A Multimodal Approach to Managing Severe Psoriasis Vulgaris: A Case Report Leveraging Natural Therapies for Flare Control
Abstract
1. Introduction
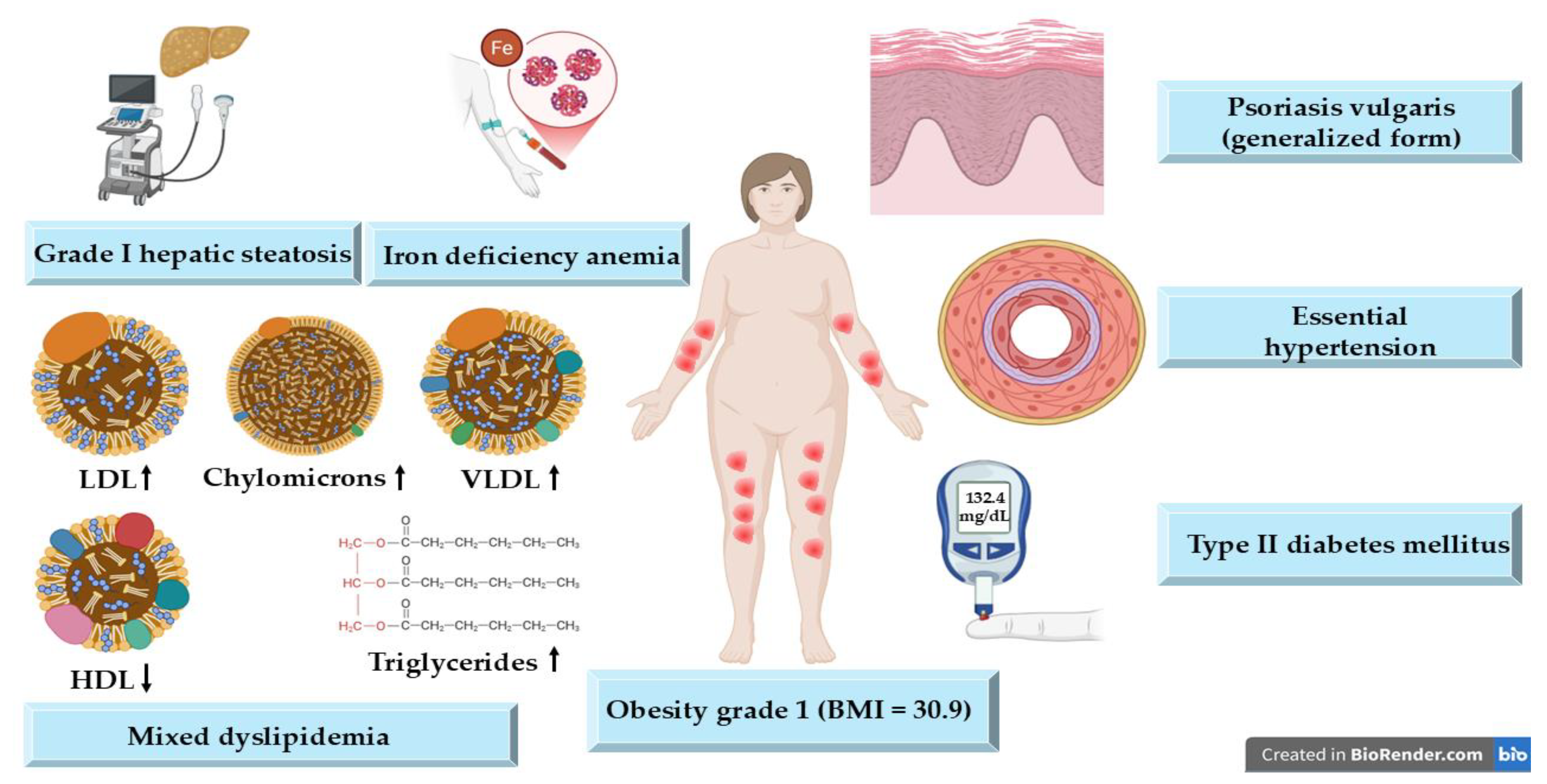
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PASI | Psoriasis Area and Severity Index |
| DLQI | Dermatology Life Quality Index |
| TNF- α | Tumor necrosis factor alpha |
| IL | Interleukin |
| ESR | Erythrocyte sedimentation rate |
| CRP | C-reactive protein |
| VLCKD | Very low-calorie ketogenic diet |
| BMI | Body mass index |
| JAK | Janus kinase |
| GPX | Glutathione peroxidase |
| ROS | Reactive oxygen species |
References
- Sieminska, I.; Pieniawska, M.; Grzywa, T.M. The Immunology of Psoriasis-Current Concepts in Pathogenesis. Clin. Rev. Allergy Immunol. 2024, 66, 164–191. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, G.W.; Lebwohl, M. Psoriasis: Overview and Diagnosis. In Evidence-Based Psoriasis Diagnosis and Treatment; Springer: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar]
- Sarac, G.; Koca, T.T.; Baglan, T. A Brief Summary of Clinical Types of Psoriasis. North Clin. Istanb. 2016, 3, 79–82. [Google Scholar] [PubMed]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and Management of Psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar] [PubMed]
- Manchanda, Y.; De, A.; Das, S.; Chakraborty, D. Disease Assessment in Psoriasis. Indian J. Dermatol. 2023, 68, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Marani, A.; Martina, E.; Diotallevi, F.; Radi, G.; Offidani, A. Psoriasis as an Immune-Mediated and Inflammatory Systemic Disease: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 1511. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moreno, A.; Krueger, J.G. The Imbalance between Type 17 T-Cells and Regulatory Immune Cell Subsets in Psoriasis Vulgaris. Front. Immunol. 2022, 13, 1005115. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Verduci, C.; Laconi, E.; Mangione, A.; Dondi, C.; Del Vecchio, M.; Carlevatti, V.; Zovi, A.; Capuozzo, M.; Langella, R. Current Therapeutic Overview and Future Perspectives Regarding the Treatment of Psoriasis. Int. Immunopharmacol. 2024, 143, 113388. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.B.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD–NPF Guidelines of Care for the Management and Treatment of Psoriasis with Topical Therapy and Alternative Medicine Modalities for Psoriasis Severity Measures. J. Am. Acad. Dermatol. 2021, 84, 432–470. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Treatment of Psoriasis: A Comprehensive Review of Entire Therapies. Curr. Drug Saf. 2020, 15, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Loft, N.; Egeberg, A.; Rasmussen, M.K.; Bryld, L.E.; Nissen, C.V.; Dam, T.N.; Ajgeiy, K.K.; Iversen, L.; Skov, L. Prevalence and Characterization of Treatment-Refractory Psoriasis and Super-Responders to Biologic Treatment: A Nationwide Study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.; Tit, D.M.; Endres, L.M.; Radu, A.-F.; Vesa, C.M.; Bungau, S.G. Naturally Derived Bioactive Compounds as Precision Modulators of Immune and Inflammatory Mechanisms in Psoriatic Conditions. Inflammopharmacology 2024, 33, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Abu-al-Basal, M.A. Histological Evaluation of the Healing Properties of Dead Sea Black Mud on Full-Thickness Excision Cutaneous Wounds in BALB/c Mice. Pakistan J. Biol. Sci. PJBS 2012, 15, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Bartholomew, E.; Yeroushalmi, S.; Hakimi, M.; Bhutani, T.; Liao, W. Dietary Intervention and Supplements in the Management of Psoriasis: Current Perspectives. Psoriasis 2022, 12, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Rousset, L.; Halioua, B. Stress and Psoriasis. Int. J. Dermatol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Olszewska, M.; Goldust, M.; Waśkiel-Burnat, A.; Warszawik-Hendzel, O.; Dorożyński, P.; Turło, J.; Rakowska, A. Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment. J. Clin. Med. 2021, 10, 5589. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Gracia-Cazaña, T.; Kurzen, H.; Galván, J. Calcipotriol/Betamethasone Dipropionate for the Treatment of Psoriasis: Mechanism of Action and Evidence of Efficacy and Safety versus Topical Corticosteroids. J. Clin. Med. 2024, 13, 4484. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q. Topical Corticosteroid Therapy for Psoriasis—A Review of Clobetasol Propionate 0.025% Cream and the Clinical Relevance of Penetration Modification. J. Clin. Aesthet. Dermatol. 2020, 13, 22–29. [Google Scholar] [PubMed]
- Shalaby, R.A.; El-Gazayerly, O.; Abdallah, M. Cubosomal Betamethasone-Salicylic Acid Nano Drug Delivery System for Enhanced Management of Scalp Psoriasis. Int. J. Nanomed. 2022, 17, 1659–1677. [Google Scholar] [CrossRef] [PubMed]
- Dallo, M.; Patel, K.; Hebert, A.A. Topical Antibiotic Treatment in Dermatology. Antibiotics 2023, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, D.; Nayak, C.; Chattopadhyay, A.; Karuppusamy, A.; Ambrose, M.M.; Kumar, A.; Singh, N.K.; Koley, M.; Saha, S. Individualized Homeopathic Medicines in the Treatment of Psoriasis Vulgaris: Double-Blind, Randomized, Placebo-Controlled Trial. Complement. Med. Res. 2023, 30, 317–331. [Google Scholar] [CrossRef] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Badri, T.; Kumar, P.; Oakley, A.M. Plaque Psoriasis. Pract. Nurs. 2023, 19, 560–565. [Google Scholar]
- Prieto-Pérez, R.; Solano-López, G.; Cabaleiro, T.; Román, M.; Ochoa, D.; Talegón, M.; Baniandrés, O.; López-Estebaranz, J.L.; de la Cueva, P.; Daudén, E.; et al. Polymorphisms Associated with Age at Onset in Patients with Moderate-to-Severe Plaque Psoriasis. J. Immunol. Res. 2015, 2015, 101879. [Google Scholar] [CrossRef] [PubMed]
- Potestio, L.; Lauletta, G.; Tommasino, N.; Portarapillo, A.; Salsano, A.; Battista, T.; Martora, F.; Megna, M. Risk Factors for Psoriasis Flares: A Narrative Review. Psoriasis 2024, 14, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Keenan, E.L.; Granstein, R.D. Proinflammatory Cytokines and Neuropeptides in Psoriasis, Depression, and Anxiety. Acta Physiol. 2025, 241, e70019. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Thaçi, D.; Warren, R.B. Addressing Challenges Associated with Long-Term Topical Treatment and Benefits of Proactive Management in Patients with Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Cîrstea, N.; Radu, A.; Vesa, C.; Radu, A.F.; Bungau, A.F.; Tit, D.M.; Cseppento, C.D.N.; Tarce, A.G.; Bungau, S.G. Current Insights on Treatment Adherence in Prevalent Dermatological Conditions and Strategies To Optimize Adherence Rates. Cureus 2024, 16, 869764. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Kaur, J.; Masand, P.; Anurag; Parihar, V.K.; Sharma, A. Vitamins Strategies for Psoriasis: An Update on Current Scientific Evidence. J. Holist. Integr. Pharm. 2023, 4, 299–309. [Google Scholar] [CrossRef]
- Elkhawaga, O.Y.; Ellety, M.M.; Mofty, S.O.; Ghanem, M.S.; Mohamed, A.O. Review of Natural Compounds for Potential Psoriasis Treatment. Inflammopharmacology 2023, 31, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, A.; Dutta, B. Correlation of Hypocalcaemia with Severity and Type of Psoriasis: A Cross-Sectional Study. IP Indian J. Clin. Exp. Dermatol. 2021, 7, 164–168. [Google Scholar] [CrossRef]
- Patra, P.; Harrison, T. Trace Elements in Psoriasis Presentation and Treatment. Arch. Dermatol. Res. 2024, 316, 728. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Saillard, C.; Ping Man, S.L.; Baggio, R.; Kammerer-Jacquet, S.-F.; Adamski, H.; Dupuy, A. A Pustular Psoriasis Flare Treated with Calcium Supplementation. JAAD Case Rep. 2021, 12, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Altemani, A.; Alsaedi, E.; Aldawish, R.; Alharbi, M.; Alzahrani, R.; Alatawi, S.; Altemani, S.; Alanazi, A.H. The Association of Psoriasis, Diabetes Mellitus, and Hypertension: A Meta-Analysis. Cureus 2023, 15, e48855. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Dębniak, T.; Baszuk, P.; Duchnik, E.; Rowińska, K.; Rogoża-Janiszewska, E.; Boer, M.; Kiedrowicz, M.; Marchlewicz, M.; Watola, D.; Feherpataky, M.; et al. Selenium and Arsenic Levels, Prevalence of Common Variants of Genes Involved in Their Metabolism, and Psoriasis Disease. Biomedicines 2024, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Ai, P.; Lei, S.; Zhou, F.; Chen, S.; Zhang, Y. Selenium Levels and Skin Diseases: Systematic Review and Meta-Analysis. J. Trace Elem. Med. Biol. 2020, 62, 126548. [Google Scholar] [CrossRef] [PubMed]
- Duchnik, E.; Kruk, J.; Tuchowska, A.; Marchlewicz, M. The Impact of Diet and Physical Activity on Psoriasis: A Narrative Review of the Current Evidence. Nutrients 2023, 15, 840. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, V.; Thatikonda, S.; Pooladanda, V.; Devabattula, G.; Godugu, C. Selenium Nanoparticles Produce a Beneficial Effect in Psoriasis by Reducing Epidermal Hyperproliferation and Inflammation. J. Nanobiotechnol. 2021, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Granger, C.; Trullàs, C.; Jesús-Silva, A.; Krutmann, J. Urea in Dermatology: A Review of Its Emollient, Moisturizing, Keratolytic, Skin Barrier Enhancing and Antimicrobial Properties. Dermatol. Ther. 2021, 11, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, T.; Petersen, A.; Houborg, H.I.; Rønsholdt, A.B.; Lybaek, D.; Steiniche, T.; Bregnhøj, A.; Iversen, L.; Johansen, C. Climatotherapy at the Dead Sea for Psoriasis Is a Highly Effective Anti-Inflammatory Treatment in the Short Term: An Immunohistochemical Study. Exp. Dermatol. 2022, 31, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Halevy, S.; Giryes, H.; Friger, M.; Sukenik, S. Dead Sea Bath Salt for the Treatment of Psoriasis Vulgaris: A Double-Blind Controlled Study. J. Eur. Acad. Dermatol. Venereol. 1997, 9, 237–242. [Google Scholar] [CrossRef]
- Hamed, S.; Almalty, A.-M.; Alkhatib, H.S. The Cutaneous Effects of Long-Term Use of Dead Sea Mud on Healthy Skin: A 4-Week Study. Int. J. Dermatol. 2021, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Ma, X.; Yan, X.; Bao, X. The Biological Role of Dead Sea Water in Skin Health: A Review. Cosmetics 2023, 10, 21. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, J. Rosemary (Rosmarinus officinalis L.) Polyphenols and Inflammatory Bowel Diseases: Major Phytochemicals, Functional Properties, and Health Effects. Fitoterapia 2024, 177, 106074. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; de Jesus, D.; Figueira, L.W.; de Oliveira, F.E.; Pacheco Soares, C.; Camargo, S.E.A.; Jorge, A.O.C.; de Oliveira, L.D. Biological Activities of Rosmarinus officinalis L. (Rosemary) Extract as Analyzed in Microorganisms and Cells. Exp. Biol. Med. 2017, 242, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Sahlabgi, A.; Lupuliasa, D.; Stanciu, G.; Lupșor, S.; Vlaia, L.L.; Rotariu, R.; Predescu, N.C.; Rădulescu, C.; Olteanu, R.-L.; Stănescu, S.-G.; et al. The Development and Comparative Evaluation of Rosemary Hydroalcoholic Macerate-Based Dermatocosmetic Preparations: A Study on Antioxidant, Antimicrobial, and Anti-Inflammatory Properties. Gels 2025, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- de Macedo, L.M.; Santos, É.M.d.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Harit, M.K.; Mundhe, N.; Tamoli, S.S.; Pawar, V.; Bhapkar, V.; Kolhe, G.; Mahadik, S.; Kulkarni, A.; Agarwal, A. Randomized, Double-Blind, Placebo-Controlled, Clinical Study of Passiflora Incarnata in Participants With Stress and Sleep Problems. Cureus 2024, 16, e56530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Sun, X.; Liu, L.; Zhang, M.; Yu, Y.; Gao, P.; Hong, S.; Li, X. Evidence-Based Dietary Recommendations for Patients with Psoriasis: A Systematic Review. Clin. Nutr. 2025, 47, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Megna, M.; Cacciapuoti, S.; Frias-Toral, E.; Fabbrocini, G.; Savastano, S.; Colao, A.; Muscogiuri, G. Very Low-Calorie Ketogenic Diet (VLCKD) in Patients with Psoriasis and Obesity: An Update for Dermatologists and Nutritionists. Crit. Rev. Food Sci. Nutr. 2022, 62, 398–414. [Google Scholar] [CrossRef] [PubMed]


| Active Substance | Concentration | Pharmaceutical Form | Mechanism of Action | Reference |
|---|---|---|---|---|
| Calcipotriol (vitamin D3 analogue) | 50 µg | Gel | Binds retinoid X receptor, regulates cell differentiation and immune function | [16] |
| Betamethasone Dipropionate | 0.5 mg | Gel | Inhibition of T-cell activation, reduced production of pro-inflammatory cytokines, induction of apoptosis and inhibition of proliferation | [17] |
| Clobetasol Propionate | 0.5 mg | Cream | Stabilizes cell membranes, modulates immune cells, reduces inflammation and proliferation | [18] |
| Salicylic acid | 20 mg | Cutaneous solution | Keratolytic action | [19] |
| Gentamicin sulphate | 1 mg | Cream | Inhibition of protein synthesis by targeting the 30S ribosomal subunit | [20] |
| Calcarea carbonica | 200 C | Granules | Overall impact on systemic predispositions, inflammation, and skin texture | [21] |
| Parameter | Value | Reference Range |
|---|---|---|
| CRP | 0.4 mg/dL | <0.5 mg/dL |
| ESR | 40 mm/h | <30 mm/h |
| Fibrinogen | 340 mg/dL | 200–400 mg/dL |
| Glucose | 132.4 mg/dL | 60–99 mg/dL |
| Serum iron | 30 µg/dL | 34–145 µg/dL |
| Total cholesterol | 280 mg/dL | <200 mg/dL |
| Triglycerides | 185 mg/dL | <150 mg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, A.; Jurca, T.; Radu, A.-F.; Bodog, T.M.; Bodog, R.F.; Endres, L. A Multimodal Approach to Managing Severe Psoriasis Vulgaris: A Case Report Leveraging Natural Therapies for Flare Control. Life 2025, 15, 1186. https://doi.org/10.3390/life15081186
Radu A, Jurca T, Radu A-F, Bodog TM, Bodog RF, Endres L. A Multimodal Approach to Managing Severe Psoriasis Vulgaris: A Case Report Leveraging Natural Therapies for Flare Control. Life. 2025; 15(8):1186. https://doi.org/10.3390/life15081186
Chicago/Turabian StyleRadu, Ada, Tunde Jurca, Andrei-Flavius Radu, Teodora Maria Bodog, Ruxandra Florina Bodog, and Laura Endres. 2025. "A Multimodal Approach to Managing Severe Psoriasis Vulgaris: A Case Report Leveraging Natural Therapies for Flare Control" Life 15, no. 8: 1186. https://doi.org/10.3390/life15081186
APA StyleRadu, A., Jurca, T., Radu, A.-F., Bodog, T. M., Bodog, R. F., & Endres, L. (2025). A Multimodal Approach to Managing Severe Psoriasis Vulgaris: A Case Report Leveraging Natural Therapies for Flare Control. Life, 15(8), 1186. https://doi.org/10.3390/life15081186








