Gastric Cancer and Microbiota: Exploring the Microbiome’s Role in Carcinogenesis and Treatment Strategies
Abstract
1. Introduction
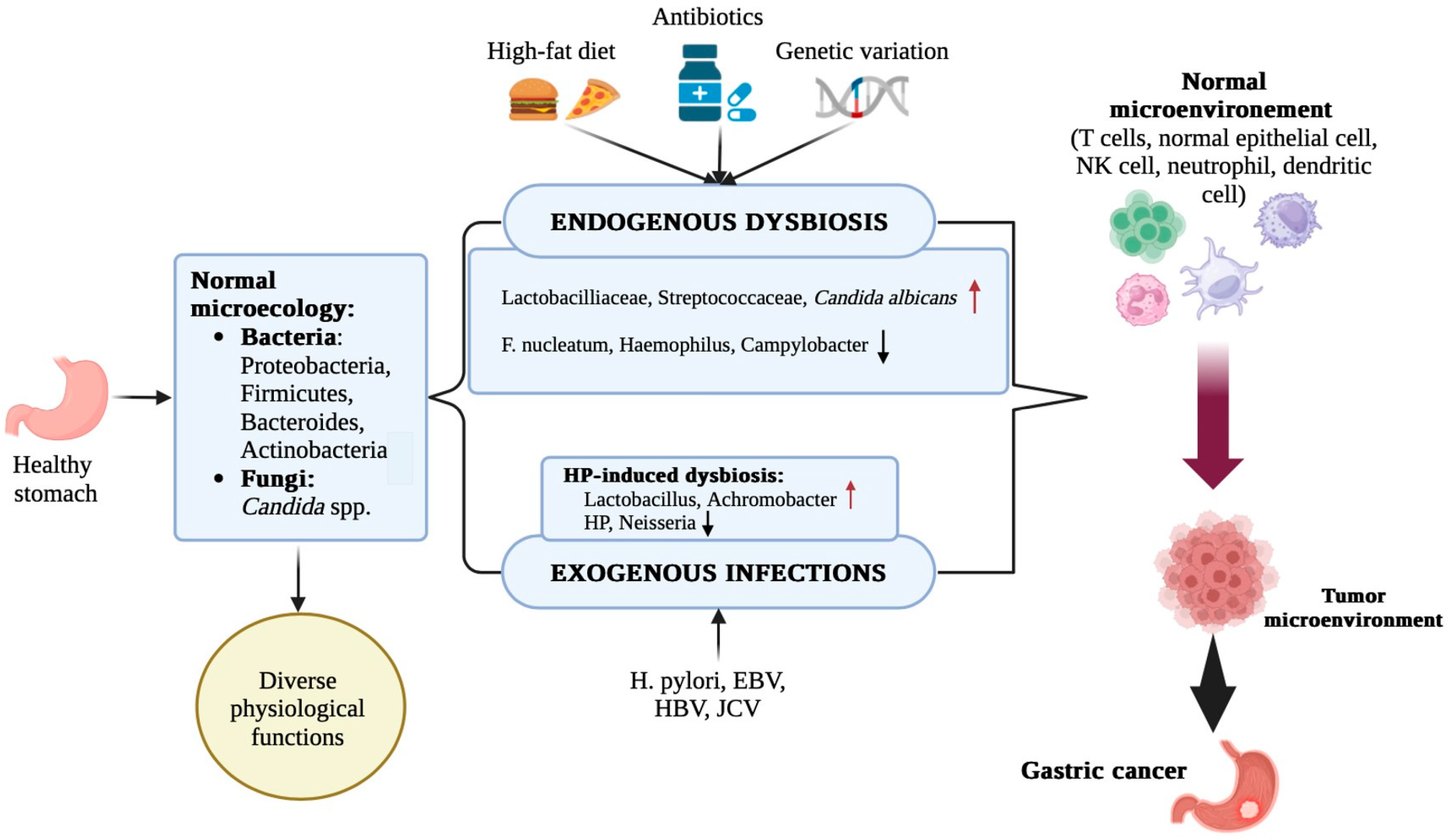
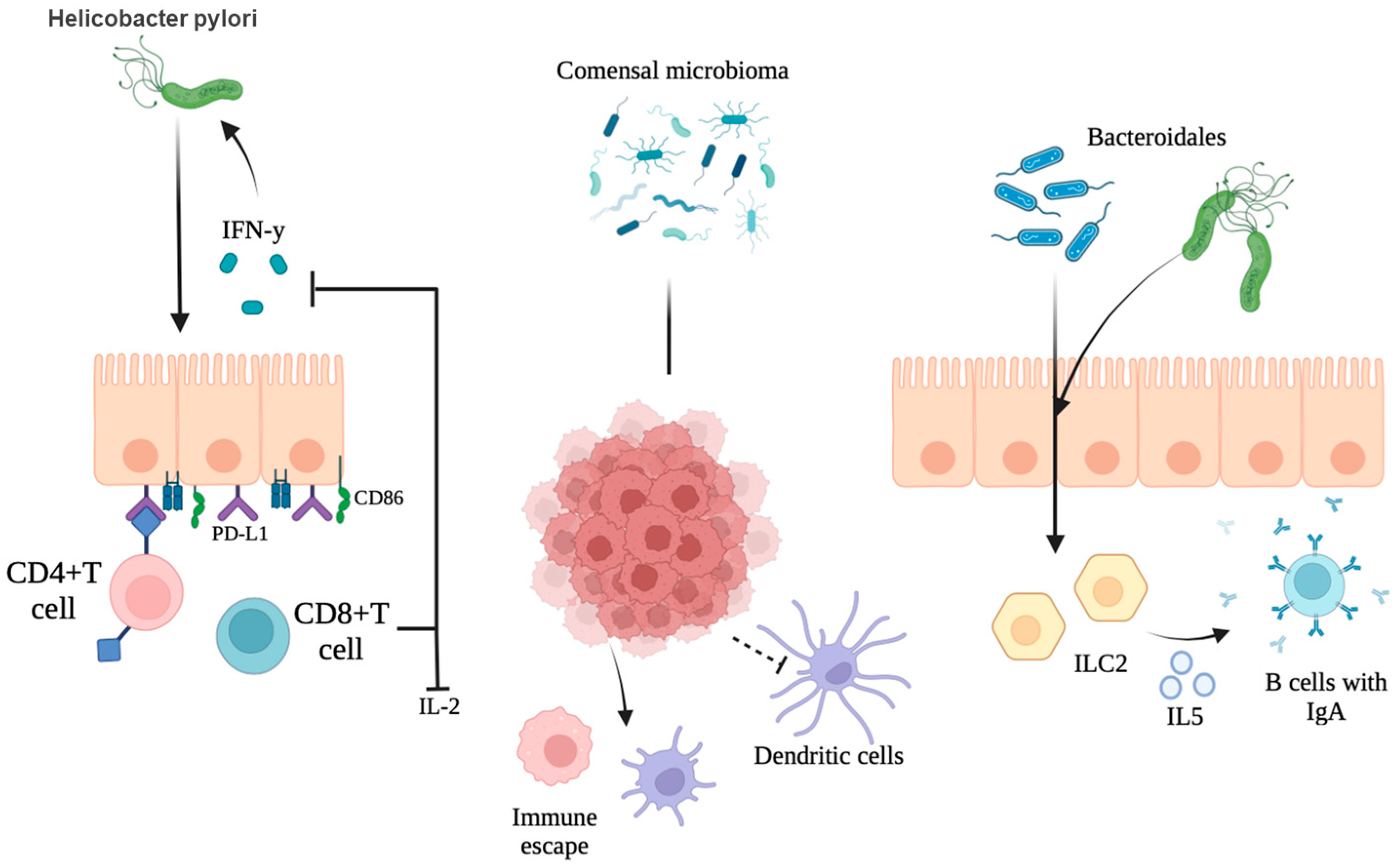
2. Helicobacter Pylori and the Gastric Microbiome
2.1. Role of H. Pylori in Gastric Carcinogenesis and the Microbial Dysbiosis Triggered by This Bacterium
2.2. Synergistic and Antagonistic Interactions with Other Microbes
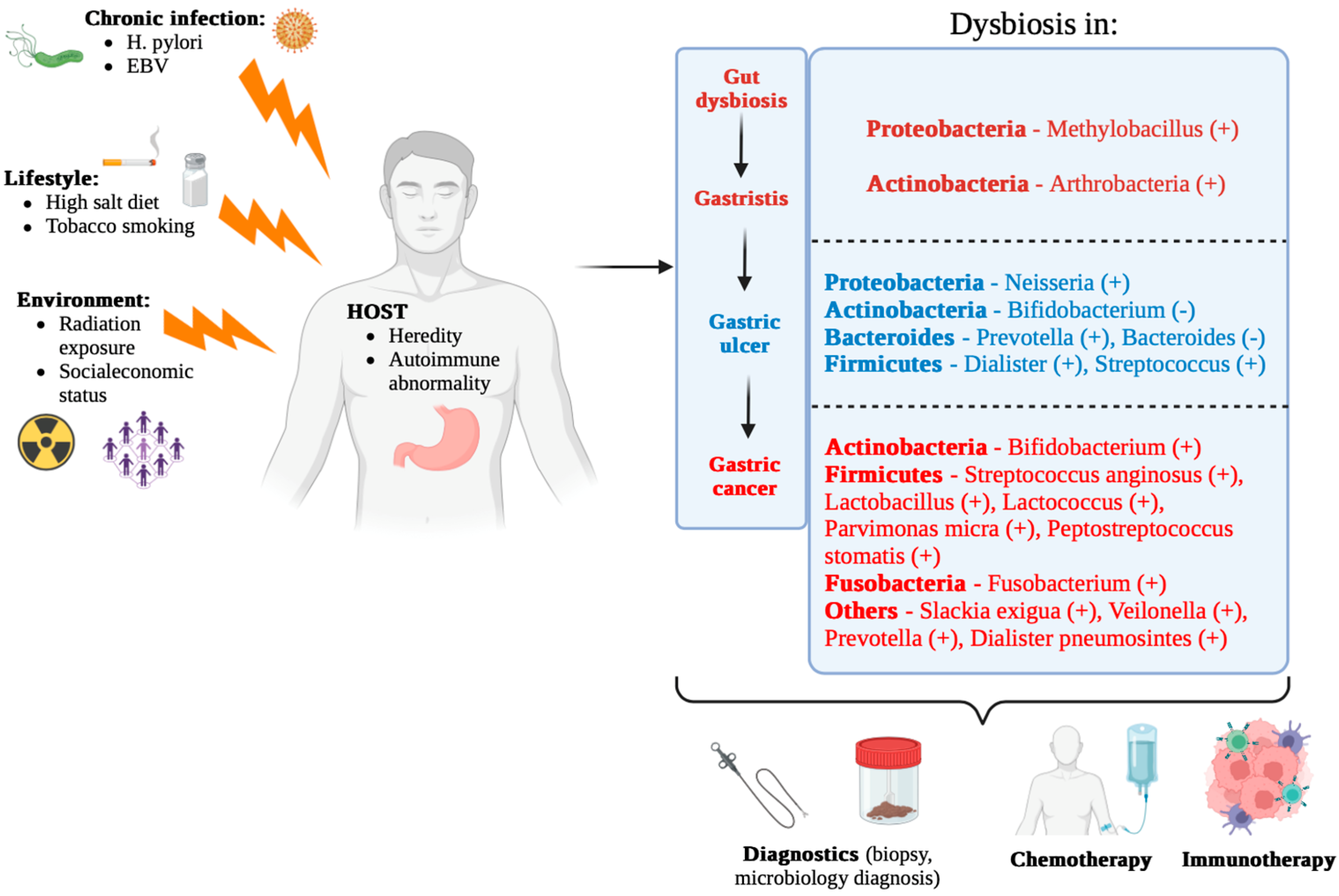
3. Gastric Microbiota Alterations in Carcinogenesis
3.1. Microbiota Composition in Precancerous and Cancerous Lesions
3.2. Fungal Microbiota and Bacterial–Fungal Interactions
4. Microbial Metabolites and Oncogenic Pathways
4.1. Key Microbial Metabolites
4.2. Metabolite-Induced Signaling in Cancer Cell Proliferation and Inflammation–the Role of Specific Metabolites as Diagnostic and Prognostic Markers
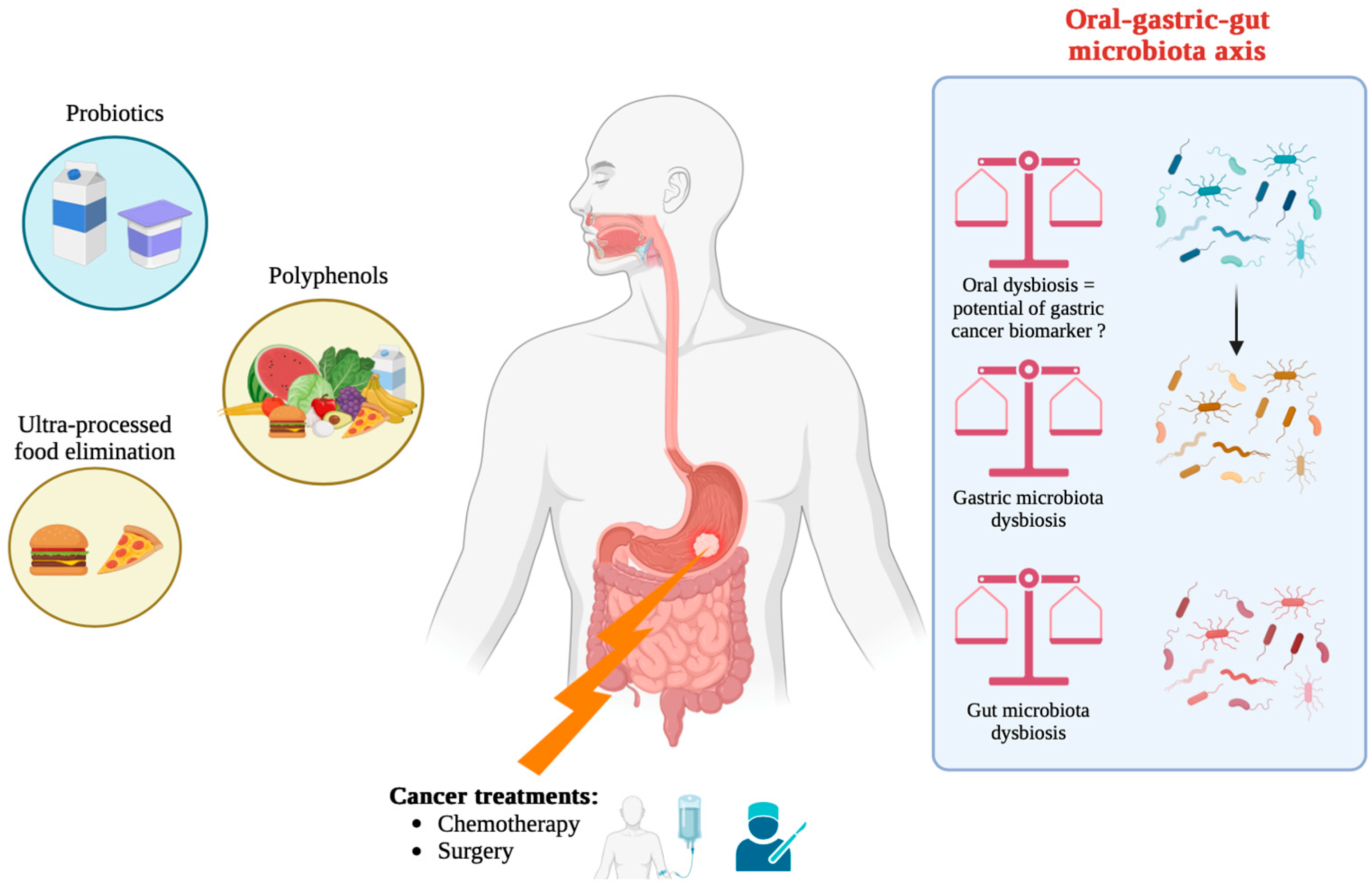
5. Oral and Gut Microbiota: Peripheral Players with Central Roles
5.1. Impact of Oral Microbiota Translocation to the Stomach
5.2. The Salivary Microbiota as a Diagnostic Avenue
5.3. The Digestive Tract Microbiota’s Systemic Immunoregulatory Effects
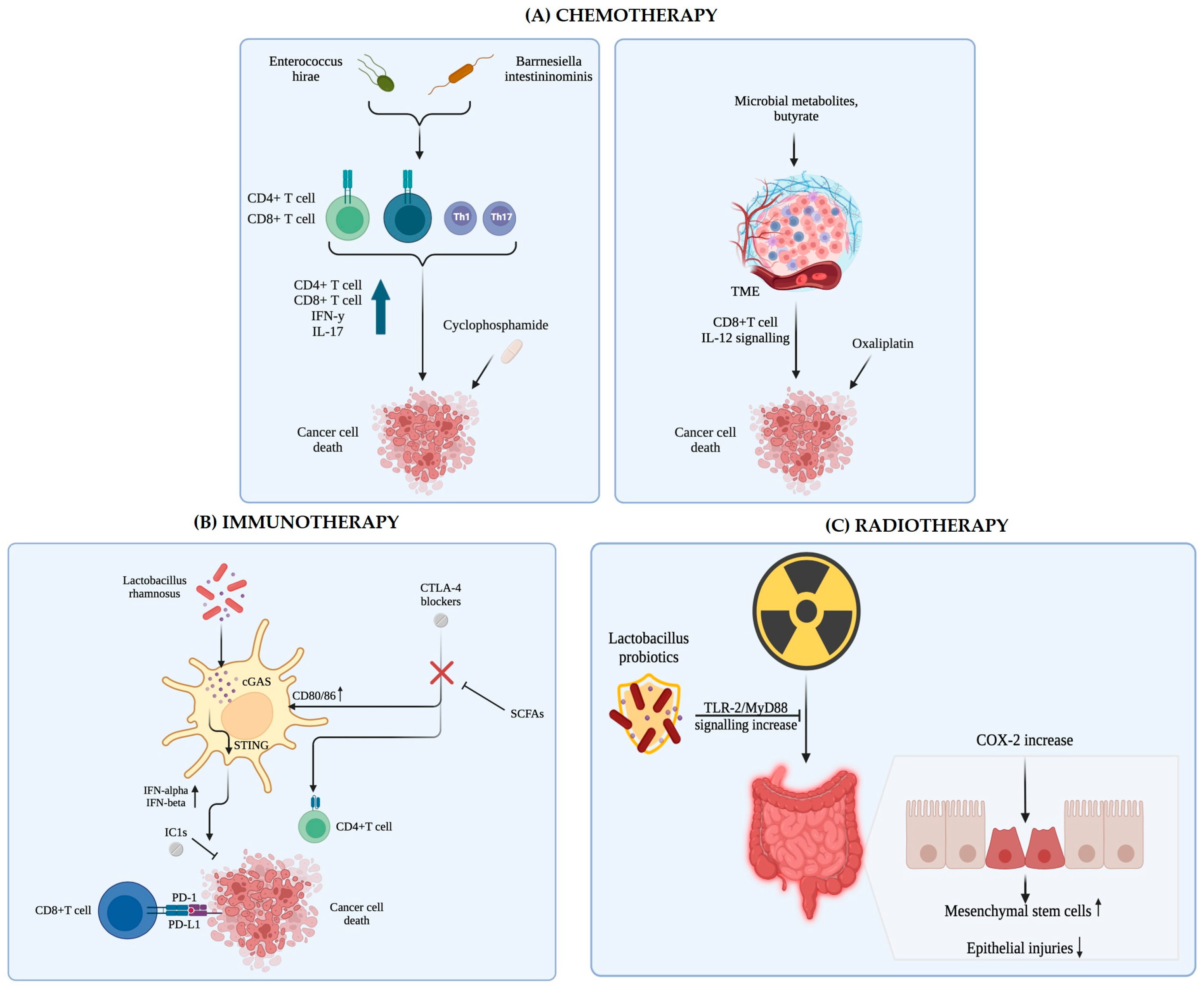
6. Microbiota, Immunity, and Therapy Response
6.1. Impact of Microbiota on Immunotherapy Outcomes
6.2. Strategies to Modulate Microbiota to Enhance Therapeutic Response
7. Clinical Perspectives and Future Directions
7.1. Clinical Aspects
7.2. Future Perspectives
7.3. Microbiome-Based Clinical Trials
8. Conclusions
- Longitudinal studies tracking microbiota changes from precancerous lesions to advanced GC to establish causal relationships and microbial signatures predictive of progression;
- Functional microbiome analyses (metatranscriptomics, metabolomics) to elucidate the active metabolic pathways driving carcinogenesis;
- Identification of microbial-derived biomarkers for early diagnosis, prognosis, and treatment stratification;
- Integration of microbiome data into personalized medicine frameworks, particularly for immunotherapy responsiveness prediction;
- Investigation of microbial–host immune interactions, including the role of microbiota in programming tissue-resident memory T cells and shaping the tumor immune landscape.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| WHO | World Health Organization |
| IARC | International Agency for Research on Cancer |
| CNS | Central nervous system |
| FMT | Fecal microbiota transplantation |
| MDIs | Microbial dysbiosis indices |
| CAG | Chronic atrophic gastritis |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| PTEN | Phosphatase and tensin homologue |
| VEGF | Vascular endothelial growth factor |
| MNU | N-Methyl-N-nitrosoureas |
| SCFAs | Short-chain fatty acids |
| HDAC | Histone deacetylase |
| TME | Tumor microenvironment |
| NK | Natural killer cells |
| DCA | Deoxycholic acid |
| COX | Cyclooxygenase |
| DFMO | α-difluoromethylornithine |
| PUFAs | ω-6 polyunsaturated fatty acids |
| PGE2 | Prostaglandin E2 |
| LPS | Lipopolysaccharide |
| PAMPs | Pathogen-associated molecular patterns |
| TLRs | Toll-like receptors |
| GPCRs | G-protein-coupled receptors |
| GC/MS | Gas chromatography/mass spectrometry |
| LC-MS | Liquid chromatography/mass spectrometry |
| NMR | Nuclear magnetic resonance spectroscopy |
| TCA | Tricarboxylic acid |
| GI | Gastrointestinal |
| PPIs | Proton pump inhibitors |
| OSCC | Oral squamous cell carcinoma |
| IBD | Inflammatory bowel disease |
| PRRs | Pathogen recognition receptors |
| TAMs | Tumor-associated macrophages |
| MDSCs | Myeloid-derived suppressor cells |
| Tregs | Regulatory T cells |
| pDCs | Plasmacytoid dendritic cells |
| ICIs | Immune checkpoint inhibitors |
| IECs | Intestinal epithelial cells |
| MHC | Major histocompatibility complex class II molecules |
| TRM | Tissue-resident memory T cells |
| NAFLD | Nonalcoholic fatty liver disease |
| ESMO | European Society of Medical Oncology |
| CSCO | Chinese Society of Clinical Oncology |
| MSI-H | Microsatellite instability-high |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
References
- Thompson, C.A.; Gomez, S.L.; Hastings, K.G.; Kapphahn, K.; Yu, P.; Shariff-Marco, S.; Bhatt, A.S.; Wakelee, H.A.; Patel, M.I.; Cullen, M.R.; et al. The Burden of Cancer in Asian Americans: A Report of National Mortality Trends by Asian Ethnicity. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.M.; Xu, R.; Tang, X.L.; Ding, Z.; Li, J.M.; Zhou, X. Conversion therapy for advanced gastric cancer with trastuzumab combined with chemotherapy: A case report. Oncol. Lett. 2018, 16, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, S.; Wang, W.; Zhao, Y.; Qiu, Y.; Jiang, X.; Lin, T.; Yang, Q. The Trends of Gastric Cancer in China From 1990 to 2019 and Predictions to 2040: A Bayesian Age-Period-Cohort Prediction Study. Cancer Control 2024, 31, 10732748241293982. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.H.; Fang, H.; Xie, C. Advanced glycation end products in gastric cancer: A promising future. World. J. Clin. Oncol. 2024, 15, 1117–1121. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Abengozar, R.; Sharma, A.; Sharma, R. Gastric cancer: Lessons learned from high-incidence geographic regions. J. Gastrointest. Oncol. 2021, 12 (Suppl. 2), S350–S360. [Google Scholar] [CrossRef]
- Echeverría-Garcés, G.; Ramos-Medina, M.; Vargas, R.; Cabrera-Andrade, A.; Altamirano-Colina, A.; Freire, M.; Montalvo-Guerrero, J.; Rivera-Orellana, S.; Echeverría-Espinoza, P.; Quiñones, L.A.; et al. Gastric cancer actionable genomic alterations across diverse populations worldwide and pharmacogenomics strategies based on precision oncology. Front. Pharmacol. 2024, 15, 1373007. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Chan, P.S.F.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef]
- Atabey, M.; Taş, A.; Ağbektaş, T.; Bostanci, M.E.; Topcu, O.; Siliğ, Y. Investigation of the relationship between β2-adrenergic receptor (β2-AR) polymorphism and gastric cancer. Cumhur. Med. J. 2018, 40, 284–290. [Google Scholar]
- Li, C.; Chen, D.; Yang, H. Trends in Incidence, Survival and Mortality of Gastric Cancer in the United States: A Population-Based Study, 2001–2015. Asian Pac. J. Cancer Prev. 2023, 24, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Bair, M.J.; Chen, P.Y.; Lee, J.Y.; Yang, T.H.; Fang, Y.J.; Chen, C.C.; Chang, A.T.; Hsiao, W.D.; Yu, J.J.; et al. Declining trends of prevalence of Helicobacter pylori infection and incidence of gastric cancer in Taiwan: An updated cross-sectional survey and meta-analysis. Helicobacter 2022, 27, e12914. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.; Chen, Y.Z.; Chou, N.H.; Chen, Y.C.; Wu, C.C.; Liu, L.F.; Yang, Y.F.; Yeh, C.Y.; Kung, M.L.; Tu, Y.T.; et al. Metformin inhibits gastric cancer cell proliferation by regulation of a novel Loc100506691-CHAC1 axis. Mol. Ther. Oncolytics 2021, 22, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Fan, Y.; Yang, T.; Yang, Z.; Fan, D. The burden of Gastric Cancer and possible risk factors from 1990 to 2021, and projections until 2035: Findings from the Global Burden of Disease Study 2021. Biomark. Res. 2025, 13, 5. [Google Scholar] [CrossRef]
- Li, Y.; Hahn, A.I.; Laszkowska, M.; Jiang, F.; Zauber, A.G.; Leung, W.K. Global burden of young-onset gastric cancer: A systematic trend analysis of the global burden of disease study 2019. Gastric Cancer 2024, 27, 684–700. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Gweon, T.G.; Yoon, K.T.; Kim, C.H.; Kim, J.J. Postgastrectomy gastric cancer patients are at high risk for colorectal neoplasia: A case control study. Intest. Res. 2021, 19, 239–246. [Google Scholar] [CrossRef]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef]
- Cover, T.L.; Blaser, M.J. Helicobacter pylori in health and disease. Gastroenterology 2009, 136, 1863–1873. [Google Scholar] [CrossRef]
- Kataoka, K. The intestinal microbiota and its role in human health and disease. J. Med. Investig. 2016, 63, 27–37. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liou, J.M.; Lee, Y.C.; Hong, T.C.; El-Omar, E.M.; Wu, M.S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021, 13, 1–22. [Google Scholar] [CrossRef]
- Nasr, R.; Shamseddine, A.; Mukherji, D.; Nassar, F.; Temraz, S. The Crosstalk between Microbiome and Immune Response in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 6586. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.; Guan, K.; Ma, Y.; Liu, R.; Liu, Y.; Lv, X.; Wang, R.; Li, Q. Therapeutic potential of Lacticaseibacillus rhamnosus grx10 and its derived postbiotic through gut microbiota and MAPK/MLCK/MLC pathway-mediated intestinal barrier repairment in ulcerative colitis. J. Food Sci. 2024, 89, 10035–10052. [Google Scholar] [CrossRef]
- Fukui, H.; Xu, X.; Miwa, H. Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Goh, K.L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef]
- Dias-Jácome, E.; Libânio, D.; Borges-Canha, M.; Galaghar, A.; Pimentel-Nunes, P. Gastric microbiota and carcinogenesis: The role of non-Helicobacter pylori bacteria-A systematic review. Rev. Esp. Enferm. Dig. 2016, 108, 530–540. [Google Scholar] [CrossRef]
- Pope, J.L.; Tomkovich, S.; Yang, Y.; Jobin, C. Microbiota as a mediator of cancer progression and therapy. Transl. Res. 2017, 179, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Noto, J.M.; Zackular, J.P.; Varga, M.G.; Delgado, A.; Romero-Gallo, J.; Scholz, M.B.; Piazuelo, M.B.; Skaar, E.P.; Peek, R.M., Jr. Modification of the Gastric Mucosal Microbiota by a Strain-Specific Helicobacter pylori Oncoprotein and Carcinogenic Histologic Phenotype. mBio 2019, 10, e00955-19. [Google Scholar] [CrossRef] [PubMed]
- Liao, O.; Ye, G.; Du, Q.; Ye, J. Gastric microbiota in gastric cancer and precancerous stages: Mechanisms of carcinogenesis and clinical value. Helicobacter 2023, 28, e12964. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Zhao, X.; Qiu, Y.; Liang, L.; Fu, X. Interkingdom signaling between gastrointestinal hormones and the gut microbiome. Gut Microbes 2025, 17, 2456592. [Google Scholar] [CrossRef]
- Thomson, P.; Núñez, P.; Quera, R.; Bay, C. Gastrointestinal microbiome, what is behind faecal microbiota transplantation? New Microbes New Infect. 2021, 42, 100898. [Google Scholar] [CrossRef]
- Lei, C.; Gong, D.; Zhuang, B.; Zhang, Z. Alterations in the gastric microbiota and metabolites in gastric cancer: An update review. Front. Oncol. 2022, 12, 960281. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Cao, W.; Zhang, Z. Alterations of Gastric Microbiota in Gastric Cancer and Precancerous Stages. Front. Cell Infect. Microbiol. 2021, 11, 559148. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, X.S.; Guo, G.Y.; Zhou, M.G.; Yu, B. Effect of Helicobacter Pylori Eradication on Human Gastric Microbiota: A Systematic Review and Meta-Analysis. Front. Cell Infect. Microbiol. 2022, 12, 899248. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tsai, W.H.; Wu, H.Y.; Chen, C.Y.; Yeh, W.L.; Chen, Y.H.; Hsu, H.Y.; Chen, W.W.; Chen, Y.W.; Chang, W.W.; et al. Probiotic Lactobacillus spp. act Against Helicobacter pylori-induced Inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef]
- Das, A.; Pereira, V.; Saxena, S.; Ghosh, T.S.; Anbumani, D.; Bag, S.; Das, B.; Nair, G.B.; Abraham, P.; Mande, S.S. Gastric microbiome of Indian patients with Helicobacter pylori infection, and their interaction networks. Sci. Rep. 2017, 7, 15438. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, N.; Ploner, A.; Andersson, A.F.; Zagai, U.; Andreasson, A.; Vieth, M.; Talley, N.J.; Agreus, L.; Ye, W. Gastric Microbiota in a Low-Helicobacter pylori Prevalence General Population and Their Associations With Gastric Lesions. Clin. Transl. Gastroenterol. 2020, 11, e00191. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Zou, K.; Xiang, C.J.; Jin, Z.J.; Ding, H.H.; Xu, S.; Wu, G.N.; Wang, Y.H.; Wu, X.Y.; Chen, C.; et al. Helicobacter pylori infection is associated with the co-occurrence of bacteria in the oral cavity and the gastric mucosa. Helicobacter 2021, 26, e12786. [Google Scholar] [CrossRef]
- Zhou, S.; Li, C.; Liu, L.; Yuan, Q.; Miao, J.; Wang, H.; Ding, C.; Guan, W. Gastric microbiota: An emerging player in gastric cancer. Front. Microbiol. 2023, 14, 1130001. [Google Scholar] [CrossRef]
- Liu, X.; Nie, W.; Liang, J.; Li, Y. Interaction of Helicobacter pylori with other microbiota species in the development of gastric cancer. Arch. Clin. Microbiol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Gao, J.J.; Zhang, Y.; Gerhard, M.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; Liu, W.D.; et al. Association Between Gut Microbiota and Helicobacter pylori-Related Gastric Lesions in a High-Risk Population of Gastric Cancer. Front. Cell Infect. Microbiol. 2018, 8, 202. [Google Scholar] [CrossRef]
- Watanabe, T.; Nadatani, Y.; Suda, W.; Higashimori, A.; Otani, K.; Fukunaga, S.; Hosomi, S.; Tanaka, F.; Nagami, Y.; Taira, K.; et al. Long-term persistence of gastric dysbiosis after eradication of Helicobacter pylori in patients who underwent endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 2021, 24, 710–720. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.M.; Cai, T.; Wang, F. Short-term and long-term alterations of gastrointestinal microbiota with different H. pylori eradication regimens: A meta-analysis. Front. Cell. Infect. Microbiol. 2022, 12, 913384. [Google Scholar] [CrossRef]
- Tao, Z.H.; Han, J.X.; Fang, J.Y. Helicobacter pylori infection and eradication: Exploring their impacts on the gastrointestinal microbiota. Helicobacter 2020, 25, e12754. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Lu, N. Impacts of Helicobacter pylori infection and eradication on gastrointestinal microbiota: An up-to-date critical review and future perspectives. Chin. Med. J. 2024, 137, 2833–2842. [Google Scholar] [CrossRef]
- Talarico, F.; Tilocca, B.; Spagnuolo, R.; Abenavoli, L.; Luzza, F.; Roncada, P. The effects of stress on gut virome: Implications on infectious disease and systemic disorders. MicrobiologyOpen 2024, 13, e1434. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Judd, L.M.; Menheniott, T.R.; Kronborg, I.; Dow, C.; Yeomans, N.D.; Boussioutas, A.; Robb, L.; Giraud, A.S. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J. Pathol. 2007, 213, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Maekita, T.; Nakazawa, K.; Mihara, M.; Nakajima, T.; Yanaoka, K.; Iguchi, M.; Arii, K.; Kaneda, A.; Tsukamoto, T.; Tatematsu, M.; et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin. Cancer Res. 2006, 12 Pt 1, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Bronte-Tinkew, D.M.; Terebiznik, M.; Franco, A.; Ang, M.; Ahn, D.; Mimuro, H.; Sasakawa, C.; Ropeleski, M.J.; Peek, R.M., Jr.; Jones, N.L. Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 2009, 69, 632–639. [Google Scholar] [CrossRef]
- Bebb, J.R.; Letley, D.P.; Thomas, R.J.; Aviles, F.; Collins, H.M.; Watson, S.A.; Hand, N.M.; Zaitoun, A.; Atherton, J.C. Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag-dependent manner. Gut 2003, 52, 1408–1413. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Y.; Yang, Z.; Hu, Y.; Shu, X.; Xie, C.; He, C.; Zhu, Y.; Lu, N. Helicobacter pylori promotes gastric epithelial cell survival through the PLK1/PI3K/Akt pathway. OncoTargets Ther. 2018, 11, 5703–5713. [Google Scholar] [CrossRef]
- McGee, D.J.; Kumar, S.; Viator, R.J.; Bolland, J.R.; Ruiz, J.; Spadafora, D.; Testerman, T.L.; Kelly, D.J.; Pannell, L.K.; Windle, H.J. Helicobacter pylori thioredoxin is an arginase chaperone and guardian against oxidative and nitrosative stresses. J. Biol. Chem. 2006, 281, 3290–3296. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, L.; Zhang, T.; Shen, L.; Liu, L.; Zhang, J.; Zhang, Y.; Wang, X.; Yang, S.; Lu, F.; et al. The involvement of Helicobacter pylori thioredoxin-1 in gastric carcinogenesis. J. Med. Microbiol. 2013, 62 Pt 8, 1226–1234. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Xu, W.; Liu, G.; Cao, X.; Li, W.; Chen, J.; Zhu, Y.; Luo, S.; Luo, Z.; et al. Phosphorylation and inactivation of PTEN at residues Ser380/Thr382/383 induced by Helicobacter pylori promotes gastric epithelial cell survival through PI3K/Akt pathway. Oncotarget 2015, 6, 31916–31926. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, N.; Chai, N.; Liu, X.; Jiang, H.; Wu, Q.; Li, Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 2016, 16, 321. [Google Scholar] [CrossRef]
- Liu, N.; Wu, Q.; Wang, Y.; Sui, H.; Liu, X.; Zhou, N.; Zhou, L.; Wang, Y.; Ye, N.; Fu, X.; et al. Helicobacter pylori promotes VEGF expression via the p38 MAPK-mediated COX-2-PGE2 pathway in MKN45 cells. Mol. Med. Rep. 2014, 10, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Wang, X.; Wang, Y.; Li, R.; Li, G.; He, Y.; Liu, S.; Luo, Y.; Wang, L.; Lei, Z. Helicobacter pylori promotes gastric cancer progression by upregulating semaphorin 5A expression via ERK/MMP9 signaling. Mol. Ther. Oncolytics 2021, 22, 256–264. [Google Scholar] [CrossRef]
- Choi, J.M.; Kim, S.G.; Yang, H.J.; Lim, J.H.; Cho, N.Y.; Kim, W.H.; Kim, J.S.; Jung, H.C. Helicobacter pylori Eradication Can Reverse the Methylation-Associated Regulation of miR-200a/b in Gastric Carcinogenesis. Gut Liver 2020, 14, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Maruta, F.; Ota, H.; Genta, R.M.; Sugiyama, A.; Tatematsu, M.; Katsuyama, T.; Kawasaki, S. Role of N-methyl-N-nitrosourea in the induction of intestinal metaplasia and gastric adenocarcinoma in Mongolian gerbils infected with Helicobacter pylori. Scand. J. Gastroenterol. 2001, 36, 283–290. [Google Scholar] [CrossRef]
- Cao, X.; Tsukamoto, T.; Nozaki, K.; Tanaka, H.; Shimizu, N.; Kaminishi, M.; Kumagai, T.; Tatematsu, M. Earlier Helicobacter pylori infection increases the risk for the N-methyl-N-nitrosourea-induced stomach carcinogenesis in Mongolian gerbils. Jpn. J. Cancer Res. 2002, 93, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Roviello, G.; Catalano, M.; Połom, K. Interdisciplinary insights into the link between gut microbiome and gastric carcinogenesis-what is currently known? Gastric Cancer 2022, 25, 1–10. [Google Scholar] [CrossRef]
- Clooney, A.G.; Sutton, T.D.S.; Shkoporov, A.N.; Holohan, R.K.; Daly, K.M.; O’Regan, O.; Ryan, F.J.; Draper, L.A.; Plevy, S.E.; Ross, R.P.; et al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe 2019, 26, 764–778. [Google Scholar] [CrossRef]
- Luo, S.; Xue, J.; Ru, J.; Mirzaei, M.; Ralser, A.; Mejias-Luque, R.; Gerhard, M.; Deng, L. Altered virome structure and function characterization in Helicobacter pylori-driven colorectal carcinogenesis and H. pylori eradication. Cold Spring Harb. Lab. 2022, 1–32. [Google Scholar] [CrossRef]
- Wang, L.; Yao, H.; Morgan, D.C.; Lau, K.S.; Leung, S.Y.; Ho, J.W.K.; Lueng, W.K. Altered human gut virome in patients undergoing antibiotics therapy for Helicobacter pylori. Nat. Commun. 2023, 14, 2196. [Google Scholar] [CrossRef]
- Sinha, A.; Li, Y.; Mirzaei, M.K.; Shamash, M.; Samadfam, R.; King, I.L.; Maurice, C.F. Transplantation of bacteriophages from ulcerative colitis patients shifts the gut bacteriome and exacerbates the severity of DSS colitis. Microbiome 2022, 10, 105. [Google Scholar] [CrossRef]
- Lin, F. Commensal virome regulates intestinal nutrient sensing via Th17 cells. Cold Spring Harb. Lab. 2024, 1–51. [Google Scholar] [CrossRef]
- He, C.; Peng, C.; Wang, H.; Ouyang, Y.; Zhu, Z.; Shu, X.; Zhu, Y.; Lu, N. The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter 2019, 24, e12590. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xie, Y.; Zhu, Y.; Zhuang, K.; Huo, L.; Yu, Y.; Lu, N. Probiotics modulate gastrointestinal microbiota after Helicobacter pylori eradication: A multicenter randomized double-blind placebo-controlled trial. Front. Immunol. 2022, 13, 1033063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, M.; Fu, X. Gastric microbiota dysbiosis and Helicobacter pylori infection. Front. Microbiol. 2023, 14, 1153269. [Google Scholar] [CrossRef]
- Gomez-Ramirez, U.; Valencia-Mayoral, P.; Mendoza-Elizalde, S.; Murillo-Eliosa, J.R.; Solórzano Santos, F.; Contreras-Rodríguez, A.; Zúñiga, G.; Aguilar-Rodea, P.; Jiménez-Rojas, V.L.; Vigueras Galindo, J.C.; et al. Role of Helicobacter pylori and other environmental factors in the development of gastric dysbiosis. Pathogens 2021, 10, 1203. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, X.; Guo, J.; Yu, D.; Xiao, Y.; Wang, H.; Li, Y. Helicobacter pylori infection alters gastric and tongue coating microbial communities. Helicobacter 2019, 24, e12567. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, X.X.; Shao, Q.Q.; Yu, X.C.; Khan, M.N.; Qi, Y.B.; Hu, R.B.; Wei, P.R.; Xiao, W.; et al. Gastric mucosal precancerous lesions in Helicobacter pylori-infected pediatric patients in central China: A single-center, retrospective investigation. World J. Gastroenterol. 2022, 28, 3682–3694. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69, 1572–1580. [Google Scholar] [CrossRef]
- Liu, C.; Ng, S.K.; Ding, Y.; Lin, Y.; Liu, W.; Wong, S.H.; Sung, J.J.; Yu, J. Meta-analysis of mucosal microbiota reveals universal microbial signatures and dysbiosis in gastric carcinogenesis. Oncogene 2022, 41, 3599–3610. [Google Scholar] [CrossRef]
- Liu, D.; Chen, S.; Gou, Y.; Yu, W.; Zhou, H.; Zhang, R.; Wang, J.; Ye, F.; Liu, Y.; Sun, B.; et al. Gastrointestinal Microbiota Changes in Patients With Gastric Precancerous Lesions. Front. Cell Infect. Microbiol. 2021, 11, 749207. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Wang, A.; Chu, A.N.; Gong, Y.H.; Yuan, Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared with Non-cancer Tissues. Front. Microbiol. 2019, 10, 1261. [Google Scholar] [CrossRef]
- Westwater, C.; Schofield, D.A.; Nicholas, P.J.; Paulling, E.E.; Balish, E. Candida glabrata and Candida albicans; dissimilar tissue tropism and infectivity in a gnotobiotic model of mucosal candidiasis. FEMS Immunol. Med. Microbiol. 2007, 51, 134–139. [Google Scholar] [CrossRef]
- Kertmen, Ö.; Gök, G.; Akçay, M. Purulent Pericarditis with Cardiac Tamponade Secondary to Candida Albicans after Total Parenteral Nutrition: A Case Report. J. Tehran Heart Cent. 2020, 15, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.R.; Smith, H.F.; Pease, F.B., Jr. Bacteriology of the stomach immediately following vagotomy: The growth of Candida albicans. Ann. Surg. 1974, 179, 859–862. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhou, Y.; Feng, Y.; Sun, T.; Xu, J. Tumor-related fungi and crosstalk with gut fungi in the tumor microenvironment. Cancer Biol. Med. 2024, 21, 977–994. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, X.; Xu, R.; Adeel, K.; Lu, X.; Chen, M.; Shen, H.; Li, Z.; Xu, Z. Fungal Microbiota Dysbiosis and Ecological Alterations in Gastric Cancer. Front. Microbiol. 2022, 13, 889694. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Li, C.; Lei, G.; Zhou, L.; Chen, X.; Jia, X.; Lu, Y. Intestinal Candida albicans promotes hepatocarcinogenesis by up-regulating NLRP6. Front. Microbiol. 2022, 13, 81277. [Google Scholar] [CrossRef]
- Wang, Y.; Han, W.; Wang, N.; Han, M.; Ban, M.; Dai, J.; Dong, Y.; Sun, T.; Xu, J. The role of microbiota in the development and treatment of gastric cancer. Front. Oncol. 2023, 13, 1224669. [Google Scholar] [CrossRef]
- Chin, V.K.; Lee, T.Y.; Rusliza, B.; Chong, P.P. Dissecting Candida albicans infection from the perspective of C. albicans virulence and omics approaches on host–pathogen interaction: A review. Int. J. Mol. Sci. 2016, 17, 1643. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Meštrović, T.; Dmitrović, B.; Juzbašić, M.; Matijević, T.; Bekić, S.; Erić, S.; Flam, J.; Belić, D.; Petek Erić, A.; et al. A putative role of Candida albicans in promoting cancer development: A current state of evidence and proposed mechanisms. Microorganisms 2023, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Barra, W.F.; Sarquis, D.P.; Khayat, A.S.; Khayat, B.C.M.; Demachki, S.; Anaissi, A.K.M.; Ishak, G.; Santos, N.P.C.; Dos Santos, S.E.B.; Burbano, R.R.; et al. Gastric Cancer Microbiome. Pathobiology 2021, 88, 156–169. [Google Scholar] [CrossRef]
- Deng, Y.; Ding, X.; Song, Q.; Zhao, G.; Han, L.; Ding, B.; Wang, X.; Hao, X.; Li, H. Alterations in mucosa-associated microbiota in the stomach of patients with gastric cancer. Cell Oncol. 2021, 44, 701–714. [Google Scholar] [CrossRef]
- Ganapathy, V.; Gopal, E.; Miyauchi, S.; Prasad, P.D. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem. Soc. Trans. 2005, 33 Pt 1, 237–240. [Google Scholar] [CrossRef]
- Anwer, E.K.E.; Ajagbe, M.; Sherif, M.; Musaibah, A.S.; Mahmoud, S.; ElBanbi, A.; Abdelnaser, A. Gut Microbiota Secondary Metabolites: Key Roles in GI Tract Cancers and Infectious Diseases. Biomedicines 2025, 13, 100. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Marano, L.; Merola, E.; Roviello, F.; Połom, K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front. Cell Infect. Microbiol. 2022, 12, 1023806. [Google Scholar] [CrossRef]
- Kumar, S.; Huang, J.; Cushnir, J.R.; Španěl, P.; Smith, D.; Hanna, G.B. Selected ion flow tube-MS analysis of headspace vapor from gastric content for the diagnosis of gastro-esophageal cancer. Anal. Chem. 2012, 84, 9550–9557. [Google Scholar] [CrossRef]
- Ye, L.; Hou, Y.; Hu, W.; Wang, H.; Yang, R.; Zhang, Q.; Feng, Q.; Zheng, X.; Yao, G.; Hao, H. Repressed Blautia-acetate immunological axis underlies breast cancer progression promoted by chronic stress. Nat. Commun. 2023, 14, 6160. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Deng, W.; Zou, Y.; Chen, Z.; Tang, H. Histone lactylation as a driver of metabolic reprogramming and immune evasion. Med. Rev. 2024, 1–4. [Google Scholar] [CrossRef]
- Sun, K.; Shen, Y.; Xiao, X.; Xu, H.; Zhang, Q.; Li, M. Crosstalk between lactate and tumor-associated immune cells: Clinical relevance and insight. Front. Oncol. 2024, 14, 1506849. [Google Scholar] [CrossRef]
- Hao, Z.N.; Tan, X.P.; Zhang, Q.; Li, J.; Xia, R.; Ma, Z. Lactate and Lactylation: Dual Regulators of T-Cell-Mediated Tumor Immunity and Immunotherapy. Biomolecules 2024, 14, 1646. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, J.; Zhang, H.; Chen, W.; Zheng, X.; Wang, J.; Huang, F.; Ge, K.; Li, M.; Zhao, M.; et al. Bile Acid-Microbiome Interaction Promotes Gastric Carcinogenesis. Adv. Sci. 2022, 9, e2200263. [Google Scholar] [CrossRef]
- Noto, J.M.; Piazuelo, M.B.; Shah, S.C.; Romero-Gallo, J.; Hart, J.L.; Di, C.; Carmichael, J.D.; Delgado, A.G.; Halvorson, A.E.; Greevy, R.A.; et al. Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis. J. Clin. Investig. 2022, 132, e147822. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, W.; Feng, Y.; Wang, Y.; Sun, T.; Xu, J. Microbial metabolites affect tumor progression, immunity and therapy prediction by reshaping the tumor microenvironment (Review). Int. J. Oncol. 2024, 65, 73. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, Y.; Zhang, T.; Zhang, J.; Wang, Y.; Ding, S. Deoxycholic Acid Could Induce Apoptosis and Trigger Gastric Carcinogenesis on Gastric Epithelial Cells by Quantitative Proteomic Analysis. Gastroenterol. Res. Pract. 2016, 2016, 9638963. [Google Scholar] [CrossRef]
- Powolny, A.; Xu, J.; Loo, G. Deoxycholate induces DNA damage and apoptosis in human colon epithelial cells expressing either mutant or wild-type p53. Int. J. Biochem. Cell Biol. 2001, 33, 193–203. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Gundamaraju, R.; Jha, N.K.; Gupta, P.K.; Dey, A.; Mandal, C.C.; Ford, B.M. Interplay between Dysbiosis of Gut Microbiome, Lipid Metabolism, and Tumorigenesis: Can Gut Dysbiosis Stand as a Prognostic Marker in Cancer? Dis. Mark. 2022, 2022, 2941248. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; Xin, M.; Gao, Y.; Chakroborty, A.; Khan, I.; Monts, J.; Monson, K.; Bode, A.M.; Dong, Z. Induction and Prevention of Gastric Cancer with Combined Helicobacter Pylori and Capsaicin Administration and DFMO Treatment, Respectively. Cancers 2020, 12, 816. [Google Scholar] [CrossRef]
- Sharma, V.; Ashawat, M.; Kumar, P. Dietary phytoactives in the management of gastric cancer-a mini review. Curr. Drug Ther. 2024, 19, 376–384. [Google Scholar] [CrossRef]
- Sarkar, A.; De, R.; Mukhopadhyay, A.K. Curcumin as a potential therapeutic candidate for Helicobacter pylori-associated diseases. World J. Gastroenterol. 2016, 22, 2736–2748. [Google Scholar] [CrossRef]
- Frankel, F.; Priven, M.; Richard, E.; Schweinshault, C.; Tongo, O.; Webster, A.; Barth, E.; Slejzer, K.; Edelstein, S. Health Functionality of Organosulfides: A Review. Int. J. Food Prop. 2015, 19, 537–548. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef]
- Candela, M.; Turroni, S.; Biagi, E.; Carbonero, F.; Rampelli, S.; Fiorentini, C.; Brigidi, P. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J. Gastroenterol. 2014, 20, 908–922. [Google Scholar] [CrossRef]
- Jiang, X.H.; Wong, B.C. Cyclooxygenase-2 inhibition and gastric cancer. Curr. Pharm. Des. 2003, 9, 2281–2288. [Google Scholar] [CrossRef]
- Prasad, S.K.; Bhat, S.; Shashank, D.; Cra, R.S.; Rachtanapun, P.; Devegowda, D.; Santhekadur, P.K.; Sommano, S.R. Bacteria-Mediated Oncogenesis and the Underlying Molecular Intricacies: What We Know So Far. Front. Oncol. 2022, 12, 836004. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, C.; Liang, W.; Li, L.; Du, J.; Pan, C.; Chen, B.; Chen, Y.; Wang, Y. ω-3 and ω-6 Polyunsaturated Fatty Acids Regulate the Proliferation, Invasion and Angiogenesis of Gastric Cancer Through COX/PGE Signaling Pathway. Front. Oncol. 2022, 12, 802009. [Google Scholar] [CrossRef]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dai, D.; Jin, W.; Huang, Y.; Zhang, Y.; Chen, Y.; Wang, W.; Lin, W.; Chen, X.; Zhang, J.; et al. Microbiota and metabolites alterations in proximal and distal gastric cancer patients. J. Transl. Med. 2022, 20, 439. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Peng, J.S.; Dong-Sheng, Y.; Yang, Z.L.; Liu, H.L.; Zeng, Y.K.; Shi, X.P.; Lu, B.Y. Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Braz. J. Med. Biol. Res. 2012, 45, 78–85. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Muszyński, D.; Styburski, D.; Makarewicz, J.; Sobocki, B.K.; Ulasiński, P.; Połom, K.; Stachowska, E.; Skonieczna-Żydecka, K.; Kalinowski, L. Untargeted metabolomics in gastric and colorectal cancer patients-preliminary results. Front. Cell Infect. Microbiol. 2024, 14, 1394038. [Google Scholar] [CrossRef]
- Wu, H.; Xue, R.; Tang, Z.; Deng, C.; Liu, T.; Zeng, H.; Sun, Y.; Shen, X. Metabolomic investigation of gastric cancer tissue using gas chromatography/mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 1385–1395. [Google Scholar] [CrossRef]
- Song, H.; Wang, L.; Liu, H.L.; Wu, X.B.; Wang, H.S.; Liu, Z.H.; Li, Y.; Diao, D.C.; Chen, H.L.; Peng, J.S. Tissue metabolomic fingerprinting reveals metabolic disorders associated with human gastric cancer morbidity. Oncol. Rep. 2011, 26, 431–438. [Google Scholar]
- Wang, C. Mass spectrometry imaging metabolomics reveals the metabolic spatial heterogeneity in gastric cancer. Theor. Nat. Sci. 2024, 49, 39–45. [Google Scholar] [CrossRef]
- Hong, L.; Tang, X.; Han, J.; Wang, J.; Xu, Q.; Zhu, X. Abnormal arginine synthesis confers worse prognosis in patients with middle third gastric cancer. Cancer Cell Int. 2024, 24, 6. [Google Scholar] [CrossRef]
- Choi, J.M.; Park, W.S.; Song, K.Y.; Lee, H.J.; Jung, B.H. Development of simultaneous analysis of tryptophan metabolites in serum and gastric juice-an investigation towards establishing a biomarker test for gastric cancer diagnosis. Biomed. Chromatogr. 2016, 30, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Lin, S.; Zhou, L.; Li, Y.; Chen, M.; Wang, Y.; Li, Y. High levels of aromatic amino acids in gastric juice during the early stages of gastric cancer progression. PLoS ONE 2012, 7, e49434. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, S.; Zhu, F.; Shen, F.; Zhang, T.; Wan, X.; Gong, S.; Liang, G.; Zhou, Y. Multi-omics Combined with Machine Learning Facilitating the Diagnosis of Gastric Cancer. Curr. Med. Chem. 2024, 31, 6692–6712. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pang, R.; Zhao, X.; Zhou, B.; Tian, Y.; Ma, Y.; Rong, L. Plasma metabolomics and lipidomics reveal potential novel biomarkers in early gastric cancer: An explorative study. Int. J. Biol. Markers 2024, 39, 226–238. [Google Scholar] [CrossRef]
- Yu, L.; Lai, Q.; Feng, Q.; Li, Y.; Feng, J.; Xu, B. Serum Metabolic Profiling Analysis of Chronic Gastritis and Gastric Cancer by Untargeted Metabolomics. Front. Oncol. 2021, 11, 636917. [Google Scholar] [CrossRef]
- Bu, F.; Shen, X.; Zhan, H.; Wang, D.; Min, L.; Song, Y.; Wang, S. Efficient Metabolomics Profiling from Plasma Extracellular Vesicles Enables Accurate Diagnosis of Early Gastric Cancer. J. Am. Chem. Soc. 2025, 147, 8672–8686. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, L.; Liu, Z.C.; Zhou, T.; Li, W.Q.; Liu, W.D.; Zhang, L.F.; You, W.C.; Zhang, Y.; Pan, K.F. Integrative metabolomics and microbiomics analysis reveals distinctive microbiota-metabolites interactions in gastric carcinogenesis. Int. J. Cancer 2025, 156, 2389–2400. [Google Scholar] [CrossRef]
- Xu, B.; Shi, Y.; Yuan, C.; Wang, Z.; Chen, O.; Wang, C.; Chai, J. Integrated gene-metabolite association network analysis reveals key metabolic pathways in gastric adenocarcinoma. Heliyon 2024, 10, e37156. [Google Scholar] [CrossRef]
- Yu, S.; Chen, M.; Zhu, X.; Chen, C.; Liang, J.; Wang, H.; Lu, J.; Ding, Y.; Kong, M.; Teng, L.; et al. The combination of exon sequencing and metabolomics to establish a molecular typing system for gastric cancer. Heliyon 2024, 10, 15. [Google Scholar] [CrossRef]
- Sun, C.; Wang, A.; Zhou, Y.; Chen, P.; Wang, X.; Huang, J.; Gao, J.; Wang, X.; Shu, L.; Lu, J.; et al. Spatially resolved multi-omics highlights cell-specific metabolic remodeling and interactions in gastric cancer. Nat. Commun. 2023, 14, 2692. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Zhao, Y.; Shao, X.; Wang, M.; Ma, F.; Yang, L.; Nie, M.; Jin, P.; Yao, K.; et al. Metabolomic machine learning predictor for diagnosis and prognosis of gastric cancer. Nat. Commun. 2024, 15, 1657. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive transmission of microbes along the gastrointestinal tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Sheng, H.; Zhen, Y.; Wu, B.; Li, Z.; Chen, D.; Zhou, H. Oral Fusobacterium nucleatum resists the acidic pH of the stomach due to membrane erucic acid synthesized via enoyl-CoA hydratase-related protein FnFabM. J. Oral. Microbiol. 2025, 17, 2453964. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G., 3rd; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef]
- Tsuda, A.; Suda, W.; Morita, H.; Takanashi, K.; Takagi, A.; Koga, Y.; Hattori, M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin. Transl. Gastroenterol. 2015, 6, e89. [Google Scholar] [CrossRef]
- Huang, C.; Chu, C.; Peng, Y.; Zhang, N.; Yang, Z.; You, J.; Wei, F. Correlations between gastrointestinal and oral microbiota in children with cerebral palsy and epilepsy. Front. Pediatr. 2022, 10, 988601. [Google Scholar] [CrossRef]
- Ji, Y.; Liang, X.; Lu, H. Analysis of by high-throughput sequencing: Helicobacter pylori infection and salivary microbiome. BMC Oral Health 2020, 20, 84. [Google Scholar] [CrossRef]
- Pivetta, G.; Dottori, L.; Fontana, F.; Cingolani, S.; Ligato, I.; Dilaghi, E.; Milani, C.; Ventura, M.; Borro, M.; Esposito, G.; et al. Gastric Microbiota Gender Differences in Subjects with Healthy Stomachs and Autoimmune Atrophic Gastritis. Microorganisms 2023, 11, 1938. [Google Scholar] [CrossRef]
- Xia, R.; Jiang, Z.; Zhou, Y.; Pan, L.; Wang, Y.; Ma, Y.; Fan, L.; Yuan, L.; Cheng, X. Oral microbiota and gastric cancer: Recent highlights and knowledge gaps. J. Oral Microbiol. 2024, 16, 2391640. [Google Scholar] [CrossRef]
- Huang, K.; Gao, X.; Wu, L.; Yan, B.; Wang, Z.; Zhang, X.; Peng, L.; Yu, J.; Sun, G.; Yang, Y. Salivary Microbiota for Gastric Cancer Prediction: An Exploratory Study. Front. Cell Infect. Microbiol. 2021, 11, 640309. [Google Scholar] [CrossRef]
- Tsuzuno, T.; Takahashi, N.; Yamada-Hara, M.; Yokoji-Takeuchi, M.; Sulijaya, B.; Aoki-Nonaka, Y.; Matsugishi, A.; Katakura, K.; Tabeta, K.; Yamazaki, K. Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J. Periodontal. Res. 2021, 56, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Rainer, F.; Bashir, M.; Leber, B.; Schmerboeck, B.; Klymiuk, I.; Groselj-Strele, A.; Durdevic, M.; Freedberg, D.E.; Abrams, J.A.; et al. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci. Rep. 2019, 9, 12000. [Google Scholar] [CrossRef] [PubMed]
- Vonaesch, P.; Morien, E.; Andrianonimiadana, L.; Sanke, H.; Mbecko, J.R.; Huus, K.E.; Naharimanananirina, T.; Gondje, B.P.; Nigatoloum, S.N.; Vondo, S.S.; et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl. Acad. Sci. USA 2018, 115, E8489–E8498. [Google Scholar] [CrossRef]
- Chen, Y.; Lei, L.; Xia, M.; Cheng, R.; Cai, H.; Hu, T. The association between oral microbiome and gastric precancerous lesions. mSystems 2025, 10, e0132224. [Google Scholar] [CrossRef]
- Guo, Z.C.; Jing, S.L.; Jumatai, S.; Gong, Z.C. Porphyromonas gingivalis promotes the progression of oral squamous cell carcinoma by activating the neutrophil chemotaxis in the tumour microenvironment. Cancer Immunol. Immunother. 2023, 72, 1523–1539. [Google Scholar] [CrossRef]
- Gao, S.; Li, S.; Ma, Z.; Liang, S.; Shan, T.; Zhang, M.; Zhu, X.; Zhang, P.; Liu, G.; Zhou, F.; et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agent Cancer 2016, 11, 3. [Google Scholar] [CrossRef]
- Olsen, I.; Yilmaz, Ö. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef]
- Reitano, E.; de’Angelis, N.; Gavriilidis, P.; Gaiani, F.; Memeo, R.; Inchingolo, R.; Bianchi, G.; de’Angelis, G.L.; Carra, M.C. Oral Bacterial Microbiota in Digestive Cancer Patients: A Systematic Review. Microorganisms. 2021, 9, 2585. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Lim, M.; Ok, S.H.; Lee, S.K.; Chun, K.S.; Park, K.K.; Hu, Y.; Chung, W.Y.; Song, N.Y. Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef] [PubMed]
- Bessède, E.; Mégraud, F. Microbiota and gastric cancer. Semin. Cancer Biol. 2022, 86, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Elghannam, M.T.; Hassanien, M.H.; Ameen, Y.A.; Turky, E.A.; ELattar, G.M.; ELRay, A.A.; ELTalkawy, M.D. Oral microbiome dysbiosis and gastrointestinal diseases: A narrative review. Egypt. Liver J. 2024, 14, 32. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Cheng, X.Q.; Li, J.Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef]
- Spielmann, N.; Ilsley, D.; Gu, J.; Lea, K.; Brockman, J.; Heater, S.; Setterquist, R.; Wong, D.T. The human salivary RNA transcriptome revealed by massively parallel sequencing. Clin. Chem. 2012, 58, 1314–1321. [Google Scholar] [CrossRef]
- Belstrøm, D.; Holmstrup, P.; Bardow, A.; Kokaras, A.; Fiehn, N.E.; Paster, B.J. Temporal Stability of the Salivary Microbiota in Oral Health. PLoS ONE 2016, 11, e0147472. [Google Scholar] [CrossRef]
- Mauceri, R.; Coppini, M.; Vacca, D.; Bertolazzi, G.; Panzarella, V.; Di Fede, O.; Tripodo, C.; Campisi, G. Salivary Microbiota Composition in Patients with Oral Squamous Cell Carcinoma: A Systematic Review. Cancers 2022, 14, 5441. [Google Scholar] [CrossRef]
- Han, Z.; Hu, Y.; Lin, X.; Cheng, H.; Dong, B.; Liu, X.; Wu, B.; Xu, Z.Z. Systematic analyses uncover robust salivary microbial signatures and host-microbiome perturbations in oral squamous cell carcinoma. mSystems 2025, 10, e0124724. [Google Scholar] [CrossRef]
- Martins, F.P.; Andrade-Silva, J.; Teixeira, B.L.; Ferrari, A.; Christoff, A.P.; Cruz, G.N.F.; Paladino, F.V.; de Oliveira, L.F.V.; Hernandes, C. Oral microbiome test as an alternative diagnostic tool for gastric alterations: A prospective, bicentric cross-sectional study. PLoS ONE 2024, 19, e0314660. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, Z.; Li, Y.; Lv, C.; Li, C.; Hu, Y.; Fu, M.; Song, L. Salivary and fecal microbiota: Potential new biomarkers for early screening of colorectal polyps. Front. Microbiol. 2023, 14, 1182346. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.; Gullickson, R.G.; Singh, R.; Ro, S.; Omaye, S.T. The Link between Oral and Gut Microbiota in Inflammatory Bowel Disease and a Synopsis of Potential Salivary Biomarkers. Appl. Sci. 2020, 10, 6421. [Google Scholar] [CrossRef]
- Contaldo, M.; Fusco, A.; Stiuso, P.; Lama, S.; Gravina, A.G.; Itro, A.; Federico, A.; Itro, A.; Dipalma, G.; Inchingolo, F.; et al. Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 2021, 9, 1064. [Google Scholar] [CrossRef]
- Wang, M.; Yan, L.Y.; Qiao, C.Y.; Zheng, C.C.; Niu, C.G.; Huang, Z.W.; Pan, Y.H. Ecological shifts of salivary microbiota associated with metabolic-associated fatty liver disease. Front. Cell Infect. Microbiol. 2023, 13, 1131255. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Liu, Y.T.; Ren, J.G.; Liu, H.M.; Fu, Q.; Yang, Y.; Fu, Q.Y.; Chen, G. Salivary Microbiome Relates to Neoadjuvant Immunotherapy Response in OSCC. J. Dent. Res. 2024, 103, 988–998. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Yu, H.; Zhou, H.; Xu, S. Oral microbiota as promising diagnostic biomarkers for gastrointestinal cancer: A systematic review. OncoTargets Ther. 2019, 12, 11131–11144. [Google Scholar] [CrossRef]
- Chen, W.D.; Zhang, X.; Zhang, M.J.; Zhang, Y.P.; Shang, Z.Q.; Xin, Y.W.; Zhang, Y. Salivary Fusobacterium nucleatum serves as a potential diagnostic biomarker for gastric cancer. World J. Gastroenterol. 2022, 28, 4120. [Google Scholar] [CrossRef]
- Saini, A.; Dalal, P.; Sharma, D. Deciphering the interdependent labyrinth between gut microbiota and the immune system. Lett. Appl. Microbiol. 2022, 75, 1122–1135. [Google Scholar] [CrossRef]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, Z.; Zhou, C.; Wang, B.; Liu, Z.; Feng, B. Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: A dynamic interplay influencing pathogenesis and therapy. Front. Med. 2024, 11, 1457218. [Google Scholar] [CrossRef] [PubMed]
- Metzger, R.N.; Krug, A.B.; Eisenächer, K. Enteric Virome Sensing-Its Role in Intestinal Homeostasis and Immunity. Viruses 2018, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Marié, I.J.; Brambilla, L.; Azzouz, D.; Chen, Z.; Baracho, G.V.; Arnett, A.; Li, H.S.; Liu, W.; Cimmino, L.; Chattopadhyay, P.; et al. Tonic interferon restricts pathogenic IL-17-driven inflammatory disease via balancing the microbiome. eLife 2021, 11, 10:e68371. [Google Scholar]
- Loh, J.T.; Lee, K.G.; Lee, A.P.; Teo, J.K.H.; Lim, H.L.; Kim, S.S.; Tan, A.H.; Lam, K.P. DOK3 maintains intestinal homeostasis by suppressing JAK2/STAT3 signaling and S100a8/9 production in neutrophils. Cell Death Dis. 2021, 12, 1054. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Chang, W.; Zhang, Y. The crosstalk between the gut microbiota and tumor immunity: Implications for cancer progression and treatment outcomes. Front. Immunol. 2023, 13, 1096551. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Dey, A.; Gautam, M.K.; Mondal, S.; Pawar, S.D.; Ranade, A.; Bora, M.; Gangwar, M.; Teli, A.; Mondal, N.S. Immune-mediated Bowel Disease: Role of Intestinal Parasites and Gut Microbiome. Curr. Pharm. Des. 2024, 30, 3164–3174. [Google Scholar] [CrossRef]
- You, H.S.; Park, J.Y.; Seo, H.; Kim, B.J.; Kim, J.G. Increasing correlation between oral and gastric microbiota during gastric carcinogenesis. Korean J. Intern. Med. 2024, 39, 590–602. [Google Scholar] [CrossRef]
- Hua, Z.; Xu, L.; Zhu, J.; Xiao, L.; Lu, B.; Wu, J.; Wu, Z.; Zhou, Q.; Zhang, J. Helicobacter pylori infection altered gastric microbiota in patients with chronic gastritis. Front. Cell Infect. Microbiol. 2023, 13, 1221433. [Google Scholar] [CrossRef]
- Bakhti, S.Z.; Latifi-Navid, S. Oral microbiota and Helicobacter pylori in gastric carcinogenesis: What do we know and where next? BMC Microbiol. 2021, 21, 71. [Google Scholar] [CrossRef]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111, 2696–2707. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, T.; Li, Q.; Chen, X.; Jiang, F.; Shen, X. The tumor immune-microenvironment in gastric cancer. Tumori 2022, 108, 541–551. [Google Scholar] [CrossRef]
- Yang, P.; Liang, G.; Ni, Y.; Chu, X.; Zhang, X.; Wang, Z.; Khan, A.; Jin, F.; Shen, H.; Li, M.; et al. Investigating the role of intratumoral Streptococcus mitis in gastric cancer progression: Insights into tumor microenvironment. J. Transl. Med. 2025, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, H.; Zhang, X.; Lu, X.; Liu, J.; Li, H.; Huang, J. Akkermansia muciniphila improves gastric cancer treatment by modulating the immune microenvironment. Future Microbiol. 2024, 19, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Shao, L.; Liu, X.; Cheng, Y.; Yan, C.; Mei, Y.; Ji, F.; Liu, X. Regulatory T Cells and Plasmacytoid Dendritic Cells Within the Tumor Microenvironment in Gastric Cancer Are Correlated With Gastric Microbiota Dysbiosis: A Preliminary Study. Front. Immunol. 2019, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Wu, P.; Guo, X.; Xu, Y.; Jin, L.; Zhao, D. The intratumoral microbiota: A new horizon in cancer immunology. Front. Cell Infect. Microbiol. 2024, 14, 1409464. [Google Scholar] [CrossRef]
- Tabrizi, E.; Pourteymour Fard Tabrizi, F.; Mahmoud Khaled, G.; Sestito, M.P.; Jamie, S.; Boone, B.A. Unraveling the gut microbiome’s contribution to pancreatic ductal adenocarcinoma: Mechanistic insights and therapeutic perspectives. Front. Immunol. 2024, 15, 1434771. [Google Scholar] [CrossRef]
- Kim, E.; Ahn, H.; Park, H. A review on the role of gut microbiota in immune checkpoint blockade therapy for cancer. Mamm. Genome 2021, 32, 223–231. [Google Scholar] [CrossRef]
- Nomura, M.; Nagatomo, R.; Doi, K.; Shimizu, J.; Baba, K.; Saito, T.; Matsumoto, S.; Inoue, K.; Muto, M. Association of Short-Chain Fatty Acids in the Gut Microbiome With Clinical Response to Treatment with Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Netw. Open 2020, 3, e202895. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, J. Exploring the regulatory mechanism of intestinal flora based on pd-1 receptor/ligand targeted cancer immunotherapy. Front. Immunol. 2024, 15, 1359029. [Google Scholar] [CrossRef]
- Kim, K.S. Regulation of T cell repertoires by commensal microbiota. Front. Cell Infect. Microbiol. 2022, 12, 1004339. [Google Scholar] [CrossRef]
- Ohnmacht, C.; Park, J.; Cording, S.; Wing, J.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Eberl, G. The microbiota regulates type 2 immunity through rorγt + T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, E.M.; Shao, T.Y.; Woo, V.; Rice, T.; Engleman, L.; Didriksen, B.J.; Whitt, J.; Haslam, D.B.; Way, S.S.; Alenghat, T. Intestinal epithelial HDAC3 and MHC class II coordinate microbiota-specific immunity. J. Clin. Investig. 2023, 133, e162190. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Boyle, F.; Pavlakis, N.; Clarke, S.; Eade, T.; Hruby, G.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; et al. The Gut Microbiome and Cancer Immunotherapy: Can We Use the Gut Microbiome as a Predictive Biomarker for Clinical Response in Cancer Immunotherapy? Cancers 2021, 13, 4824. [Google Scholar] [CrossRef]
- Davar, D.; Zarour, H.M. Facts and Hopes for Gut Microbiota Interventions in Cancer Immunotherapy. Clin. Cancer Res. 2022, 28, 4370–4384. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Novisedlakova, M.; Mego, M. Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors. Cancers 2024, 16, 4271. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Chen, T. Gut Microbiota: A Promising Milestone in Enhancing the Efficacy of PD1/PD-L1 Blockade Therapy. Front. Oncol. 2022, 12, 847350. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, R.; Liu, F.; Lee, S.; Zhang, L. Modulation of gut microbiota: A novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy. Front. Immunol. 2018, 9, 374. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Frąk, M.; Grenda, A.; Krawczyk, P.; Milanowski, J.; Kalinka, E. Interactions between Dietary Micronutrients, Composition of the Microbiome and Efficacy of Immunotherapy in Cancer Patients. Cancers 2022, 14, 5577. [Google Scholar] [CrossRef] [PubMed]
- Buchta Rosean, C.; Bostic, R.R.; Ferey, J.C.M.; Feng, T.Y.; Azar, F.N.; Tung, K.S.; Dozmorov, M.G.; Smirnova, E.; Bos, P.D.; Rutkowski, M.R. Preexisting Commensal Dysbiosis Is a Host-Intrinsic Regulator of Tissue Inflammation and Tumor Cell Dissemination in Hormone Receptor-Positive Breast Cancer. Cancer Res. 2019, 79, 3662–3675. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.Y.; Do, E.J.; Bae, D.J.; Kim, S.; Kweon, M.N.; Song, J.S.; et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Sun, Z.; Cao, Y.; Mu, Z.; Ji, X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci. 2021, 112, 3005–3017. [Google Scholar] [CrossRef]
- Xiong, H.; Shen, Z. Tissue-resident memory T cells in immunotherapy and immune-related adverse events by immune checkpoint inhibitor. Int. J. Cancer 2024, 155, 193–202. [Google Scholar] [CrossRef]
- Shen, Y.C.; Yeh, C.P.; Jeng, Y.M.; Hsu, C.; Hsu, C.H.; Lin, Z.Z.; Shao, Y.Y.; Lu, L.C.; Liu, T.H.; Chen, C.H.; et al. Limited Predictive or Prognostic Role of Tumor-Infiltrating Tissue-Resident Memory CD8 T Cells in Patients with Hepatocellular Carcinoma Receiving Immunotherapy. Cancers 2021, 13, 5142. [Google Scholar] [CrossRef]
- Nawal, H. Exploring the role of the gut microbiome in modulating response to anti-pd-1 immunotherapy in melanoma patients. Int. J. Clin. Biochem. Res. 2025, 11, 223–228. [Google Scholar] [CrossRef]
- Lee, P.C.; Wu, C.J.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Yu-Lun, K.; Chou, S.H.; Luo, J.C.; Hou, M.C.; et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004779. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, J.; Woo, M.-Y.; Lee, M.J.; Shin, H.-J.; Kim, K.; Park, S. Modulation of the Gut Microbiota Alters the Tumour-Suppressive Efficacy of Tim-3 Pathway Blockade in a Bacterial Species- and Host Factor-Dependent Manner. Microorganisms 2020, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Firwana, B.; Avaritt, N.; Shields, B.; Ravilla, R.; Makhoul, I.; Hutchins, L.; Tackett, A.J.; Mahmoud, F. Do checkpoint inhibitors rely on gut microbiota to fight cancer? J. Oncol. Pharm. Pract. 2018, 24, 468–472. [Google Scholar] [CrossRef]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- David, A.; Lev-Ari, S. Targeting the Gut Microbiome to Improve Immunotherapy Outcomes: A Review. Integr. Cancer Ther. 2024, 23, 15347354241269870. [Google Scholar] [CrossRef]
- Ting, N.L.; Lau, H.C.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Raoul, P.; Maccauro, V.; Cintoni, M.; Scarpellini, E.; Ianiro, G.; Gasbarrini, A.; Mele, M.C.; Rinninella, E. Microbiota-Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies. Int. J. Mol. Sci. 2024, 25, 1679. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kolat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Sig. Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Fiorentini, E.; Russo, E.; Amedei, A.; Bellando Randone, S. Fecal microbiome in systemic sclerosis, in search for the best candidate for microbiota-targeted therapy for small intestinal bacterial overgrowth control. J. Scleroderma Relat. Disord. 2022, 7, 163–167. [Google Scholar] [CrossRef]
- Craciun, C.I.; Neag, M.A.; Catinean, A.; Mitre, A.O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.D.; Muntean, D.M.; Craciun, A.E. The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines 2022, 10, 308. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef]
- Mishra, S.P.; Jain, S.; Taraphder, S.; Yadav, H. New Horizons in Microbiota and Metabolic Health Research. J. Clin. Endocrinol. Metab. 2021, 106, e1052–e1059. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, V.; Cassard, A.; Mas, E.; Barreau, F. Multi-Omics Analysis of Gut Microbiota in Inflammatory Bowel Diseases: What Benefits for Diagnostic, Prognostic and Therapeutic Tools? Int. J. Mol. Sci. 2021, 22, 11255. [Google Scholar] [CrossRef] [PubMed]
- Toumi, E.; Goutorbe, B.; Plauzolles, A.; Bonnet, M.; Mezouar, S.; Militello, M.; Mege, J.L.; Chiche, L.; Halfon, P. Gut microbiota in systemic lupus erythematosus patients and lupus mouse model: A cross species comparative analysis for biomarker discovery. Front. Immunol. 2022, 13, 943241. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Jain, D.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut microbiota in pathophysiology, diagnosis, and therapeutics of inflammatory bowel disease. Intest. Res. 2024, 22, 15–43. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, Y.; Xu, J.; Shu, S.; Wang, P.; Ding, S.; Huang, Y.; Zheng, L.; Yang, Y.; et al. Promising dawn in the management of pulmonary hypertension: The mystery veil of gut microbiota. Imeta 2024, 3, e159. [Google Scholar] [CrossRef]
- Binda, C.; Gibiino, G.; Sbrancia, M.; Coluccio, C.; Cazzato, M.; Carloni, L.; Cucchetti, A.; Ercolani, G.; Sambri, V.; Fabbri, C. Microbiota in the Natural History of Pancreatic Cancer: From Predisposition to Therapy. Cancers 2023, 15, 1. [Google Scholar] [CrossRef]
- Zhang, F.; Ferrero, M.; Dong, N.; D’Auria, G.; Reyes-Prieto, M.; Herreros-Pomares, A.; Calabuig-Fariñas, S.; Duréndez, E.; Aparisi, F.; Blasco, A.; et al. Analysis of the Gut Microbiota: An Emerging Source of Biomarkers for Immune Checkpoint Blockade Therapy in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Gajjar, D.; Joshi, A. Gut Microbiota Dysbiosis and Its Potential Application as Biomarker in Various Cancer. In Futuristic Trends in Biotechnology Volume 3 Book 10; IIP: Novi, MI, USA, 2024; pp. 172–181. [Google Scholar]
- Sun, J.; Song, S.; Liu, J.; Chen, F.; Li, X.; Wu, G. Gut microbiota as a new target for anticancer therapy: From mechanism to means of regulation. npj Biofilms Microbiomes 2025, 11, 43. [Google Scholar] [CrossRef]
- Xu, Z.F.; Zhao, D.; Wei, C.; Wang, W.; Zhang, Y.; Zhang, W.; Zhou, D. Characteristics and prognostic value of gut microbiota in follicular lymphoma. Oncol. Lett. 2024, 27, 207. [Google Scholar] [CrossRef]
- Maccauro, V.; Fianchi, F.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota in Primary Sclerosing Cholangitis: From Prognostic Role to Therapeutic Implications. Dig. Dis. 2024, 42, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Chambers, S.; Scott-Thomas, A.; Bhatia, M. Gut Microbiota and Liver Dysfunction in Sepsis: The Role of Inflammatory Mediators and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 13415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ghosh, T.S.; McCann, R.; Mallon, P.; Hill, C.; Draper, L.; Schult, D.; Fanning, L.J.; Shannon, R.; Sadlier, C.; et al. Robust cross-cohort gut microbiome associations with COVID-19 severity. Gut Microbes 2023, 15, 2242615. [Google Scholar] [CrossRef]
- Zhong, J.; Guo, L.; Wang, Y.; Jiang, X.; Wang, C.; Xiao, Y.; Wang, Y.; Zhou, F.; Wu, C.; Chen, L.; et al. Gut Microbiota Improves Prognostic Prediction in Critically Ill COVID-19 Patients Alongside Immunological and Hematological Indicators. Research 2024, 7, 0389. [Google Scholar] [CrossRef]
- Farsi, Y.; Tahvildari, A.; Arbabi, M.; Vazife, F.; Sechi, L.A.; Shahidi Bonjar, A.H.; Jamshidi, P.; Nasiri, M.J.; Mirsaeidi, M. Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Front. Cell Infect. Microbiol. 2022, 12, 804644. [Google Scholar] [CrossRef]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases-A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Kageyama, S.; Takeshita, T.; Asakawa, M.; Shibata, Y.; Takeuchi, K.; Yamanaka, W.; Yamashita, Y. Relative abundance of total subgingival plaque-specific bacteria in salivary microbiota reflects the overall periodontal condition in patients with periodontitis. PLoS ONE 2017, 12, e0174782. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Lin, C.C.; Abemayor, E.; Wang, M.B.; Wong, D.T. The emerging landscape of salivary diagnostics. Periodontol 2016, 70, 38–52. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, T.; Dong, M.; Ahamed, M.A.; Guan, W. Sample-to-answer salivary miRNA testing: New frontiers in point-of-care diagnostic technologies. Wiley Interdiscip. Rev. Nanomed Nanobiotechnol. 2024, 16, e1969. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; Martínez-Reglero, C.; Salgado-Barreira, Á.; Takkouche, B.; López-López, R.; Suárez-Cunqueiro, M.M.; Muinelo-Romay, L. Salivary biomarkers for cancer diagnosis: A meta-analysis. Ann. Med. 2020, 52, 131–144. [Google Scholar] [CrossRef]
- Koga, Y. Microbiota in the stomach and application of probiotics to gastroduodenal diseases. World J. Gastroenterol. 2022, 28, 6702–6715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ji, X.; Song, Z.; Wu, F.; Qu, Y.; Jin, X.; Xue, X.; Wang, F.; Huang, Y. Butyrate Inhibits Gastric Cancer Cells by Inducing Mitochondriamediated Apoptosis. Comb. Chem. High Throughput Screen. 2023, 26, 630–638. [Google Scholar] [PubMed]
- Han, Z.; Li, Y.; Nan, X.; Zhou, T.; Li, L.; Li, Y. Effect of probiotic supplementation combined with bismuth-containing quadruple therapy on gut microbiota during Helicobacter pylori eradication: A randomized, double-blind, placebo-controlled trial. Front. Nutr. 2024, 11, 1484646. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.V.; Leite, G.; Resende, A.; Blachier, F.; Lancha, A.H., Jr. Exercise, Nutrition and Gut Microbiota: Possible Links and Consequences. Int. J. Sports Exerc. Med. 2017, 3, 69. [Google Scholar] [CrossRef]
- Sugihara, K.; Kamada, N. Diet-Microbiota Interactions in Inflammatory Bowel Disease. Nutrients 2021, 13, 1533. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Zhao, J.; Liu, M.; Shu, X.; Li, Q.; Wang, Y.; Zhou, Y. Polyphenol Mechanisms against Gastric Cancer and Their Interactions with Gut Microbiota: A Review. Curr. Oncol. 2022, 29, 5247–5261. [Google Scholar] [CrossRef]
- Fakharian, F.; Asgari, B.; Nabavi-Rad, A.; Sadeghi, A.; Soleimani, N.; Yadegar, A.; Zali, M.R. The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front. Cell Infect. Microbiol. 2022, 12, 953718. [Google Scholar] [CrossRef]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. 1), S23–S31. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dabrowska, B.; Jończyk-Matysiak, E.; Wojciechowska, R.; Górski, A. Bacteriophages in the gastrointestinal tract and their implications. Gut Pathog. 2017, 9, 44. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Zheng, D.W.; Bao, P.; Zeng, X.; Zhang, X.Z. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci. Adv. 2020, 6, eaba1590. [Google Scholar] [CrossRef]
- Cuomo, P.; Papaianni, M.; Fulgione, A.; Guerra, F.; Capparelli, R.; Medaglia, C. An Innovative Approach to Control H. pylori-Induced Persistent Inflammation and Colonization. Microorganisms 2020, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Kleber, K.T.; Iranpur, K.R.; Perry, L.M.; Cruz, S.M.; Razmara, A.M.; Culp, W.T.N.; Kent, M.S.; Eisen, J.A.; Rebhun, R.B.; Canter, R.J. Using the canine microbiome to bridge translation of cancer immunotherapy from pre-clinical murine models to human clinical trials. Front. Immunol. 2022, 13, 983344. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, J.; Li, Q.; Xia, W.; Zhang, C.; Liu, Z.; Xiao, J.; Yi, Z.; Deng, H.; Xiao, Z.; et al. Gut Microbiota and Tumor Immune Escape: A New Perspective for Improving Tumor Immunotherapy. Cancers 2022, 14, 5317. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Wang, F.H.; Zhang, X.T.; Tang, L.; Wu, Q.; Cai, M.Y.; Li, Y.F.; Qu, X.J.; Qiu, H.; Zhang, Y.J.; Ying, J.E.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun. 2024, 44, 127–172. [Google Scholar] [CrossRef]
- Yu, G.; Torres, J.; Hu, N.; Medrano-Guzman, R.; Herrera-Goepfert, R.; Humphrys, M.S.; Wang, L.; Wang, C.; Ding, T.; Ravel, J.; et al. Molecular Characterization of the Human Stomach Microbiota in Gastric Cancer Patients. Front. Cell Infect. Microbiol. 2017, 7, 302. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Ling, Z.; Zhou, X.; Si, Y.; Liu, X.; Ji, F. Prognostic effects of the gastric mucosal microbiota in gastric cancer. Cancer Sci. 2022, 114, 1075–1085. [Google Scholar] [CrossRef]
- Wu, Z.F.; Zou, K.; Wu, G.N.; Jin, Z.J.; Xiang, C.J.; Xu, S.; Wang, Y.H.; Wu, X.Y.; Chen, C.; Xu, Z.; et al. A Comparison of Tumor-Associated and Non-Tumor-Associated Gastric Microbiota in Gastric Cancer Patients. Dig. Dis. Sci. 2020, 66, 1673–1682. [Google Scholar] [CrossRef]
- Gunathilake, M.N.; Lee, J.; Choi, I.J.; Kim, Y.I.; Ahn, Y.; Park, C.; Kim, J. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: A case-control study. Sci. Rep. 2019, 9, 13589. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Shi, X.; Du, Y.; Niu, Y.; Jin, G.; Wang, Z.; Lyu, J. Gut microbiome analysis as a predictive marker for the gastric cancer patients. Appl. Microbiol. Biotechnol. 2021, 105, 803–814. [Google Scholar] [CrossRef]
- Liu, K.; Jiang, Z.; Ma, Y.; Xia, R.; Zheng, Y.; Yin, K.; Pang, C.; Yuan, L.; Cheng, X.; Liu, Z.; et al. Multiomics insights into BMI-related intratumoral microbiota in gastric cancer. Front. Cell Infect. Microbiol. 2025, 15, 1511900. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.Y.; Tung, S.Y.; Pan, H.Y.; Yen, C.W.; Xu, H.W.; Lin, Y.J.; Deng, Y.F.; Hsu, W.T.; Wu, C.S.; Li, C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, F.; Yang, J.; Yang, S. Explore and Analyze the Composition and Characteristics of Intestinal Microbiota between Gastric Cancer Patients and Healthy People. Evid.-Based Complement. Altern. Med. 2022, 2022, 5834293. [Google Scholar] [CrossRef]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [PubMed]
- Tang, Z.; Wang, Y.; Liu, D.; Wang, X.; Xu, C.; Yu, Y.; Cui, Y.; Tang, C.; Li, Q.; Sun, J.; et al. The Neo-PLANET phase II trial of neoadjuvant camrelizumab plus concurrent chemoradiotherapy in locally advanced adenocarcinoma of stomach or gastroesophageal junction. Nat. Commun. 2022, 13, 6807. [Google Scholar] [CrossRef]
- André, T.; Tougeron, D.; Piessen, G.; de la Fouchardière, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J. Clin. Oncol. 2023, 41, 255–265. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Hu, G.; Wang, J.; Chen, D.; Song, C.; Cai, Y.; Zhai, C.; Xu, W. Circulating memory PD-1+CD8+ T cells and PD-1+CD8+T/PD-1+CD4+T cell ratio predict response and outcome to immunotherapy in advanced gastric cancer patients. Cancer Cell Int. 2023, 23, 274. [Google Scholar] [CrossRef]
- Chung, D.T.; Tung, D.S.; Dung, T.N. Harnessing Immune Checkpoint Inhibitors Against Gastric Cancer: Charting the Course to Expanded Therapeutic Benefit. Biomed. Res. Ther. 2024, 11, 6305–6325. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Wang, H.; Dai, J.; Li, C.; Hao, Y.; Jiang, D. Exploring the immune escape mechanisms in gastric cancer patients based on the deep ai algorithms and single-cell sequencing analysis. J. Cell Mol. Med. 2024, 28, e18379. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Z.; Yang, X.; Li, Z.; Sang, S.; Islam, M.T.; Guo, A.A.; Li, Z.; Wang, X.; Wang, J.; et al. Development and interpretation of a pathomics-driven ensemble model for predicting the response to immunotherapy in gastric cancer. J. Immunother. Cancer 2024, 12, e008927. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, L.; Gao, M.; Zhang, N.; Wang, R.; Liu, Y.; Niu, Y.; Jia, L. Role of tertiary lymphoid structures and b cells in clinical immunotherapy of gastric cancer. Front. Immunol. 2025, 15, 1519034. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, X.; Shen, F.; Ma, J.; Li, Z. Prognostic value of peripheral blood neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, pan-immune-inflammation value and systemic immune-inflammation index for the efficacy of immunotherapy in patients with advanced gastric cancer. Immunotherapy online ahead of print. 2024. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Cheng, S.; Dai, D.; Kou, Y.; Zhang, X.; Li, F.; Yin, X.; Ji, J.; Zhang, Z.; Wang, X.; et al. The gut microbiome affects response of treatments in HER2-negative advanced gastric cancer. Clin. Transl. Med. 2023, 13, e1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Guan, Y.; Wu, M.; Hu, M.; Fang, C.; Wu, H.; Yang, M. Long-term survival in metastatic gastric cancer patient with Apatinib plus S-1 maintenance treatment following first-line chemotherapy-case report. Front. Oncol. 2024, 14, 478719. [Google Scholar] [CrossRef]
- Wang, J.; Tong, T.; Zhang, G.; Jin, C.; Guo, H.; Liu, X.; Zhang, Z.; Li, J.; Zhao, Y. Evaluation of neoadjuvant immunotherapy in resectable gastric/gastroesophageal junction tumors: A meta-analysis and systematic review. Front. Immunol. 2024, 15, 1339757. [Google Scholar] [CrossRef]
- Ilson, D.H. Advances in the treatment of gastric cancer: 2022–2023. Curr. Opin. Gastroenterol. 2023, 39, 517–521. [Google Scholar] [CrossRef]
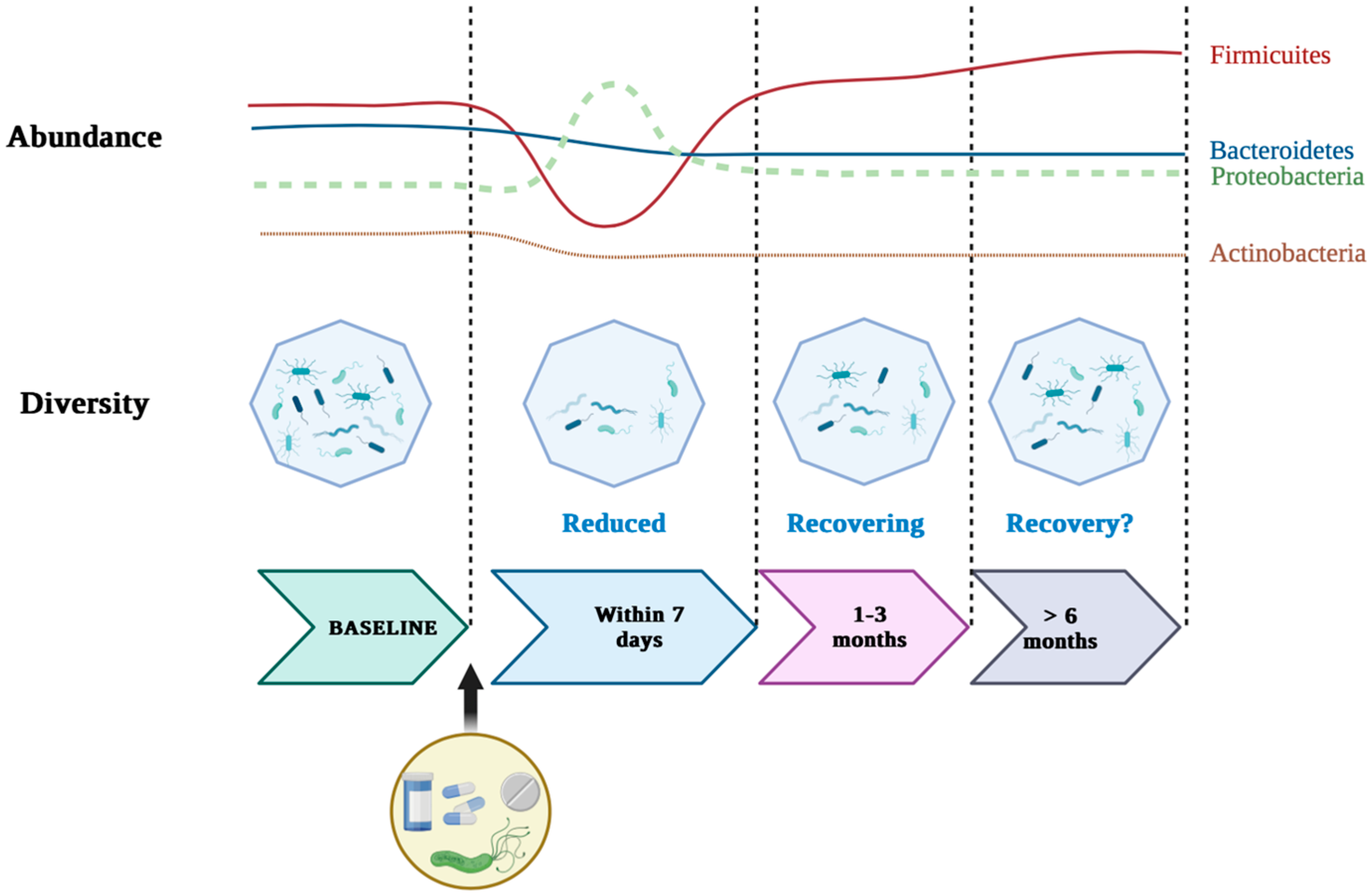
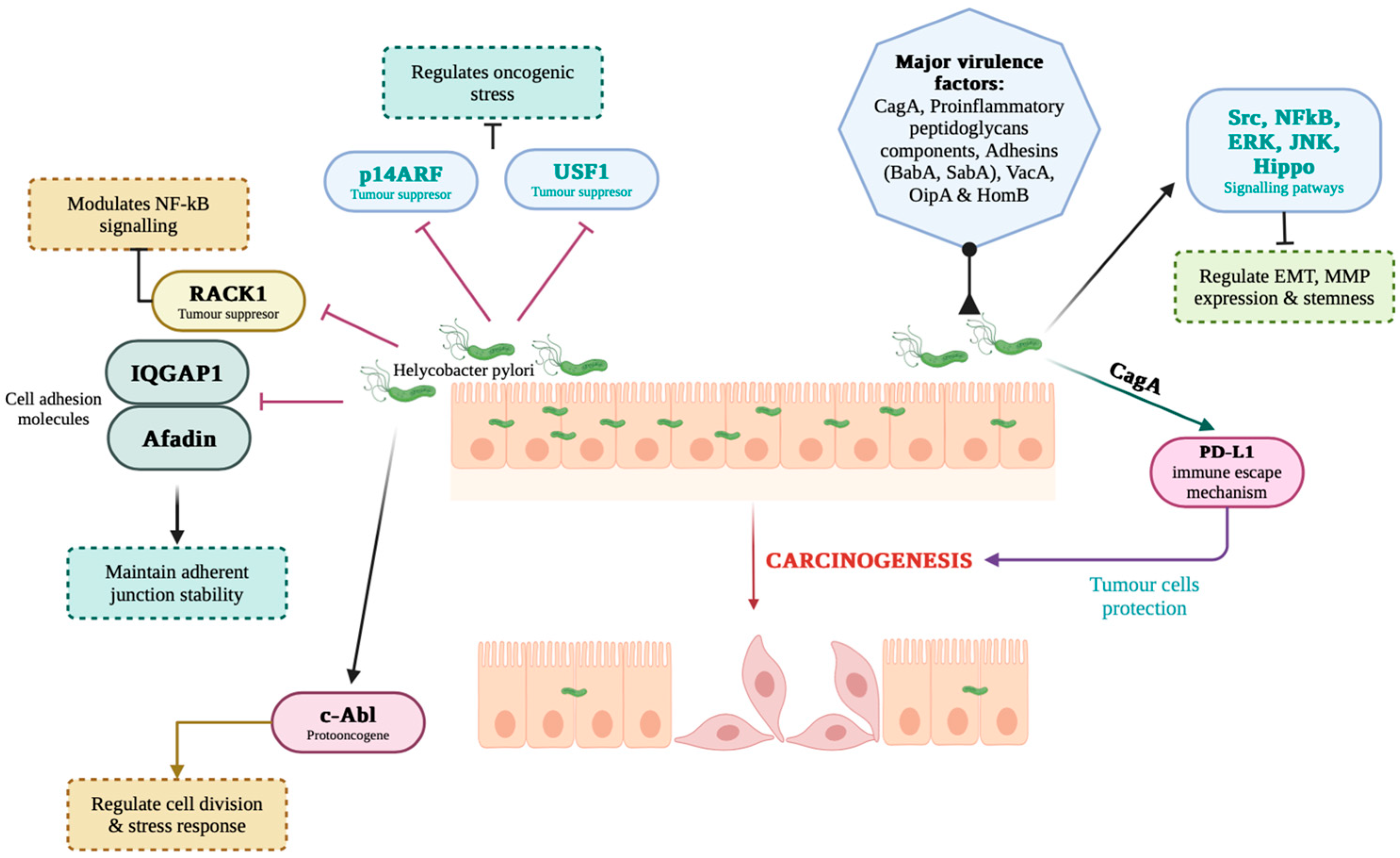
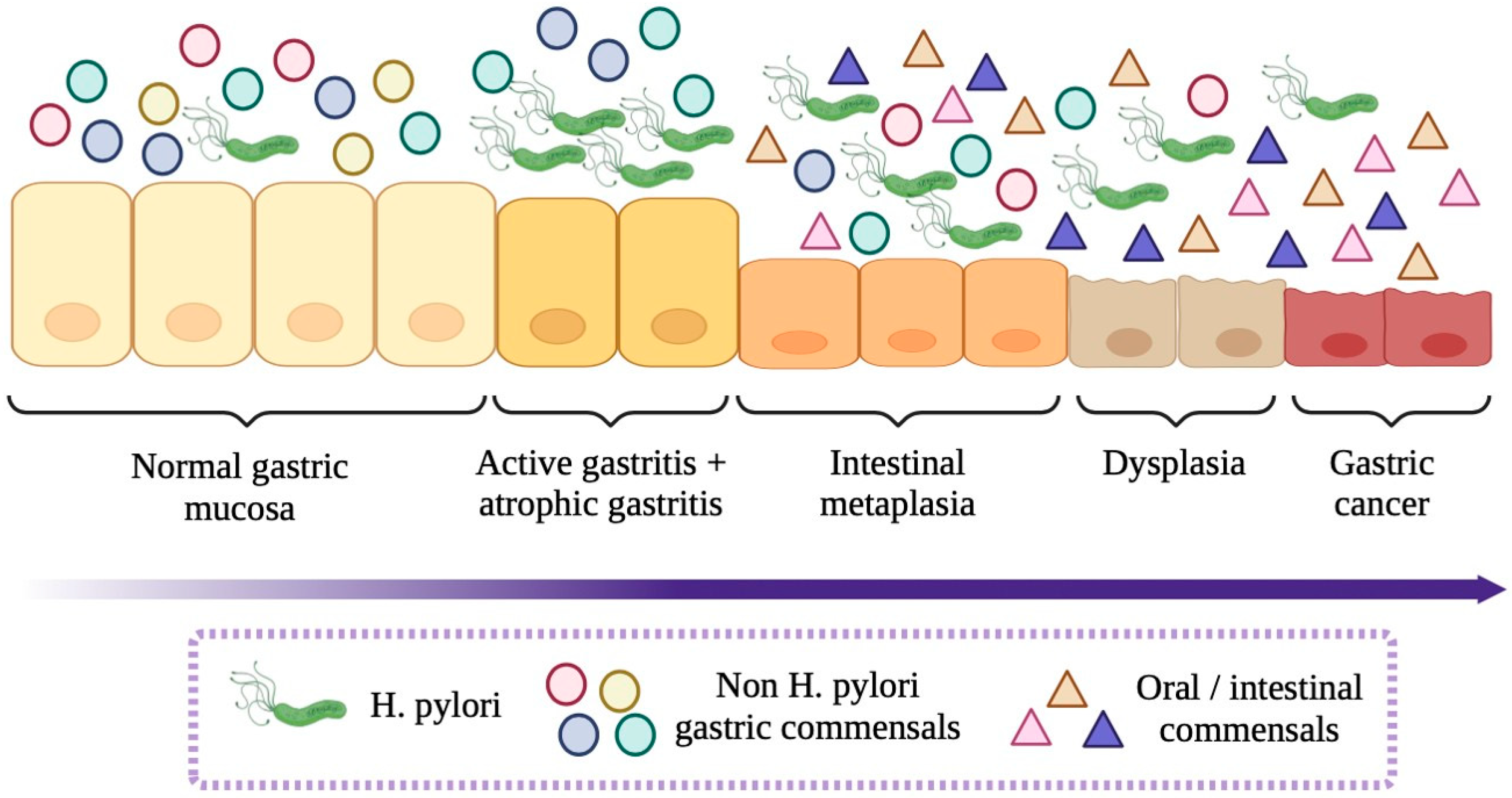
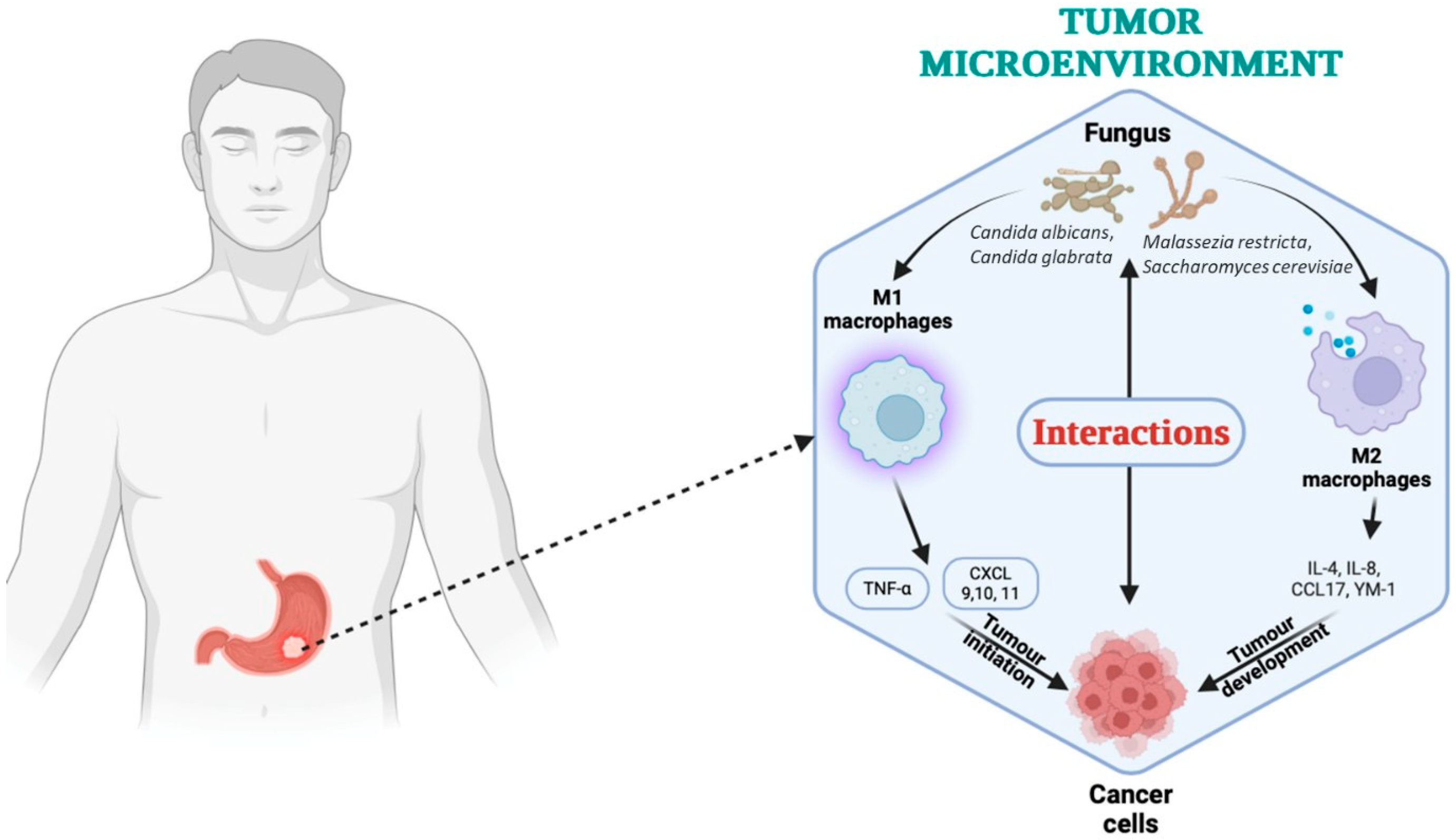
| Model | Biological System | Study Objective | Key Findings | Ref. |
|---|---|---|---|---|
| Human clinical observational study | Human gastric mucosal biopsies obtained via endoscopic procedures from 47 patients at various stages of gastric disease: SG (superficial gastritis), AG (atrophic gastritis), GIN (gastric intraepithelial neoplasia), GC (gastric cancer). Human gastric microbiota analyzed using 16S rRNA gene sequencing (targeting regions V3–V4). | To characterize alterations in gastric microbiota associated with different stages of gastric carcinogenesis, identify potential biomarkers, and compare microbiota profiles between cardia and non-cardia gastric cancers. | The study suggested potential microbial biomarkers for early detection. No significant trend in overall microbial richness or diversity across stages. The Shannon index was higher in GIN compared to other groups. The top dominant phyla included Firmicutes, Proteobacteria, Bacteroidetes, Fusobacteria, and Actinobacteria. Enrichment of oral bacteria (e.g., Slackia, Selenomonas) increased progressively from gastritis to GC, which may suggest oral flora involvement. Microbiota profiles differed significantly in cardia vs. non-cardia gastric cancer, with higher Helicobacter abundance in cardia cancers. | [38] |
| Human clinical comparative study | Gastric non-malignant and tumor tissue samples from GC patients in China (cardia) and Mexico (non-cardia). | To characterize the human stomach microbiota in gastric cancer patients, compare microbiota between non-malignant and tumor tissues, and assess microbial differences across populations and tissue types. | Microbial diversity and richness were significantly higher in tumor tissues compared to non-tumor tissues. Helicobacter was enriched in non-tumor tissues; Lactobacillus, Streptococcus, Acinetobacter, Prevotella, and six additional genera were enriched in tumor tissues. Untargeted metabolomics identified 150 discriminative metabolites with higher relative abundance of amino acids, carbohydrates and carbohydrate conjugates, glycerophospholipids, and nucleosides in tumor tissues. Targeted metabolomics revealed that 1-methylnicotinamide and N-acetyl-D-glucosamine-6-phosphate combined serve as robust biomarkers for distinguishing tumor from non-tumor tissue. Correlations suggest that specific bacteria like Helicobacter and Lactobacillus influence the tumor metabolome, potentially promoting gastric cancer development. | [267] |
| Human clinical study | Human salivary microbiota characterized through 16S rRNA gene sequencing; a total of 293 patients undergoing endoscopic examination grouped into superficial gastritis (SG), atrophic gastritis (AG), and gastric cancer (GC) stages. | To characterize salivary microbiota changes across progressive stages of gastric carcinogenesis and identify saliva bacterial markers that can be used to detect gastric cancer. | Distinct salivary microbiota profile observed in GC patients with enrichment of proinflammatory taxa, such as Corynebacterium and Streptococcus. Reduction of bacteria that reduce carcinogenic N-nitroso compounds (e.g., Haemophilus, Neisseria) in GC. Salivary microbiota profiles distinguished GC from SG and AG with high accuracy (AUC = 0.91). Potential diagnostic biomarkers identified among taxa like unclassified Streptophyta and Streptococcus. Proposed mechanisms include the accumulation of proinflammatory bacteria and a decline in bacteria that reduce carcinogens, contributing to gastric carcinogenesis. | [150] |
| Human clinical study (retrospective cohort study) | Gastric mucosal microbiota from patients with gastric cancer (tissues: normal, peritumoral, tumoral). | To investigate the prognostic value of gastric mucosal microbiota in different stomach microhabitats of gastric cancer patients. | Patients with different prognoses showed distinct gastric microbiota compositions and diversity. In peritumoral microhabitats, Helicobacter abundance was higher in patients with good prognoses, while Halomonas and Shewanella were lower. The gastric microbiota association network was more complex in patients with poor prognoses. Predicted microbiota functions varied by microhabitat, notably in peritumoral tissues. Gastric mucosal microbiota alterations may serve as prognostic biomarkers for clinical outcomes in gastric cancer. | [268] |
| Human clinical study | Human gastric mucosa biopsy samples from 18 gastric cancer (GC) patients and 32 superficial gastritis (SG) patients. Paired tumor and paracancerous tissue samples were collected from GC patients. 16S rRNA gene sequencing for bacterial profiling. | To investigate and compare the gastric mucosal microbiome composition in GC patients and SG patients, assessing differences in bacterial diversity, specific taxa abundance, and predictive functional profiles. To evaluate the microbial dysbiosis index (MDI) as a discriminant metric for gastric-cancer-associated dysbiosis. | GC patients exhibit distinct gastric microbiome profiles compared to SG patients, with significant microbial dysbiosis evident in both tumor and paracancerous tissues. Six bacterial genera were enriched and eighteen were depleted in GC tissues relative to SG. The microbial dysbiosis index (MDI) was significantly higher in GC patients, negatively correlated with microbial diversity (Shannon index), and positively correlated with Helicobacter spp. abundance. Functional predictions suggest nitrosating microbial community enrichment in GC patients, implicating microbial metabolism in carcinogenesis. The microbiome differences in GC patients are not limited to tumor sites but also present in adjacent normal tissue, suggesting early microbiome changes in gastric carcinogenesis. | [269] |
| Human clinical study (case-control study) | Human stomach gastric microbiota in a Korean population involving 556 participants (268 gastric cancer patients and 288 controls). DNA extracted from gastric biopsy samples was analyzed using 16S rRNA gene sequencing to characterize gastric microbiota composition. | The study focused on identifying the relative abundance of specific bacterial species in the gastric mucosa and their association with gastric cancer risk. | Higher relative abundance of Helicobacter pylori, Propionibacterium acnes, and Prevotella copri was significantly associated with increased gastric cancer risk. Higher relative abundance of Lactococcus lactis was associated with decreased gastric cancer risk. These bacterial species combined predicted gastric cancer with about 79.7% sensitivity. The study supports microbial profiles as potential diagnostic markers for gastric cancer risk in Koreans. | [270] |
| Human clinical study | The study involved 227 participants, including 83 gastric cancer patients, 54 gastritis patients, 29 colorectal cancer patients, and 61 healthy controls. Human gut microbiota analyzed via 16S rRNA gene sequencing of fecal samples. | To investigate alterations in gut microbiota composition during gastric cancer progression and evaluate the gut microbiome as a non-invasive predictive marker for gastric cancer diagnosis. | Gut microbiota composition and diversity significantly differ between gastric cancer patients and healthy controls. Random forest model using microbial taxa classified gastric cancer with an AUC of 0.91 and a true positive rate of 0.83 in validation. Gastritis shares some microbiome features with gastric cancer. Chemotherapy reduces microbial abundance and diversity in gastric cancer patients. The genera Lactobacillus and Megasphaera are significantly associated with gastric cancer and serve as predictive markers. | [271] |
| Retrospective clinical cohort study | Human gastric cancer patients were classified by body mass index (BMI), specifically low BMI (LBMI) and non-low BMI (NLBMI) groups. Tumor tissues and adjacent normal tissues from these patients were analyzed for intratumoral microbiota, immune cell infiltration, gene expression, and metabolite profiling. | To investigate how BMI relates to intratumoral microbiota and the tumor microenvironment in gastric cancer patients and to identify specific microbial features associated with prognosis in low-BMI patients. | GC patients with low BMI had poorer clinical outcomes and pathological features, and low BMI was an independent risk factor for poor prognosis. 16S rRNA microbial diversity was similar between BMI groups, but 32 bacterial taxa differed, with the genus Abiotrophia significantly enriched in LBMI tumors. Abiotrophia was negatively correlated with eosinophils and certain genes and positively correlated with others in LBMI tumors. LBMI was linked with increased purine metabolites (guanine, IDP). Low BMI may suppress immune responses and affect chemotherapy efficacy, possibly via changes in intratumoral microbiota and metabolism. | [272] |
| Human clinical study | Human gastric tissue biopsies from patients with gastritis, intestinal metaplasia, and gastric cancer. | To profile and compare the gastric-epithelium-associated bacterial species among patients with gastritis, intestinal metaplasia, and gastric cancer, aiming to identify additional potential pathogenic bacteria beyond Helicobacter pylori. | Helicobacter pylori abundance significantly decreased in gastric cancer patients compared to non-cancer patients. Clostridium (notably Clostridium colicanis), Fusobacterium (notably Fusobacterium nucleatum), and Lactobacillus species were enriched in gastric cancer tissues. Clostridium and Fusobacterium may serve as diagnostic markers for gastric cancer. The gastric microbiota’s composition differs significantly between cancer and non-cancer groups, suggesting a cancer-specific bacterial signature. | [273] |
| Human subjects: 30 gastric cancer patients and 30 healthy controls. Intestinal microbiota analyzed through fecal samples using 16S rRNA gene sequencing technology. | To explore and analyze the composition and characteristics of intestinal microbiota differences between gastric cancer patients and healthy people and to identify specific bacteria associated with gastric cancer. | No significant difference in overall diversity and abundance of intestinal flora between groups. Significant decrease in Faecalibacterium, Bifidobacterium, and Subdoligranulum in gastric cancer patients. Significant increase in Enterococcus, Streptococcus, and Bacteroides in gastric cancer patients. Identified six key intestinal bacterial genera closely related to gastric cancer. Functional pathways enriched in gastric cancer patients’ gut flora include two-component systems, glycolysis/gluconeogenesis, transporters, and others. | [274] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr, D.-C.; Chiriac, S.-D.; Drăghici, G.-A.; Moacă, E.-A.; Faur, A.C.; Avram, M.-F.; Turi, V.-R.; Nicolin, M.-R.; Goldiș, A.; Salehi, M.A.; et al. Gastric Cancer and Microbiota: Exploring the Microbiome’s Role in Carcinogenesis and Treatment Strategies. Life 2025, 15, 999. https://doi.org/10.3390/life15070999
Lazăr D-C, Chiriac S-D, Drăghici G-A, Moacă E-A, Faur AC, Avram M-F, Turi V-R, Nicolin M-R, Goldiș A, Salehi MA, et al. Gastric Cancer and Microbiota: Exploring the Microbiome’s Role in Carcinogenesis and Treatment Strategies. Life. 2025; 15(7):999. https://doi.org/10.3390/life15070999
Chicago/Turabian StyleLazăr, Daniela-Cornelia, Sorin-Dan Chiriac, George-Andrei Drăghici, Elena-Alina Moacă, Alexandra Corina Faur, Mihaela-Flavia Avram, Vladiana-Romina Turi, Mihaela-Roxana Nicolin, Adrian Goldiș, Matin Asad Salehi, and et al. 2025. "Gastric Cancer and Microbiota: Exploring the Microbiome’s Role in Carcinogenesis and Treatment Strategies" Life 15, no. 7: 999. https://doi.org/10.3390/life15070999
APA StyleLazăr, D.-C., Chiriac, S.-D., Drăghici, G.-A., Moacă, E.-A., Faur, A. C., Avram, M.-F., Turi, V.-R., Nicolin, M.-R., Goldiș, A., Salehi, M. A., & Jipa, R. (2025). Gastric Cancer and Microbiota: Exploring the Microbiome’s Role in Carcinogenesis and Treatment Strategies. Life, 15(7), 999. https://doi.org/10.3390/life15070999








