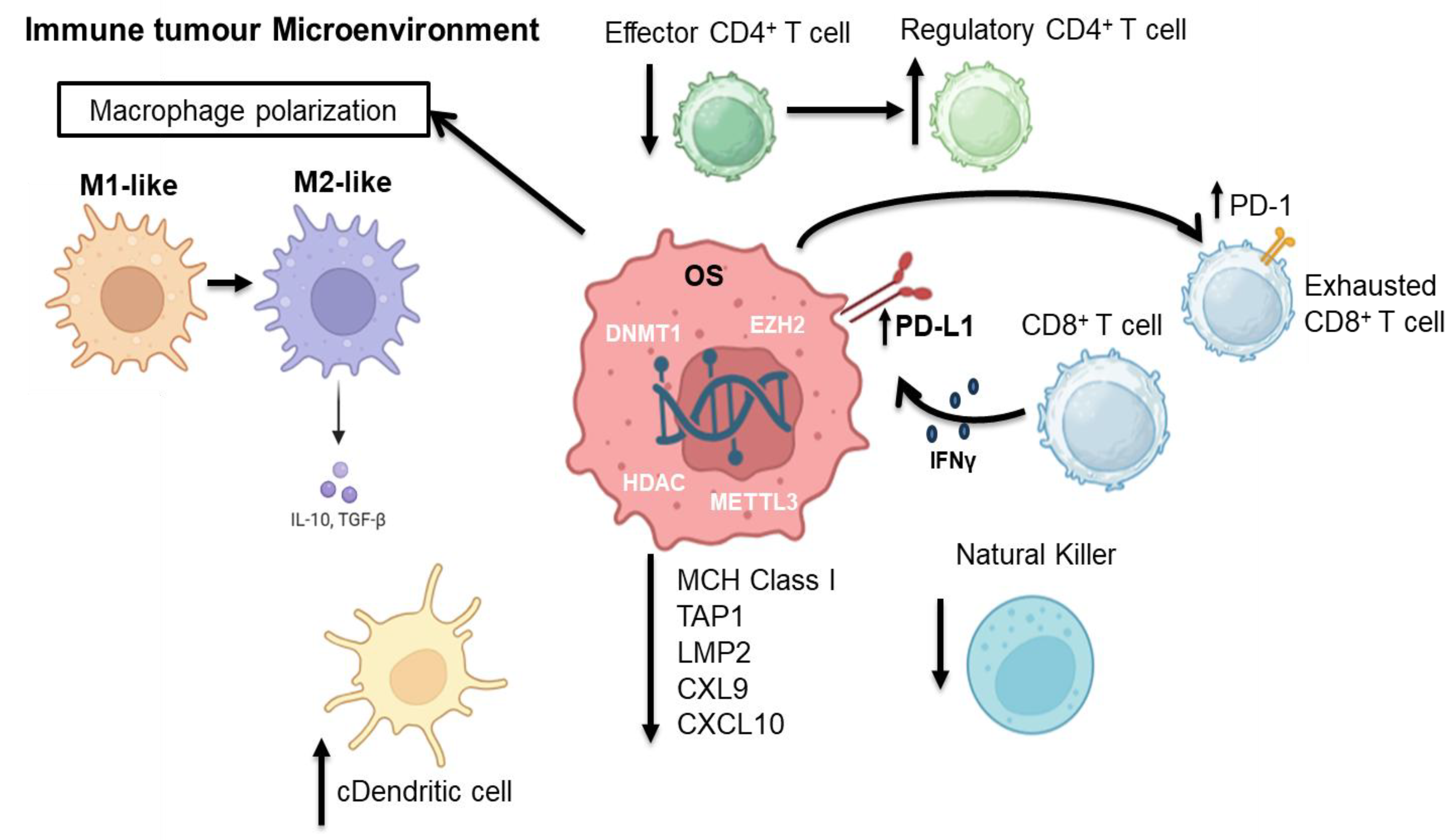
4. Epigenetic Modifications and Immune Cells in Osteosarcoma
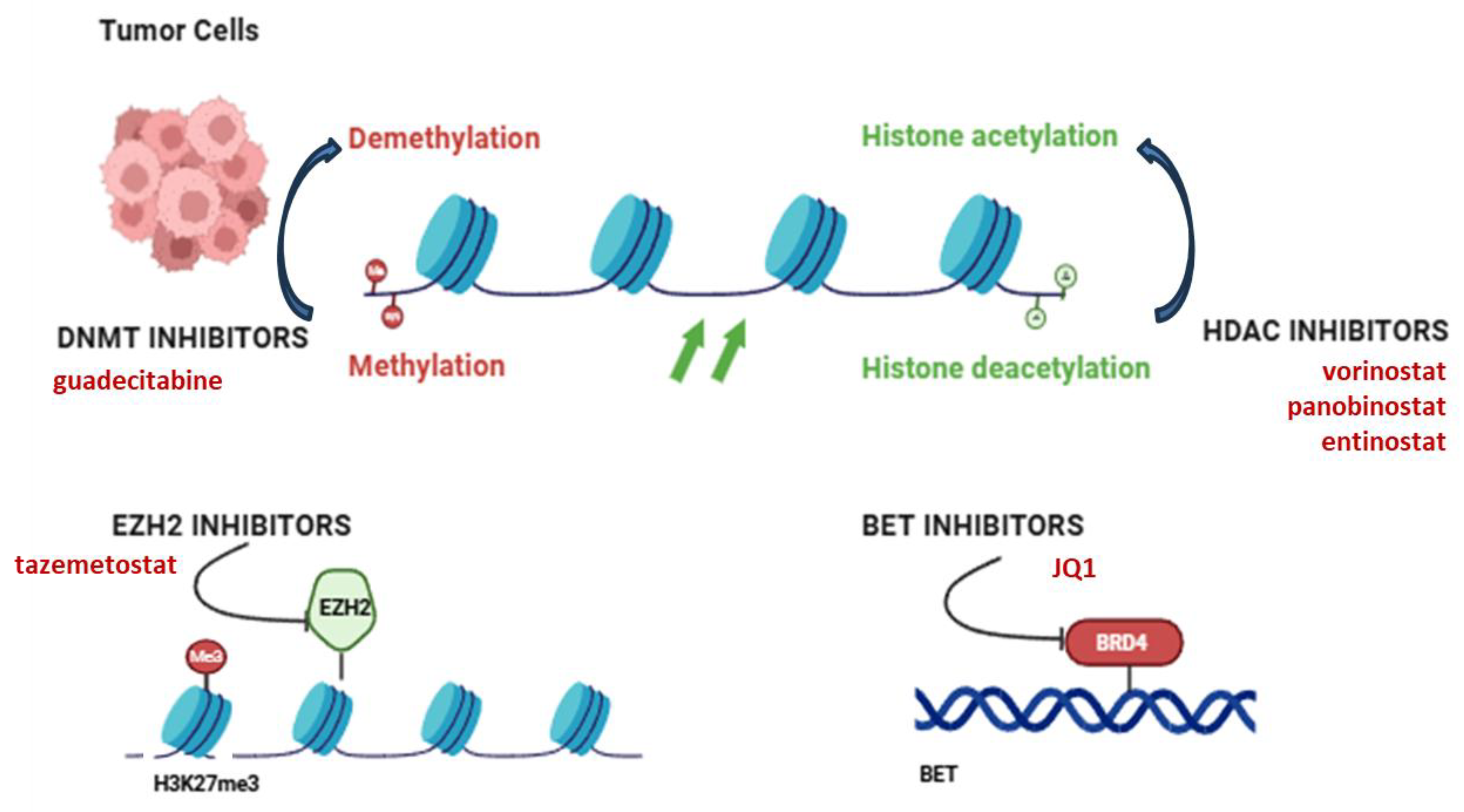
5. Epigenetic Therapeutic Targets in OS
| Epigenetic Modification | Target | Role in Therapy | Emerging Therapies/Clinical Trials | References |
|---|---|---|---|---|
| DNA Methylation | DNMTs (DNMT1/3A/3B) | DNMT inhibitors restore silenced tumor suppressors | Guadecitabine in preclinical OS models; synergistic effects with chemotherapy | [127] |
| Histone Modifications | HDACs | Reactivate silenced genes; reduce metastasis and resistance | Vorinostat, panobinostat and entinostat in phase I/II trials (NCT04308330) | [123] |
| EZH2 (HMT) | Mediates H3K27me3 silencing; promotes progression | Tazemetostat tested in pediatric sarcomas including OS (NCT02601950) | [133,134] | |
| KDM6B (JMJD3) | Demethylates H3K27me3; upregulation linked to metastasis | Experimental inhibitors under development | [135,136] | |
| LSD1/KDM1A | Demethylates H3K4me1/2; promotes proliferation | ORY-1001 (iadademstat) in hematologic/solid tumors (exploratory for sarcoma) | [137] | |
| Non-coding RNAs | Oncogenic lncRNAs/miRNAs (e.g., THAP9-AS1) | Modulate epigenetic silencing via DNMT recruitment or miRNA targeting | Development of miRNA mimics (e.g., miR-34a) and anti-lncRNA ASOs | [138] |
| RNA Modifications | METTL3, FTO, ALKBH5 | Alter mRNA stability/translation; affect immune regulation | Targeted inhibitors in discovery (e.g., STM2457 for METTL3) | [129] |
| Epigenetic Modifier Genes | DDX24, HDAC4, SP140, UHRF2, etc. | Involved in gene regulation and prognosis; predict therapy response | Incorporated into risk scoring systems for immunotherapy stratification | [139] |
| Enhancer Elements | Metastatic enhancer regions | Control metastasis-related gene expression | BET inhibitors (e.g., JQ1) show promise in blocking enhancer activity | [140] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Taran, S.J.; Taran, R.; Malipatil, N.B. Pediatric Osteosarcoma: An Updated Review. Indian. J. Med. Paediatr. Oncol. 2017, 38, 33. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Squire, J.A.; Zielenska, M. The Genetics of Osteosarcoma. Sarcoma 2012, 2012, 627254. [Google Scholar] [CrossRef]
- De Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araújo, J.M.G.; Fernandes, J.V. Biology and Pathogenesis of Human Osteosarcoma. Oncol. Lett. 2019, 19, 1099. [Google Scholar] [CrossRef] [PubMed]
- Chaiyawat, P.; Pruksakorn, D.; Phanphaisarn, A.; Teeyakasem, P.; Klangjorhor, J.; Settakorn, J. Expression Patterns of Class I Histone Deacetylases in Osteosarcoma: A Novel Prognostic Marker with Potential Therapeutic Implications. Mod. Pathol. 2018, 31, 264–274. [Google Scholar] [CrossRef]
- Sangle, N.A.; Layfield, L.J.; Osteosarcoma, N. Telangiectatic Osteosarcoma. Arch. Pathol. Lab. Med. 2012, 136, 572–576. [Google Scholar] [CrossRef]
- Klein, M.J.; Siegal, G.P. Osteosarcoma: Anatomic and Histologic Variants. Am. J. Clin. Pathol. 2006, 125, 555–581. [Google Scholar] [CrossRef]
- Harris, S.L.; Levine, A.J. The P53 Pathway: Positive and Negative Feedback Loops. Oncogene 2005, 24, 2899–2908. [Google Scholar] [CrossRef]
- Nevins, J.R. The Rb/E2F Pathway and Cancer. Hum. Mol. Genet. 2001, 10, 699–703. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; Chai, J.; Xing, L. RUNX2 as a Promising Therapeutic Target for Malignant Tumors. Cancer Manag. Res. 2021, 13, 2539–2548. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lovén, J.; Rahl, P.B.; Paranal, R.M.; Burge, C.B.; Bradner, J.E.; Lee, T.I.; Young, R.A. Transcriptional Amplification in Tumor Cells with Elevated C-Myc. Cell 2012, 151, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Song, W.X.; Luo, J.; Haydon, R.C.; He, T.C. Osteosarcoma Development and Stem Cell Differentiation. Clin. Orthop. Relat. Res. 2008, 466, 2114. [Google Scholar] [CrossRef]
- Rickel, K.; Fang, F.; Tao, J. Molecular Genetics of Osteosarcoma. Bone 2017, 102, 69–79. [Google Scholar] [CrossRef]
- Twenhafel, L.; Moreno, D.A.; Punt, T.; Kinney, M.; Ryznar, R. Epigenetic Changes Associated with Osteosarcoma: A Comprehensive Review. Cells 2023, 12, 1595. [Google Scholar] [CrossRef]
- Lopes-Júnior, L.C.; Silveira, D.; Vulczak, A.; Dos Santos, J.; Chain Veronez, L.; Fisch, A.; Flória-Santos, M.; Garcia de Lima, R.A.; Pereira-da-Silva, G. Emerging Cytokine Networks in Osteosarcoma. Cancer Cell Microenviron. 2017, 4, 1–11. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.; Yang, Y.; Yuan, K.; Yang, S.; Zhang, S.; Li, H.; Tang, T. Multi-Omics Analysis Based on 3D-Bioprinted Models Innovates Therapeutic Target Discovery of Osteosarcoma. Bioact. Mater. 2022, 18, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Sakthikumar, S.; Elvers, I.; Kim, J.; Arendt, M.L.; Thomas, R.; Turner-Maier, J.; Swofford, R.; Johnson, J.; Schumacher, S.E.; Alfoldi, J.; et al. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 2018, 78, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic Modifications: Basic Mechanisms and Role in Cardiovascular Disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A.; Kouzarides, T. Cancer Epigenetics: From Mechanism to Therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. A Decade of Exploring the Cancer Epigenome—Biological and Translational Implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef]
- Nowacka-Zawisza, M.; Wiśnik, E. DNA Methylation and Histone Modifications as Epigenetic Regulation in Prostate Cancer (Review). Oncol. Rep. 2017, 38, 2587–2596. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.D. DNA Methylation and Human Disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef]
- Voskarides, K.; Giannopoulou, N. The Role of TP53 in Adaptation and Evolution. Cells 2023, 12, 512. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Toyooka, S.; Maitra, A.; Maruyama, R.; Toyooka, K.O.; Timmons, C.F.; Tomlinson, G.E.; Mastrangelo, D.; Hay, R.J.; Minna, J.D.; et al. Aberrant Promoter Methylation and Silencing of the RASSF1A Gene in Pediatric Tumors and Cell Lines. Oncogene 2002, 21, 4345–4349. [Google Scholar] [CrossRef]
- Bu, J.; Li, H.; Liu, L.-H.; Ouyang, Y.-R.; Guo, H.-B.; Li, X.-Y.; Xiao, T. P16INK4a Overexpression and Survival in Osteosarcoma Patients: A Meta Analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 6091–6096. [Google Scholar]
- Kelly, T.K.; De Carvalho, D.D.; Jones, P.A. Epigenetic Modifications as Therapeutic Targets. Nat. Biotechnol. 2010, 28, 1069–1078. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Arfuso, F.; Arumugam, S.; Chinnathambi, A.; Jinsong, B.; Warrier, S.; Wang, L.Z.; Kumar, A.P.; Ahn, K.S.; Sethi, G.; et al. Role of Novel Histone Modifications in Cancer. Oncotarget 2018, 9, 11414–11426. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the Histone Code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Zhao, Z.; Shilatifard, A. Epigenetic Modifications of Histones in Cancer. Genome Biol. 2019, 20, 245. [Google Scholar] [CrossRef]
- Piao, L.; Yuan, X.; Wang, L.; Xu, X.; Zhuang, M.; Li, J.; Kong, R.; Liu, Z. Loss of Histone H4 Lysine 20 Trimethylation in Osteosarcoma Is Associated with Aberrant Expression Ofhistone Methyltransferase SUV420H2. Oncol. Lett. 2020, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, E.R.; Thiele, C.J. Epigenetic Changes in Pediatric Solid Tumors: Promising New Targets. Clin. Cancer Res. 2012, 18, 2768–2779. [Google Scholar] [CrossRef]
- Vaidya, H.; Rumph, C.; Katula, K.S. Inactivation of the WNT5A Alternative Promoter B Is Associated with DNA Methylation and Histone Modification in Osteosarcoma Cell Lines U2OS and SaOS-2. PLoS ONE 2016, 11, e0151392. [Google Scholar] [CrossRef]
- Duan, X.; Yu, X.; Li, Z. Circular RNA Hsa_circ_0001658 Regulates Apoptosis and Autophagy in Gastric Cancer through MicroRNA-182/Ras-Related Protein Rab-10 Signaling Axis. Bioengineered 2022, 13, 2387–2397. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Sun, W.; Zheng, B.; Wang, C.; Luo, Z.; Wang, J.; Yan, W. LncRNA MALAT1 Promotes Cancer Metastasis in Osteosarcoma via Activation of the PI3K-Akt Signaling Pathway. Cell. Physiol. Biochem. 2018, 51, 1313–1326. [Google Scholar] [CrossRef]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of Transcription in Human Cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long Non-Coding RNAs: Insights into Functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-Coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 612393. [Google Scholar] [CrossRef]
- Ahmad, M.; Weiswald, L.B.; Poulain, L.; Denoyelle, C.; Meryet-Figuiere, M. Involvement of LncRNAs in Cancer Cells Migration, Invasion and Metastasis: Cytoskeleton and ECM Crosstalk. J. Exp. Clin. Cancer Res. 2023, 42, 173. [Google Scholar] [CrossRef]
- Kun-Peng, Z.; Xiao-Long, M.; Chun-Lin, Z. Overexpressed CircPVT1, a Potential New Circular RNA Biomarker, Contributes to Doxorubicin and Cisplatin Resistance of Osteosarcoma Cells by Regulating ABCB1. Int. J. Biol. Sci. 2018, 14, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, Y.; Ma, C.; Lv, Q. Competitive Endogenous Network of CircRNA, LncRNA, and MiRNA in Osteosarcoma Chemoresistance. Eur. J. Med. Res. 2023, 28, 354. [Google Scholar] [CrossRef] [PubMed]
- Wiman, K.G. The Retinoblastoma Gene: Role in Cell Cycle Control and Cell Differentiation. FASEB J. 1993, 7, 841–845. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, H.S.; Kim, H.H.; Kim, W.H.; Lee, S.H. Aberrant Methylation of P14ARF Gene Correlates with Poor Survival in Osteosarcoma. Clin. Orthop. Relat. Res. 2006, 442, 216–222. [Google Scholar] [CrossRef]
- Badal, V.; Menendez, S.; Coomber, D.; Lane, D.P. Regulation of the P14ARF Promoter by DNA Methylation. Cell Cycle 2008, 7, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Virmani, A.K.; Harada, K.; Timmons, C.F.; Miyajima, K.; Hay, R.J.; Mastrangelo, D.; Maitra, A.; Tomlinson, G.E.; Gazdar, A.F. Aberrant Methylation of the HIC1 Promoter Is a Frequent Event in Specific Pediatric Neoplasms. Clin. Cancer Res. 2003, 9, 3674–3678. [Google Scholar] [PubMed]
- Zhang, B.; Li, Y.-L.; Zhao, J.-L.; Zhen, O.; Yu, C.; Yang, B.-H.; Yu, X.-R. Hypoxia-Inducible Factor-1 Promotes Cancer Progression through Activating AKT/Cyclin D1 Signaling Pathway in Osteosarcoma. Biomed. Pharmacother. 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.J.; Khanna, C. Osteosarcoma Genetics and Epigenetics: Emerging Biology and Candidate Therapies. Crit. Rev. Trade Oncog. 2015, 20, 173–197. [Google Scholar] [CrossRef]
- Shi, Y.-K.; Guo, Y.-H. MiR-139-5p Suppresses Osteosarcoma Cell Growth and Invasion through Regulating DNMT1. Biochem. Biophys. Res. Commun. 2018, 503, 459–466. [Google Scholar] [CrossRef]
- Pires, S.F.; de Barros, J.S.; da Costa, S.S.; de Oliveira Scliar, M.; Van Helvoort Lengert, A.; Boldrini, É.; da Silva, S.R.M.; Tasic, L.; Vidal, D.O.; Krepischi, A.C.V.; et al. DNA Methylation Patterns Suggest the Involvement of DNMT3B and TET1 in Osteosarcoma Development. Mol. Genet. Genom. 2023, 298, 721–733. [Google Scholar] [CrossRef]
- Parker, A.C.; Quinteros, B.I.; Piccolo, S.R. The DNA Methylation Landscape of Five Pediatric-Tumor Types. PeerJ 2022, 10, e13516. [Google Scholar] [CrossRef]
- Al-Romaih, K.; Sadikovic, B.; Yoshimoto, M.; Wang, Y.; Zielenska, M.; Squire, J.A. Decitabine-Induced Demethylation of 5′ CpG Island in GADD45A Leads to Apoptosis in Osteosarcoma Cells. Neoplasia 2008, 10, 471–480. [Google Scholar] [CrossRef]
- Guo, X.; Liu, W.; Pan, Y.; Ni, P.; Ji, J.; Guo, L.; Zhang, J.; Wu, J.; Jiang, J.; Chen, X.; et al. Homeobox Gene IRX1 Is a Tumor Suppressor Gene in Gastric Carcinoma. Oncogene 2010, 29, 3908–3920. [Google Scholar] [CrossRef]
- Xu, J.; Li, D.; Cai, Z.; Zhang, Y.; Huang, Y.; Su, B.; Ma, R. An Integrative Analysis of DNA Methylation in Osteosarcoma. J. Bone Oncol. 2017, 9, 34–40. [Google Scholar] [CrossRef]
- Donninger, H.; Vos, M.D.; Clark, G.J. The RASSF1A Tumor Suppressor. J. Cell Sci. 2007, 120, 3163–3172. [Google Scholar] [CrossRef]
- Rosenblum, J.M.; Ari Wijetunga, N.A.; Fazzari, M.J.; Krailo, M.; Barkauskas, D.A.; Gorlick, R.; Greally, J.M. Predictive Properties of DNA Methylation Patterns in Primary Tumor Samples for Osteosarcoma Relapse Status. Epigenetics 2015, 10, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Qing, Y.; Horne, D.; Huang, H.; Chen, J. The Roles and Implications of RNA M6A Modification in Cancer. Nat. Rev. Clin. Oncol. 2023, 20, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Chen, J.; Jia, L.; Ma, J.; Song, D. The M6A Methyltransferase METTL3 Promotes Osteosarcoma Progression by Regulating the M6A Level of LEF1. Biochem. Biophys. Res. Commun. 2019, 516, 719–725. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Bao, Y.; Luo, Y.; Qiu, G.; He, M.; Lu, J.; Xu, J.; Chen, B.; Wang, Y. N6-Methyladenosine (M6A) Modification in Osteosarcoma: Expression, Function and Interaction with Noncoding RNAs-an Updated Review. Epigenetics 2023, 18, 2260213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Li, L.M.; Sun, H.L.; Liu, S.M. Link Between M6A Modification and Cancers. Front. Bioeng. Biotechnol. 2018, 6, 360732. [Google Scholar] [CrossRef]
- Li, N.; Wei, X.; Dai, J.; Yang, J.; Xiong, S. METTL3: A Multifunctional Regulator in Diseases. Mol. Cell Biochem. 2025, 480, 3429–3454. [Google Scholar] [CrossRef]
- Yang, M.; Wei, R.; Zhang, S.; Hu, S.; Liang, X.; Yang, Z.; Zhang, C.; Zhang, Y.; Cai, L.; Xie, Y. NSUN2 Promotes Osteosarcoma Progression by Enhancing the Stability of FABP5 MRNA via M5C Methylation. Cell Death Dis. 2023, 14, 125. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, D.; Zheng, L.; Zhao, J.; Tan, M. Advances in prognostic models for osteosarcoma risk. Heliyon 2024, 10, e28493. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, P.; Song, Y. Machine-Learning-Based M5C Score for the Prognosis Diagnosis of Osteosarcoma. J. Oncol. 2021, 2021, 11. [Google Scholar] [CrossRef]
- Calo, E.; Quintero-Estades, J.A.; Danielian, P.S.; Nedelcu, S.; Berman, S.D.; Lees, J.A. Rb Regulates Fate Choice and Lineage Commitment in Vivo. Nature 2010, 466, 1110–1114. [Google Scholar] [CrossRef]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone Lysine Methylation Dynamics: Establishment, Regulation, and Biological Impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Liu, C.; Jiang, Y.; Hao, Y. Elevated H3K27me3 Levels Sensitize Osteosarcoma to Cisplatin. Clin. Epigenetics 2019, 11, 8. [Google Scholar] [CrossRef]
- Yang, H.; Salz, T.; Zajac-Kaye, M.; Liao, D.; Huang, S.; Qiu, Y. Overexpression of Histone Deacetylases in Cancer Cells Is Controlled by Interplay of Transcription Factors and Epigenetic Modulators. FASEB J. 2014, 28, 4265–4279. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Cubizolles, F.; Zhang, Y.; Reichert, N.; Kohler, H.; Seiser, C.; Matthias, P. Histone Deacetylases 1 and 2 Act in Concert to Promote the G1-to-S Progression. Genes Dev. 2010, 24, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Xue, J.; Niu, N. SETD2 in Cancer: Functions, Molecular Mechanisms, and Therapeutic Regimens. Cancer Biol. Med. 2024, 21, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, E.; Salvati, A.; Pecoraro, G.; Lamberti, J.; Melone, V.; Sellitto, A.; Rizzo, F.; Giurato, G.; Tarallo, R.; Nassa, G.; et al. Histone Methyltransferase DOT1L as a Promising Epigenetic Target for Treatment of Solid Tumors. Front. Genet. 2022, 13, 864612. [Google Scholar] [CrossRef]
- Piao, L.; Yuan, X.; Zhuang, M.; Qiu, X.; Xu, X.; Kong, R.; Liu, Z. Histone Methyltransferase SUV39H2 Serves Oncogenic Roles in Osteosarcoma. Oncol. Rep. 2019, 41, 325–332. [Google Scholar] [CrossRef]
- Lakiotaki, E.; Kanakoglou, D.S.; Pampalou, A.; Karatrasoglou, E.A.; Piperi, C.; Korkolopoulou, P. Dissecting the Role of Circular RNAs in Sarcomas with Emphasis on Osteosarcomas. Biomedicines 2021, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, S.W.; Gruhl, F.; Mattick, J.S.; Dinger, M.E. Long Noncoding RNAs and the Genetics of Cancer. Br. J. Cancer 2013, 108, 2419–2425. [Google Scholar] [CrossRef]
- Salah, Z.; Arafeh, R.; Maximov, V.; Galasso, M.; Khawaled, S.; Abou-Sharieha, S.; Volinia, S.; Jones, K.B.; Croce, C.M.; Aqeilan, R.I. MiR-27a and MiR-27a* Contribute to Metastatic Properties of Osteosarcoma Cells. Oncotarget 2015, 6, 4920–4935. [Google Scholar] [CrossRef]
- Ren, X.; Shen, Y.; Zheng, S.; Liu, J.; Jiang, X. MiR-21 Predicts Poor Prognosis in Patients with Osteosarcoma. Br. J. Biomed. Sci. 2016, 73, 158–162. [Google Scholar] [CrossRef]
- Gang, W.; Tanjun, W.; Yong, H.; Jiajun, Q.; Yi, Z.; Hao, H. Inhibition of MiR-9 Decreases Osteosarcoma Cell Proliferation. Bosn. J. Basic. Med. Sci. 2020, 20, 218–225. [Google Scholar] [CrossRef]
- He, C.; Xiong, J.; Xu, X.; Lu, W.; Liu, L.; Xiao, D.; Wang, D. Functional Elucidation of MiR-34 in Osteosarcoma Cells and Primary Tumor Samples. Biochem. Biophys. Res. Commun. 2009, 388, 35–40. [Google Scholar] [CrossRef]
- Jones, P.L.; Veenstra, G.J.C.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 Recruit Histone Deacetylase to Repress Transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, L.; Zhang, Z.; Chen, X.; Luo, J.; Zhang, Z.; Chen, S.; Shu, Y.; Han, Z.; Cao, K. Overexpression of MiR-140 Inhibits Proliferation of Osteosarcoma Cells via Suppression of Histone Deacetylase 4. Oncol. Res. 2017, 25, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Zhang, C.; Liu, G.; Gu, R.; Wu, H. MicroRNA-101 Inhibits Proliferation, Migration and Invasion in Osteosarcoma Cells by Targeting ROCK1. Am. J. Cancer Res. 2017, 7, 88. [Google Scholar] [PubMed]
- Huang, G.; Nishimoto, K.; Zhou, Z.; Hughes, D.; Kleinerman, E.S. MiR-20a Encoded by the MiR-17–92 Cluster Increases the Metastatic Potential of Osteosarcoma Cells by Regulating Fas Expression. Cancer Res. 2012, 72, 908–916. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Zhao, X.; Wang, B.; Zhang, L.; Zhang, C.; Zhang, F. DNA Methylation Mediated Downregulation of MiR-449c Controls Osteosarcoma Cell Cycle Progression by Directly Targeting Oncogene c-Myc. Int. J. Biol. Sci. 2017, 13, 1038–1050. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, X.; Wang, Q.; Lu, Q.; He, F.; Li, J.; Li, X.; Jin, M.; Xu, J. The impact of miR-9 in osteosarcoma: A study based on meta-analysis, TCGA data, and bioinformatics analysis. Medicine 2020, 99, e21902. [Google Scholar] [CrossRef]
- Lv, C.; Hao, Y.; Tu, G. MicroRNA-21 promotes proliferation, invasion and suppresses apoptosis in human osteosarcoma line MG63 through PTEN/Akt pathway. Tumour Biol. 2016, 37, 9333–9342. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.; Gong, M. MiRNA-221 from Tissue May Predict the Prognosis of Patients with Osteosarcoma. Medicine 2018, 97, e11100. [Google Scholar] [CrossRef]
- Xu, W.; Li, Z.; Zhu, X.; Xu, R.; Xu, Y. MiR-29 Family Inhibits Resistance to Methotrexate and Promotes Cell Apoptosis by Targeting COL3A1 and MCL1 in Osteosarcoma. Med. Sci. Monit. 2018, 24, 8812–8821. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, T.; Ren, X.; Yang, M.; Tu, C.; Li, Z. Mir-34a: A Regulatory Hub with Versatile Functions That Controls Osteosarcoma Networks. Cell Cycle 2022, 21, 2121–2131. [Google Scholar] [CrossRef]
- Zhao, H.; Li, M.; Li, L.; Yang, X.; Lan, G.; Zhang, Y. MiR-133b Is Down-Regulated in Human Osteosarcoma and Inhibits Osteosarcoma Cells Proliferation, Migration and Invasion, and Promotes Apoptosis. PLoS ONE 2013, 8, e83571. [Google Scholar] [CrossRef] [PubMed]
- Shang, G.; Mi, Y.; Mei, Y.; Wang, G.; Wang, Y.; Li, X.; Wang, Y.; Li, Y.; Zhao, G. MicroRNA-192 Inhibits the Proliferation, Migration and Invasion of Osteosarcoma Cells and Promotes Apoptosis by Targeting Matrix Metalloproteinase-11. Oncol. Lett. 2018, 15, 7265–7272. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, J.; Wu, C.; Hu, C.; Wang, J.; Bao, Q.; Wang, H.; Wang, J.; Zhou, Q.; Wei, L.; et al. MicroRNA-200a Induces Immunosuppression by Promoting PTEN-Mediated PD-L1 Upregulation in Osteosarcoma. Aging 2020, 12, 1213–1236. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dou, P.; Liu, T.; He, S. Application of Long Noncoding RNAs in Osteosarcoma: Biomarkers and Therapeutic Targets. Cell. Physiol. Biochem. 2017, 42, 1407–1419. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Wang, W. Long Non-Coding RNA HOTAIR Promotes Human Osteosarcoma Proliferation, Migration through Activation of the Wnt/b-Catenin Signaling Pathway. J. Oncol. 2023, 2023, 9667920. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, E.; Song, L.; Tu, L.; Wang, Z.; Tian, F.; Aikenmu, K.; Chu, G.; Zhao, J. Long Noncoding RNA HOXD-AS1 Aggravates Osteosarcoma Carcinogenesis through Epigenetically Inhibiting P57 via EZH2. Biomed. Pharmacother. 2018, 106, 890–895. [Google Scholar] [CrossRef]
- Yu, X.; Hu, L.; Li, S.; Shen, J.; Wang, D.; Xu, R.; Yang, H. Long Non-Coding RNA Taurine Upregulated Gene 1 Promotes Osteosarcoma Cell Metastasis by Mediating HIF-1α via MiR-143-5p. Cell Death Dis. 2019, 10, 280. [Google Scholar] [CrossRef]
- Deng, R.; Zhang, J.; Chen, J. LncRNA SNHG1 Negatively Regulates MiRNA-101-3p to Enhance the Expression of ROCK1 and Promote Cell proliferation, Migration and Invasion in Osteosarcoma. Int. J. Mol. Med. 2018, 43, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tang, X.; Xie, Y.; Zhang, H.; Huang, Z.; Huang, C. Long Non-Coding RNA BCAR4 Regulates Osteosarcoma Progression by Targeting MicroRNA-1260a. Bull. Cancer 2025, 112, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, L.; Tong, G.; Zeng, Z.; Tan, J.; Su, Z.; Liu, Z.; Lin, J.; Gao, W.; Chen, J.; et al. Circular RNA Circ_001422 Promotes the Progression and Metastasis of Osteosarcoma via the MiR-195-5p/FGF2/PI3K/Akt Axis. J. Exp. Clin. Cancer Res. 2021, 40, 235. [Google Scholar] [CrossRef]
- Li, H.; Lan, M.; Liao, X.; Tang, Z.; Yang, C. Circular RNA Cir-ITCH Promotes Osteosarcoma Migration and Invasion through Cir-ITCH /MiR-7/EGFR Pathway. Technol. Cancer Res. Treat. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Li, D.; Huang, Y.; Wang, G. Circular RNA CircPVT1 Contributes to Doxorubicin (DXR) Resistance of Osteosarcoma Cells by Regulating TRIAP1 via MiR-137. Biomed. Res. Int. 2021, 2021, 7463867. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, J.; Lang, X.; Zhuang, Y. Expression of Circ_001569 Is Upregulated in Osteosarcoma and Promotes Cell Proliferation and Cisplatin Resistance by Activating the Wnt/β-Catenin Signaling Pathway. Oncol. Lett. 2018, 16, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xie, Z.; Chen, J.; Chen, J.; Ni, W.; Ma, Y.; Huang, K.; Wang, G.; Wang, J.; Ma, J.; et al. Circular RNA CircTADA2A Promotes Osteosarcoma Progression and Metastasis by Sponging MiR-203a-3p and Regulating CREB3 Expression. Mol. Cancer 2019, 18, 73. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, J.; Shen, H.; Shao, T.; Li, S.; Wang, W.; Yu, Z. Circular RNA LARP4 Correlates with Decreased Enneking Stage, Better Histological Response, and Prolonged Survival Profiles, and It Elevates Chemosensitivity to Cisplatin and Doxorubicin via Sponging MicroRNA-424 in Osteosarcoma. J. Clin. Lab. Anal. 2020, 34, e23045. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zou, C.; Xie, X.; Wang, Y.; Wang, B.; Zhao, Z.; Tu, J.; Wang, X.; Li, H.; et al. Microarray Expression Profile and Functional Analysis of Circular RNAs in Osteosarcoma. Cell. Physiol. Biochem. 2017, 43, 969–985. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhang, X.; Huang, Q.; Diao, Y.; Yin, H.; Liu, H. Long non-coding RNA HOXD-AS1 in cancer. Clin. Chim. Acta 2018, 487, 197–201. [Google Scholar] [CrossRef]
- Huo, Y.; Li, Q.; Wang, X.; Jiao, X.; Zheng, J.; Li, Z.; Pan, X. MALAT1 predicts poor survival in osteosarcoma patients and promotes cell metastasis through associating with EZH2. Oncotarget 2017, 8, 46993–47006. [Google Scholar] [CrossRef]
- Ju, L.; Zhou, Y.M.; Yang, G.S. Up-regulation of long non-coding RNA BCAR4 predicts a poor prognosis in patients with osteosarcoma, and promotes cell invasion and metastasis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4445–4451. [Google Scholar]
- Shi, Y.; Lv, C.; Shi, L.; Tu, G. MEG3 inhibits proliferation and invasion and promotes apoptosis of human osteosarcoma cells. Oncol. Lett. 2018, 15, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, J.; Zhou, N.; Zhang, Z.; Zhang, A.; Lu, Z.; Wu, F.; Mo, Y.Y. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res. 2013, 41, 4976–4987. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Y.; Li, Z.; Zhang, K.; Jiao, N.; Lu, D.-G.; Zhou, D.-W.; Meng, Y.-B.; Sun, L. Circular RNA CircMTO1 Suppressed Proliferation and Metastasis of Osteosarcoma through MiR-630/KLF6 Axis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 86–93. [Google Scholar] [CrossRef]
- Orrapin, S.; Moonmuang, S.; Udomruk, S.; Yongpitakwattana, P.; Pruksakorn, D.; Chaiyawat, P. Unlocking the Tumor-Immune Microenvironment in Osteosarcoma: Insights into the Immune Landscape and Mechanisms. Front. Immunol. 2024, 15, 1394284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Y.; Hu, Y. Metabolites in the Tumor Microenvironment Reprogram Functions of Immune Effector Cells Through Epigenetic Modifications. Front. Immunol. 2021, 12, 641883. [Google Scholar] [CrossRef]
- Yu, B.; Geng, C.; Wu, Z.; Zhang, Z.; Zhang, A.; Yang, Z.; Huang, J.; Xiong, Y.; Yang, H.; Chen, Z. A CIC-Related-Epigenetic Factors-Based Model Associated with Prediction, the Tumor Microenvironment and Drug Sensitivity in Osteosarcoma. Sci. Rep. 2024, 14, 1308. [Google Scholar] [CrossRef]
- Tien, F.M.; Lu, H.H.; Lin, S.Y.; Tsai, H.C. Epigenetic Remodeling of the Immune Landscape in Cancer: Therapeutic Hurdles and Opportunities. J. Biomed. Sci. 2023, 30, 3. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Wu, H.; Xue, M.; Lin, P.; Wang, S.; Lin, N.; Huang, X.; Pan, W.; Liu, M.; et al. Epigenetic Regulation of CXCL12 Plays a Critical Role in Mediating Tumor Progression and the Immune Response in Osteosarcoma. Cancer Res. 2018, 78, 3938–3953. [Google Scholar] [CrossRef]
- Li, B.; Zhu, X.; Sun, L.; Yuan, L.; Zhang, J.; Li, H.; Ye, Z.; Li, B.; Zhu, X.; Sun, L.; et al. Induction of a Specific CD8+ T-Cell Response to Cancer/Testis Antigens by Demethylating Pre-Treatment against Osteosarcoma. Oncotarget 2014, 5, 10791–10802. [Google Scholar] [CrossRef]
- Keremu, A.; Aimaiti, A.; Liang, Z.; Zou, X. Role of the HDAC6/STAT3 Pathway in Regulating PD-L1 Expression in Osteosarcoma Cell Lines. Cancer Chemother. Pharmacol. 2019, 83, 255–264. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, X.; Liu, Z. Multi-Omics Analysis of Histone-Related Genes in Osteosarcoma: A Multidimensional Integrated Study Revealing Drug Sensitivity and Immune Microenvironment Characteristics. Technol. Cancer Res. Treat. 2025, 24, 15330338251336275. [Google Scholar] [CrossRef]
- Licht, J.D.; Bennett, R.L. Leveraging Epigenetics to Enhance the Efficacy of Immunotherapy. Clin. Epigenetics 2021, 13, 115. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via DsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zeng, J.; Huang, L.; Peng, Y.; Yan, Z.; Zhang, A.; Zhao, X.; Li, J.; Zhou, Z.; Wang, S.; et al. RNA Adenosine Modifications Related to Prognosis and Immune Infiltration in Osteosarcoma. J. Transl. Med. 2022, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, D.; Piao, Y.; Chen, M.; Wang, D.; Jiang, Z.; Liu, B. Modulation of Immunosuppressive Cells and Noncoding RNAs as Immunotherapy in Osteosarcoma. Front. Immunol. 2022, 13, 1025532. [Google Scholar] [CrossRef] [PubMed]
- Khanna, C.; Fan, T.M.; Gorlick, R.; Helman, L.J.; Kleinerman, E.S.; Adamson, P.C.; Houghton, P.J.; Tap, W.D.; Welch, D.R.; Steeg, P.S.; et al. Toward a Drug Development Path That Targets Metastatic Progression in Osteosarcoma. Clin. Cancer Res. 2014, 20, 4200–4209. [Google Scholar] [CrossRef]
- He, C.; Liu, C.; Wang, L.; Sun, Y.; Jiang, Y.; Hao, Y. Histone Methyltransferase NSD2 Regulates Apoptosis and Chemosensitivity in Osteosarcoma. Cell Death Dis. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Brynien, D.; Weiss, K.R. The HDAC Inhibitor Vorinostat Diminishes the In Vitro Metastatic Behavior of Osteosarcoma Cells. Biomed. Res. Int. 2015, 2015, 290368. [Google Scholar] [CrossRef] [PubMed]
- Galvan, M.L.; Paradise, C.R.; Kubrova, E.; Jerez, S.; Khani, F.; Thaler, R.; Dudakovic, A.; van Wijnen, A.J. Multiple pharmacological inhibitors targeting the epigenetic suppressor enhancer of zeste homolog 2 (Ezh2) accelerate osteoblast differentiation. Bone. 2021, 150, 115993. [Google Scholar] [CrossRef]
- Strepkos, D.; Markouli, M.; Klonou, A.; Papavassiliou, A.G.; Piperi, C. Histone Methyltransferase SETDB1: A Common Denominator of Tumorigenesis with Therapeutic Potential. Cancer Res. 2021, 81, 525–534. [Google Scholar] [CrossRef]
- Nan, X.; Ng, H.H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional Repression by the Methyl-CpG-Binding Protein MeCP2 Involves a Histone Deacetylase Complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef]
- Al-Romaih, K.; Somers, G.R.; Bayani, J.; Hughes, S.; Prasad, M.; Cutz, J.C.; Xue, H.; Zielenska, M.; Wang, Y.; Squire, J.A. Modulation by Decitabine of Gene Expression and Growth of Osteosarcoma U2OS Cells in Vitro and in Xenografts: Identification of Apoptotic Genes as Targets for Demethylation. Cancer Cell Int. 2007, 7, 14. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Dong, L.; Chang, Y.; Zhang, X.; Wang, C.; Chen, M.; Bo, X.; Chen, H.; Han, W.; et al. Decitabine priming increases anti-PD-1 antitumor efficacy by promoting CD8+ progenitor exhausted T cell expansion in tumor models. J. Clin. Invest. 2023, 133, e165673. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, J.; Ni, Y.; Chen, W.; Rong, W.; Zhang, X.; Guo, C.; Kong, X.; Tang, S. Synthesis of STM2457, a Selective Small-Molecule Inhibitor of METTL3. Tetrahedron Lett. 2024, 141, 155077. [Google Scholar] [CrossRef]
- Asano, N.; Takeshima, H.; Yamashita, S.; Takamatsu, H.; Hattori, N.; Kubo, T.; Yoshida, A.; Kobayashi, E.; Nakayama, R.; Matsumoto, M.; et al. Epigenetic Reprogramming Underlies Efficacy of DNA Demethylation Therapy in Osteosarcomas. Sci. Rep. 2019, 9, 20360. [Google Scholar] [CrossRef] [PubMed]
- Roudi, R.; Pisani, L.; Pisani, F.; Kiru, L.; Daldrup-Link, H.E. Novel Clinically Translatable Iron Oxide Nanoparticle for Monitoring Anti-CD47 Cancer Immunotherapy. Investig. Radiol. 2023, 59, 391. [Google Scholar] [CrossRef] [PubMed]
- Ahvati, H.; Roudi, R.; Sobhani, N.; Safari, F. CD47 as a Potent Target in Cancer Immunotherapy: A Review. Biochim. Biophys. Acta (BBA) Rev. Cancer 2025, 1880, 189294. [Google Scholar] [CrossRef]
- Kita, S.; Shimoi, T.; Komine, K.; Ando, M.; Ariyama, H.; Okita, N.; Sadachi, R.; So, N.; Azuma, S.; Kazumi, Y.; et al. O17-2 A Phase II Trial of Tazemetostat for Patients with Unresectable or Metastatic Epithelioid Sarcoma (TAZETTA Trial). Ann. Oncol. 2023, 34, S1392. [Google Scholar] [CrossRef]
- Kurmasheva, R.T.; Erickson, S.W.; Earley, E.; Smith, M.A.; Houghton, P.J. In Vivo Evaluation of the EZH2 Inhibitor (EPZ011989) Alone or in Combination with Standard of Care Cytotoxic Agents against Pediatric Malignant Rhabdoid Tumor Preclinical Models—A Report from the Pediatric Preclinical Testing Consortium. Pediatr. Blood Cancer 2021, 68, e28772. [Google Scholar] [CrossRef]
- Shoaib, Z.; Fan, T.M.; Irudayaraj, J.M.K. Osteosarcoma Mechanobiology and Therapeutic Targets. Br. J. Pharmacol. 2021, 2, 201–217. [Google Scholar] [CrossRef]
- Hua, C.; Chen, J.; Li, S.; Zhou, J.; Fu, J.; Sun, W.; Wang, W. KDM6 Demethylases and Their Roles in Human Cancers. Front. Oncol. 2021, 11, 779918. [Google Scholar] [CrossRef]
- Majello, B.; Gorini, F.; Saccà, C.D.; Amente, S. Expanding the Role of the Histone Lysine-Specific Demethylase LSD1 in Cancer. Cancers 2019, 11, 324. [Google Scholar] [CrossRef]
- Farooqi, A.; Tabassum, S.; Ahmad, A. MicroRNA-34a: A Versatile Regulator of Myriads of Targets in Different Cancers. Int. J. Mol. Sci. 2017, 18, 2089. [Google Scholar] [CrossRef]
- Li, Z.; Xue, Y.; Huang, X.; Xiao, G. Stratifying Osteosarcoma Patients Using an Epigenetic Modification-Related Prognostic Signature: Implications for Immunotherapy and Chemotherapy Selection. Transl. Cancer Res. 2024, 13, 3556–3574. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, H.R.; Mi, D.J.; Farley, V.M.; Esmond, T.; Kaood, M.B.; Aune, T.M. Bromodomain Inhibitor JQ1 Reversibly Blocks IFN-γ Production. Sci. Rep. 2019, 9, 10280. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Jiang, J.; Zhan, X.; Liang, Y.; Guo, Q.; Liu, P.; Lu, L.; Yang, Y.; Xu, W.; Zhang, Y.; et al. Integrating Artificial Intelligence in Osteosarcoma Prognosis: The Prognostic Significance of SERPINE2 and CPT1B Biomarkers. Sci. Rep. 2024, 14, 4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yi, C.; Gong, D.; Zhao, Q.; Xie, H.; Zhao, S.; Yu, H.; Lv, J.; Bian, E.; Tian, D. Construction of a 5-Gene super-enhancer-related signature for osteosarcoma prognosis and the regulatory role of TNFRSF11B in osteosarcoma. Transl Oncol. 2024, 47, 102047. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Chi, K.; Chen, Z.; Zhuang, S.; Ye, Y.; Zhang, B.; Cai, C. Development and Pan-Cancer Validation of an Epigenetics-Based Random Survival Forest Model for Prognosis Prediction and Drug Response in OS. Front. Pharmacol. 2025, 16, 1529525. [Google Scholar] [CrossRef]
- Zhra, M.; Akhund, S.A.; Mohammad, K.S. Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies. Pharmaceuticals 2025, 18, 520. [Google Scholar] [CrossRef]
- Dutour, A.; Pasello, M.; Farrow, L.; Amer, M.H.; Entz-Werlé, N.; Nathrath, M.; Scotlandi, K.; Mittnacht, S.; Gomez-Mascard, A. Microenvironment Matters: Insights from the FOSTER Consortium on Microenvironment-Driven Approaches to Osteosarcoma Therapy. Cancer Metastasis Rev. 2025, 44, 44. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsianou, M.A.; Andreou, D.; Korkolopoulou, P.; Vetsika, E.-K.; Piperi, C. Epigenetic Modifications in Osteosarcoma: Mechanisms and Therapeutic Strategies. Life 2025, 15, 1202. https://doi.org/10.3390/life15081202
Katsianou MA, Andreou D, Korkolopoulou P, Vetsika E-K, Piperi C. Epigenetic Modifications in Osteosarcoma: Mechanisms and Therapeutic Strategies. Life. 2025; 15(8):1202. https://doi.org/10.3390/life15081202
Chicago/Turabian StyleKatsianou, Maria A., Dimitrios Andreou, Penelope Korkolopoulou, Eleni-Kyriaki Vetsika, and Christina Piperi. 2025. "Epigenetic Modifications in Osteosarcoma: Mechanisms and Therapeutic Strategies" Life 15, no. 8: 1202. https://doi.org/10.3390/life15081202
APA StyleKatsianou, M. A., Andreou, D., Korkolopoulou, P., Vetsika, E.-K., & Piperi, C. (2025). Epigenetic Modifications in Osteosarcoma: Mechanisms and Therapeutic Strategies. Life, 15(8), 1202. https://doi.org/10.3390/life15081202







