Human Immune System Reconstitution in NOD/Shi-Prkdcscid Il2rgem1/Cyagen Mice to Study HIV Infection: Challenges and Pitfalls
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Cultures
- 24 h hPBMCs after thawing and incubation in culture medium;
- Activated 10-day-old hT-cells mixed with autologous hPBMCs.
2.3. Myeloablative Conditioning of Mice
2.4. Administration of the Cells
2.5. Determining the Effect of Engraftment on the Welfare of Mice
- The mice were deprived of food and water. Their activity was monitored for 5 min. Scoring was as follows: 0 = mouse started moving within 2 min and continued moving; 1 = mouse took more than 2 min to get up and moved slowly; 2 = mouse did not get up within 5 min and moved only when touched;
- Weight loss: 0 = <10%, 1 = 10–20% and 2 = >20%;
- Mouse posture: 0 = no change, 1 = hunched posture at rest, 2 = hunched posture makes movement difficult;
- Fur texture: 0 = unchanged, 1 = light to moderate ruffle, 2 = heavy ruffle;
- Skin condition: 0 = no change, 1 = peeling in hairless areas, 2 = obvious peeling of the skin in areas of hair loss.
- Assigning a mouse 7 or more scores;
- Body weight loss > 25%.
2.6. Blood Samples Collection
2.7. Evaluation of Engraftment
- Determination of the lymphocyte fraction;
- Determination of the single lymphocyte fractions;
- Determination of the fraction of viable lymphocytes;
- Determination of the population of hT-cells (hCD3+mCD45−) and mouse lymphocytes (hCD3−mCD45+);
- Determination of the fraction of human T-helper cells (hT-helper cells) in the hT-cell population (Figure S1).
2.8. Evaluation of the Effect of Grafts on Mouse Hematopoiesis
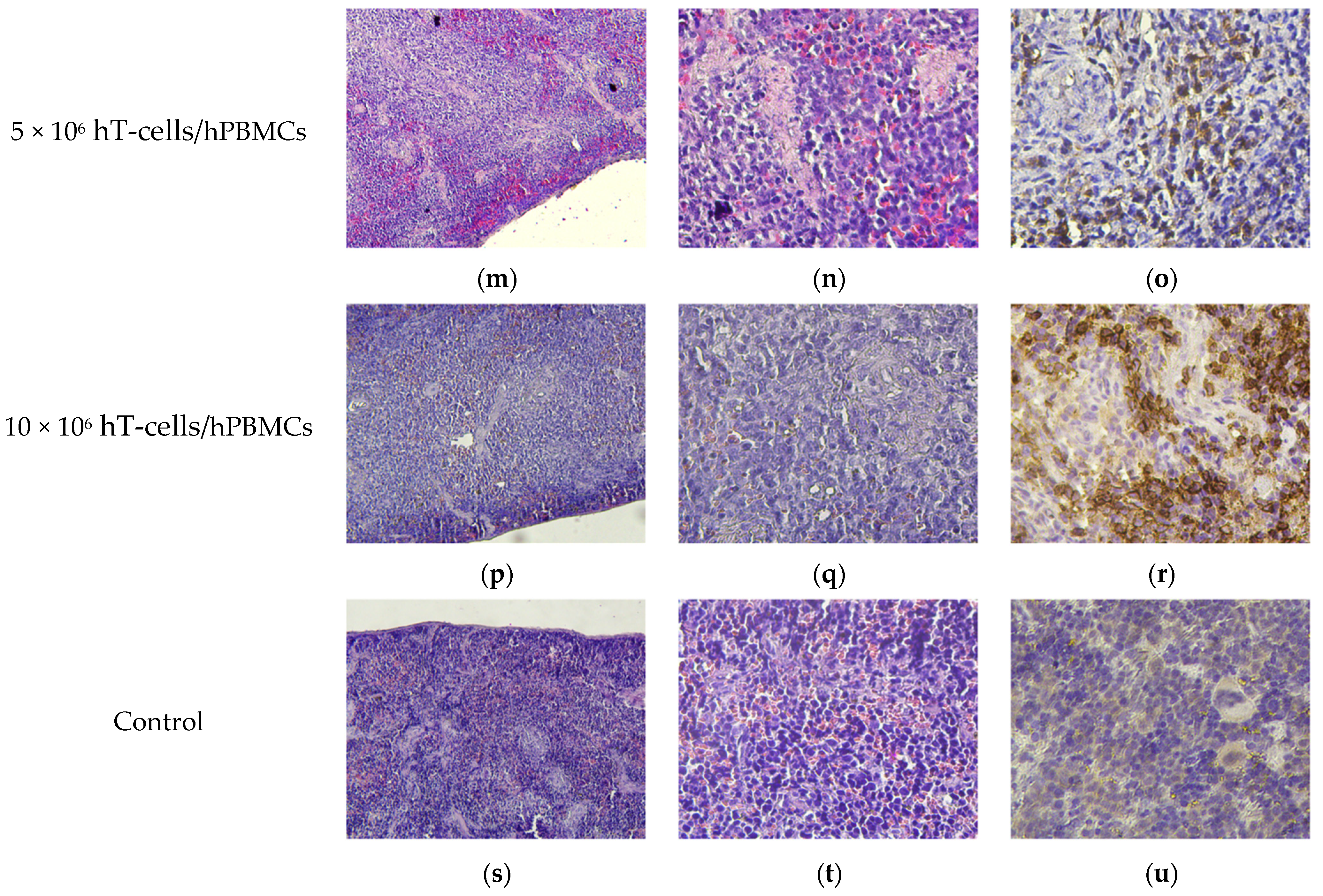
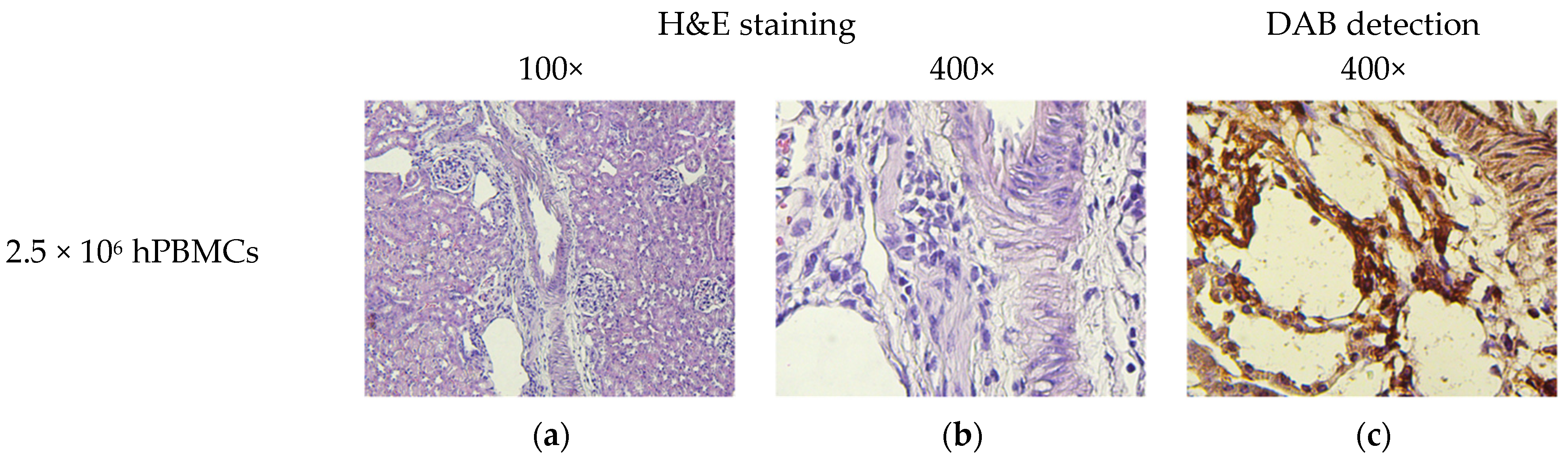
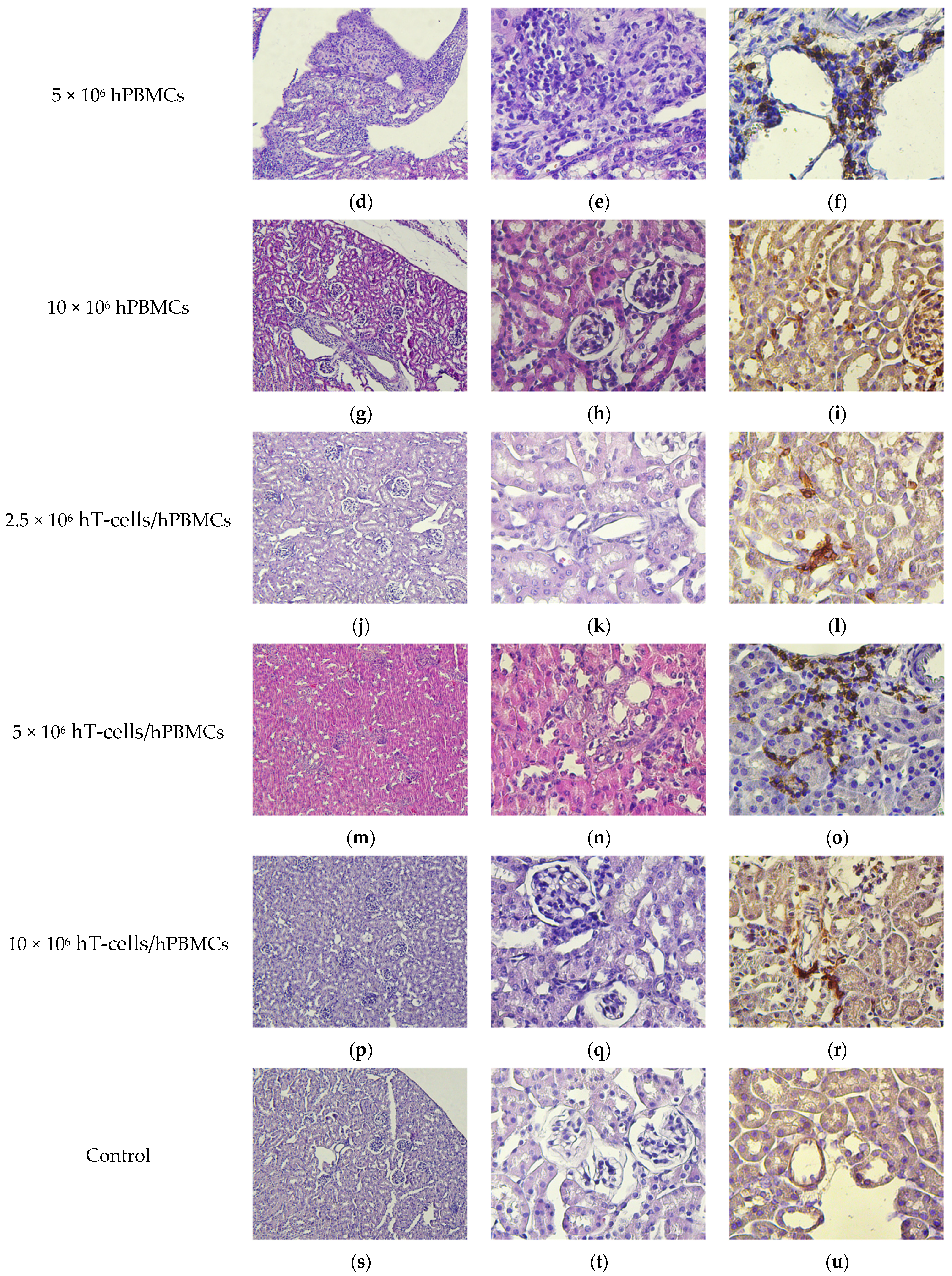
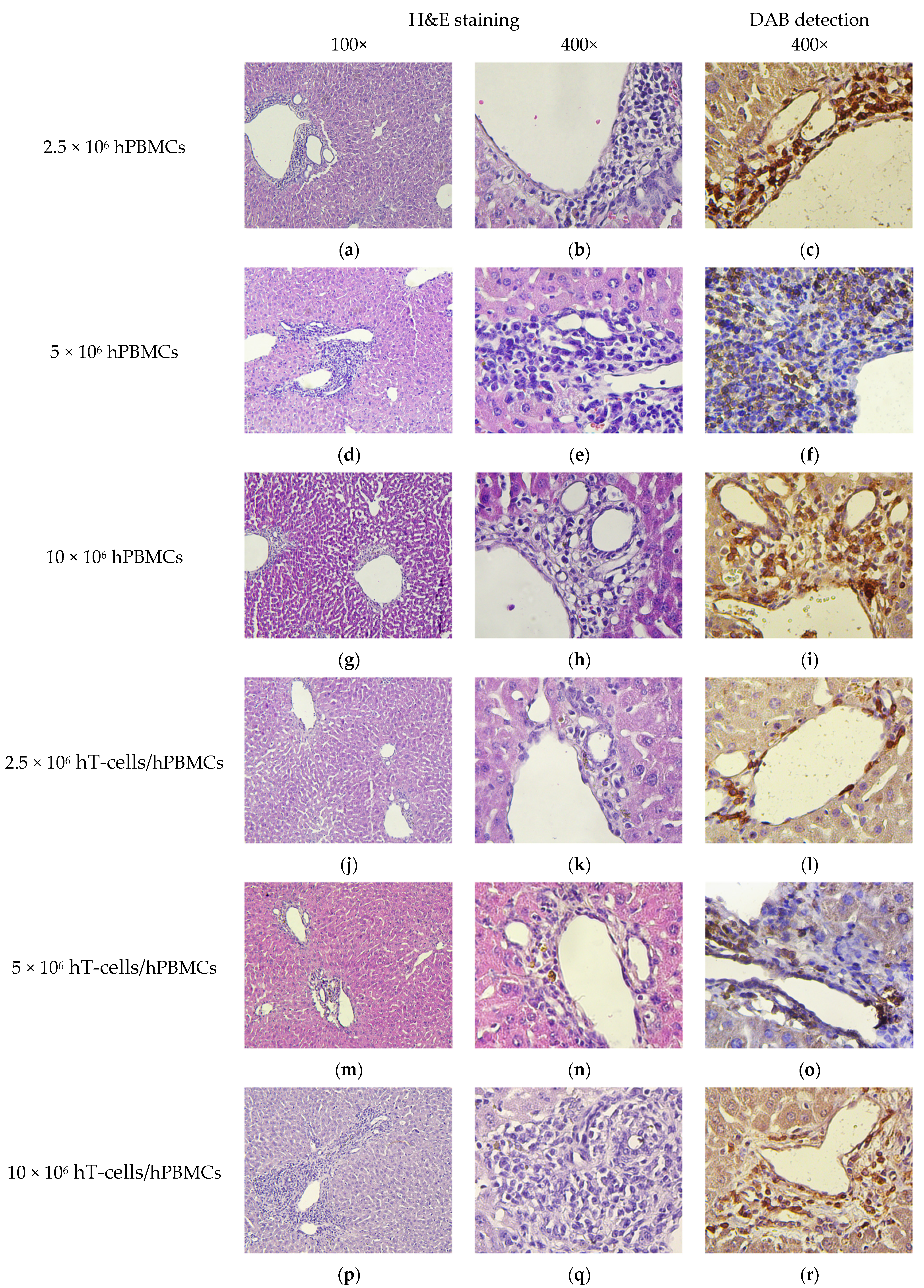
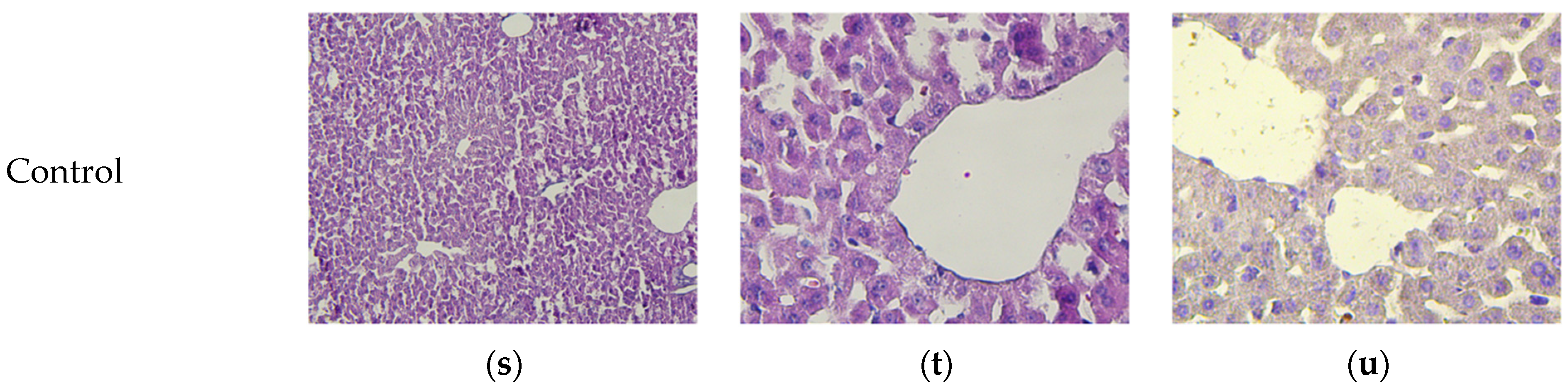
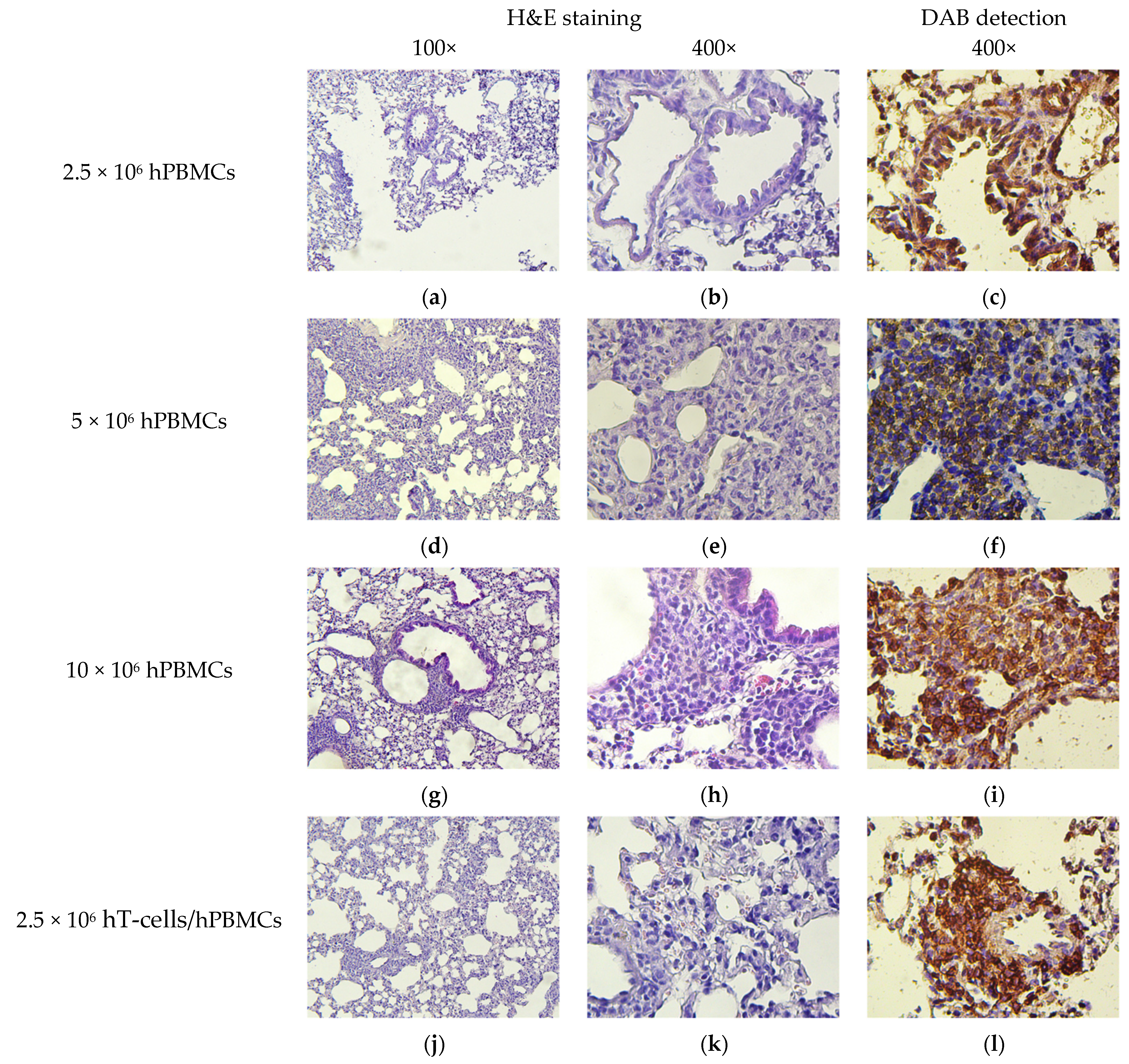
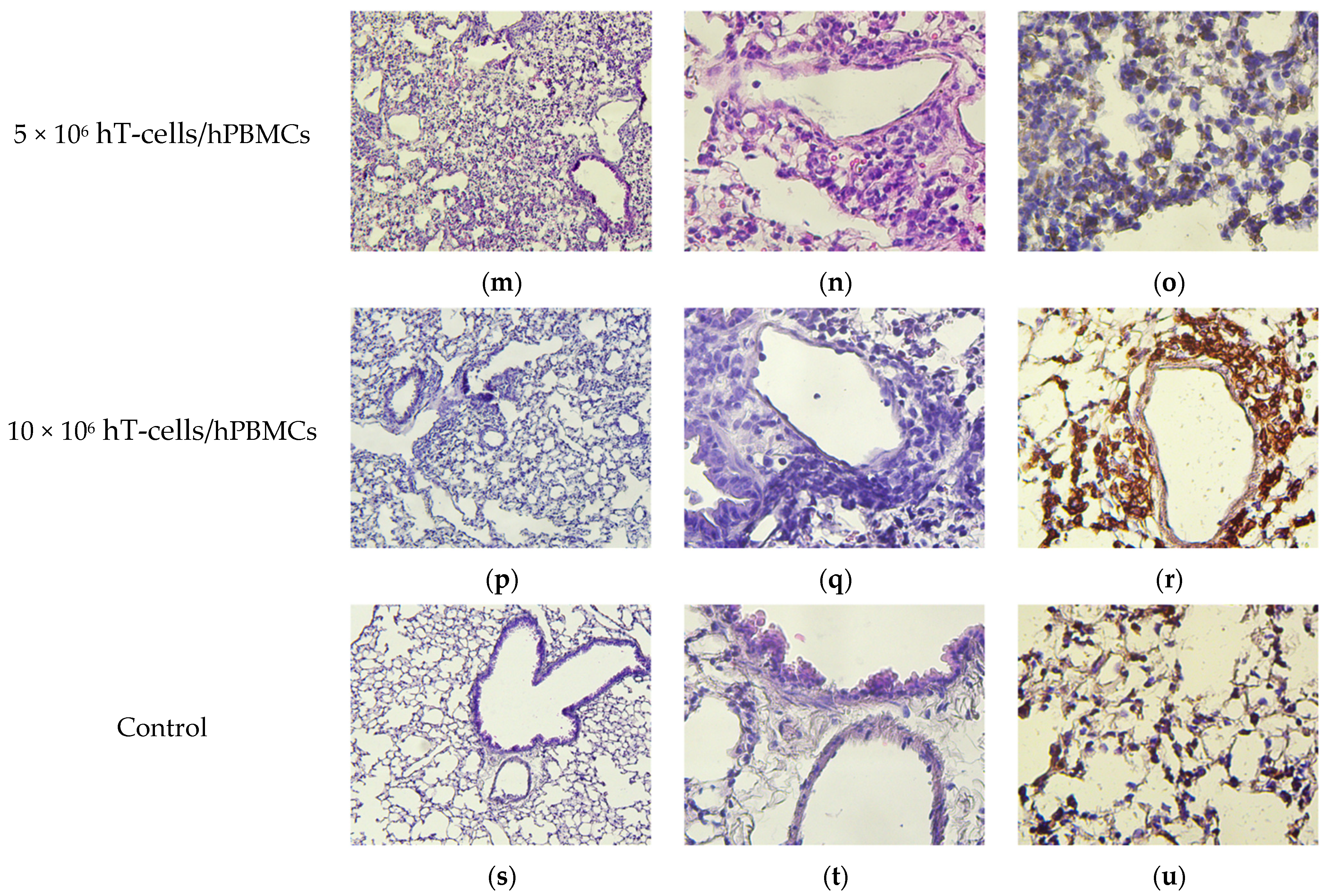
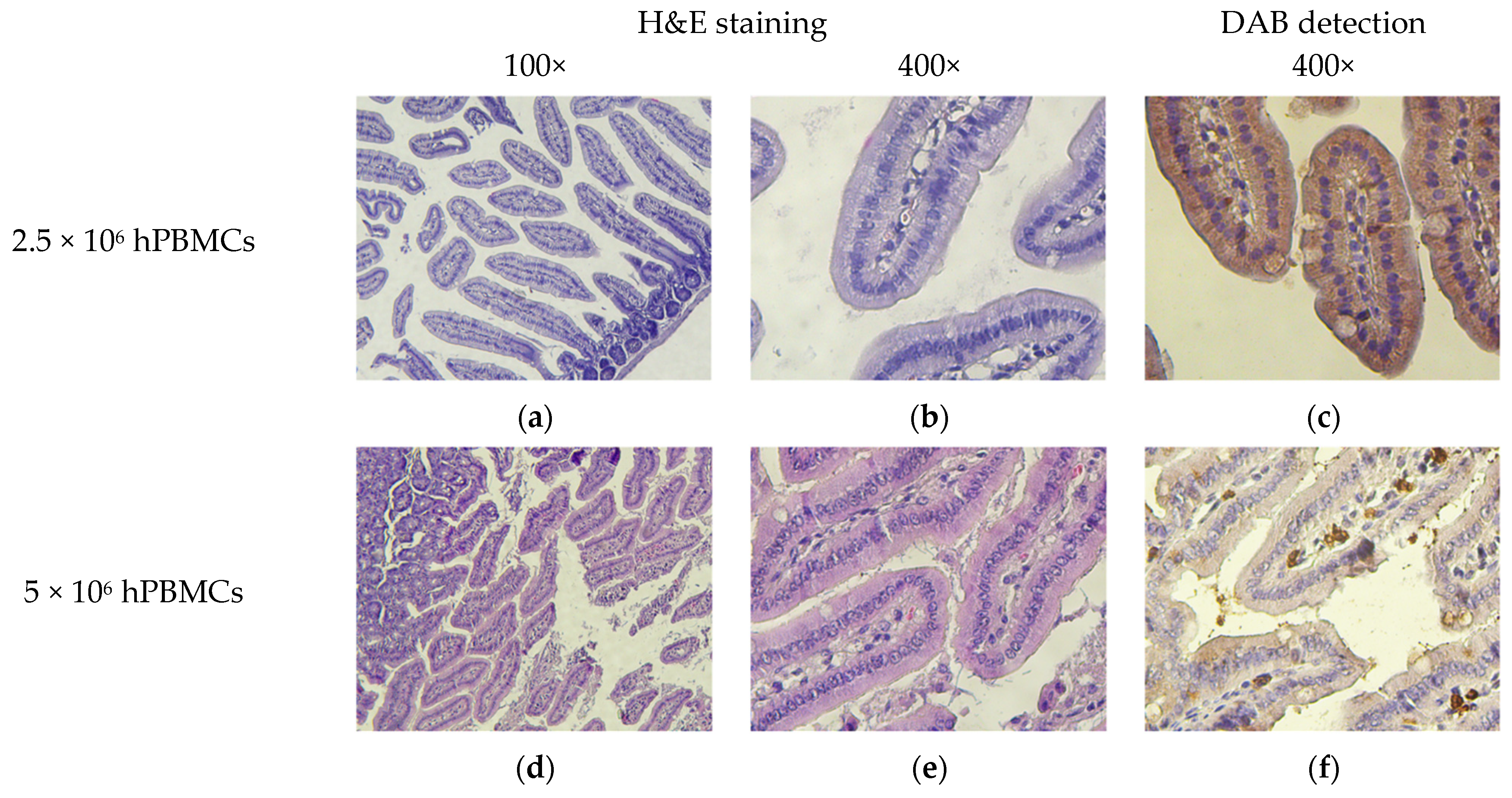
2.9. Pathomorphology
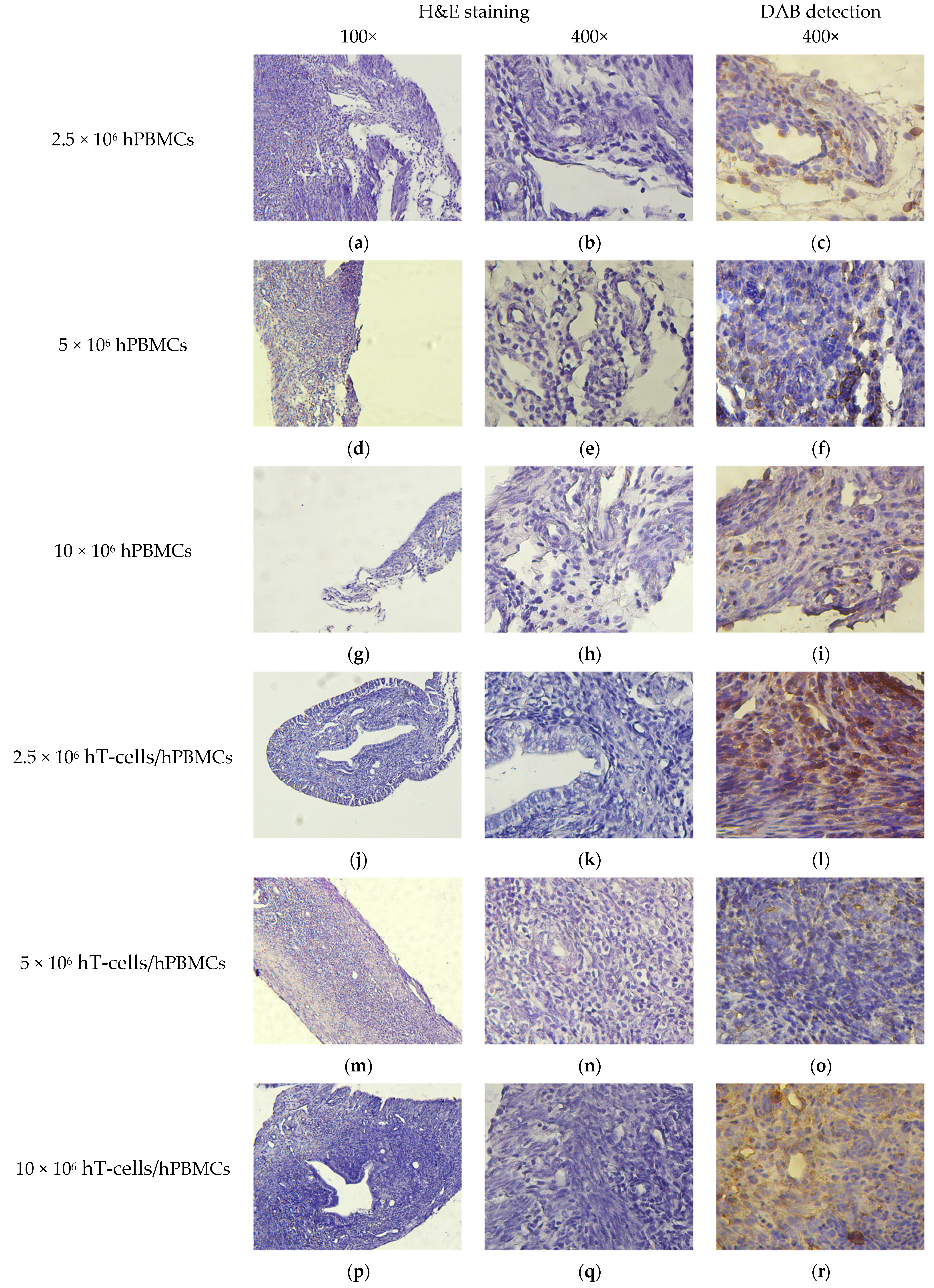
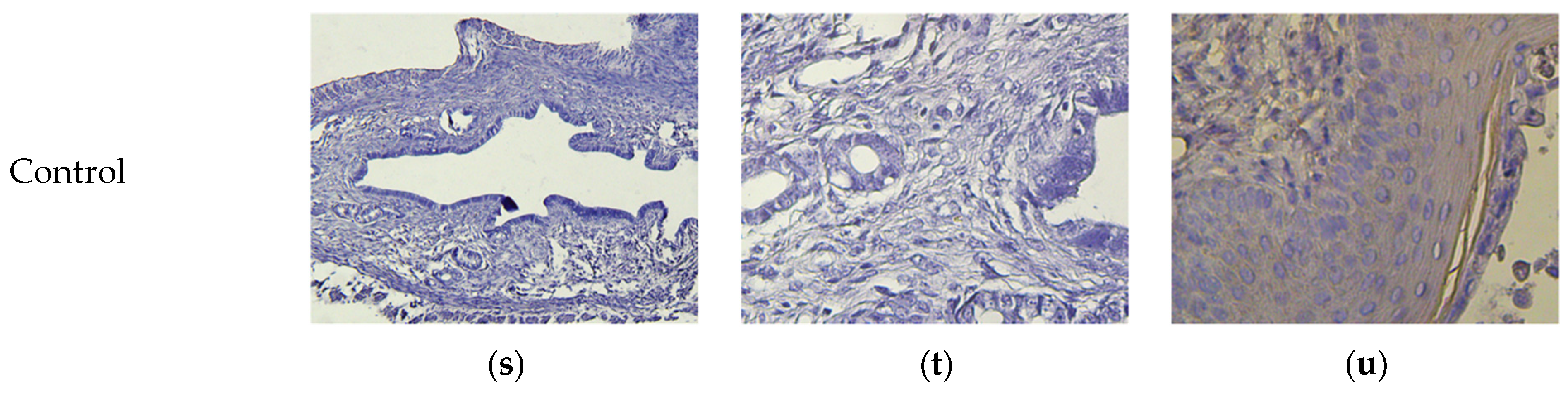
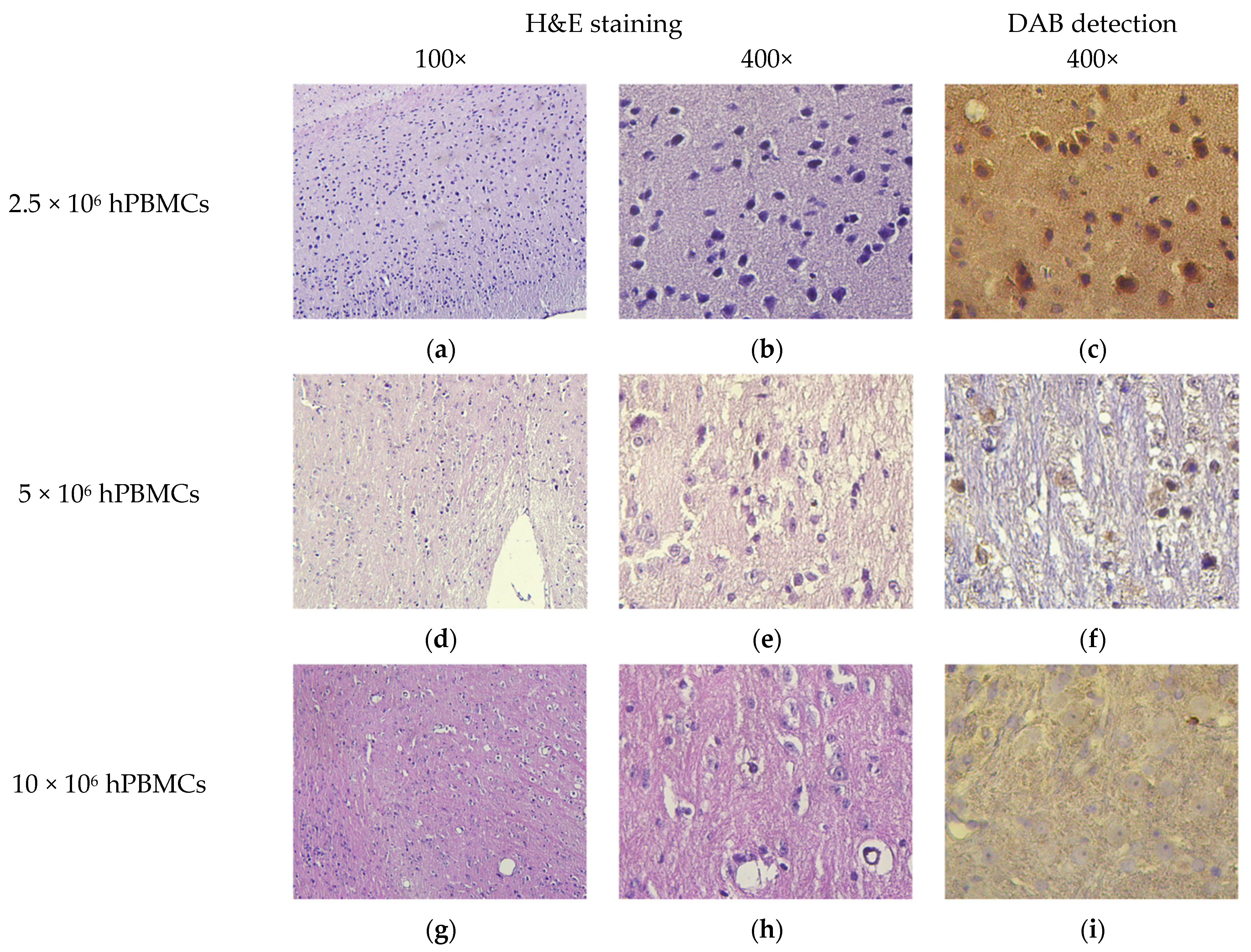
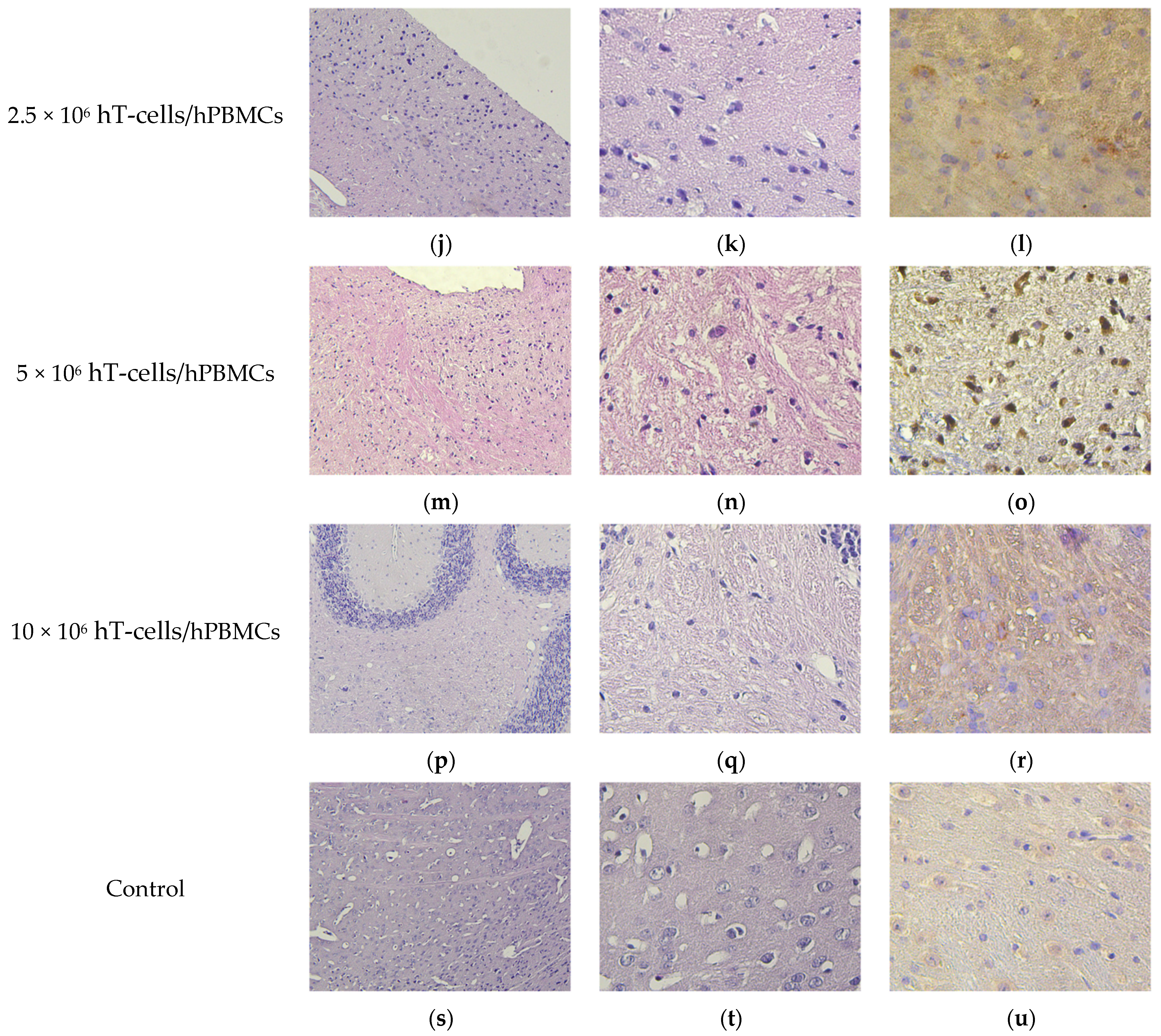
2.10. Histology and Immunohistochemistry
2.11. Statistical Analysis
3. Results
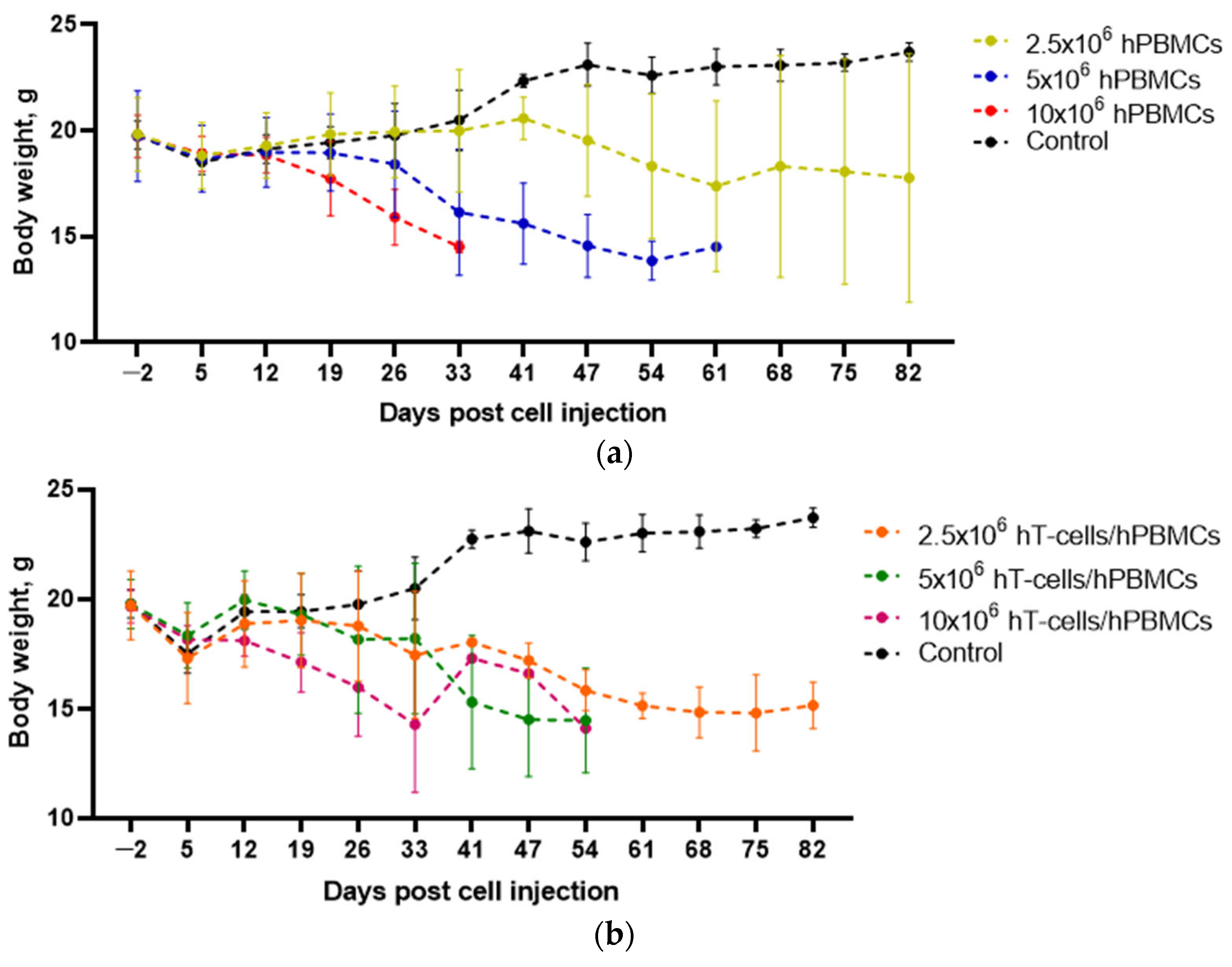
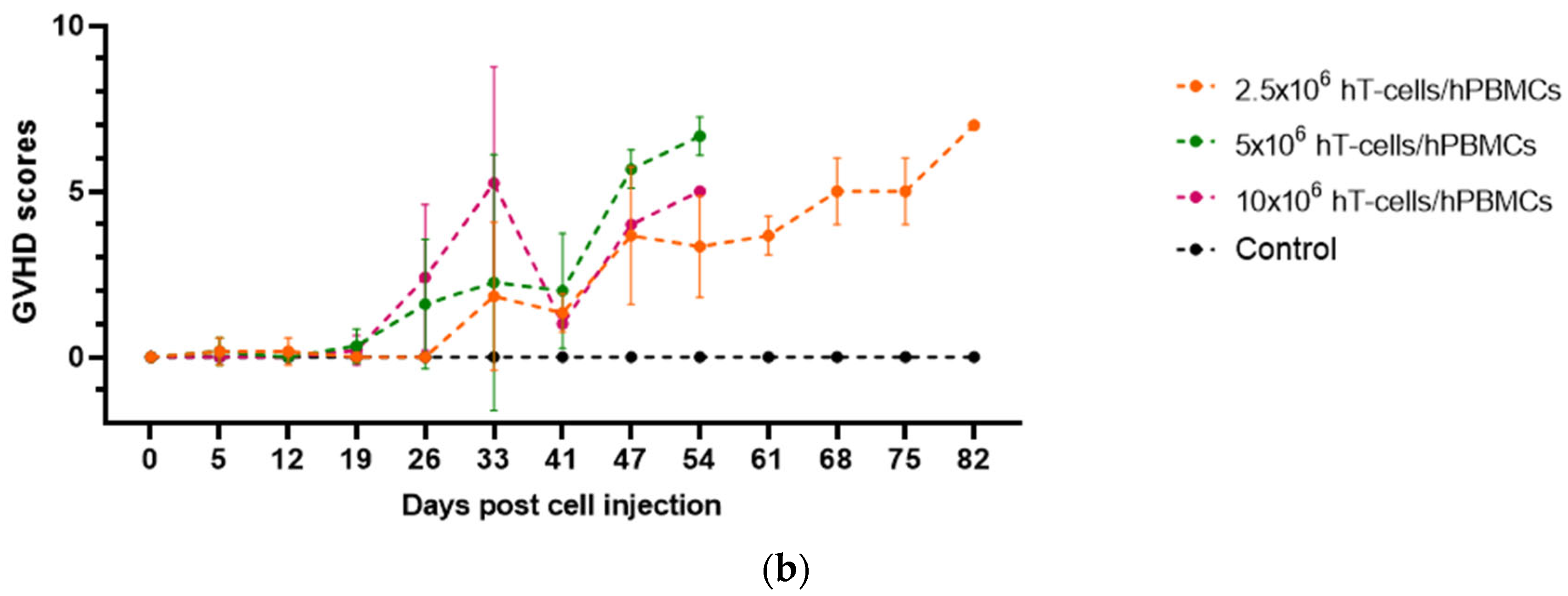
3.1. Effects of the Type and Concentration of the Graft on the GVHD Progression
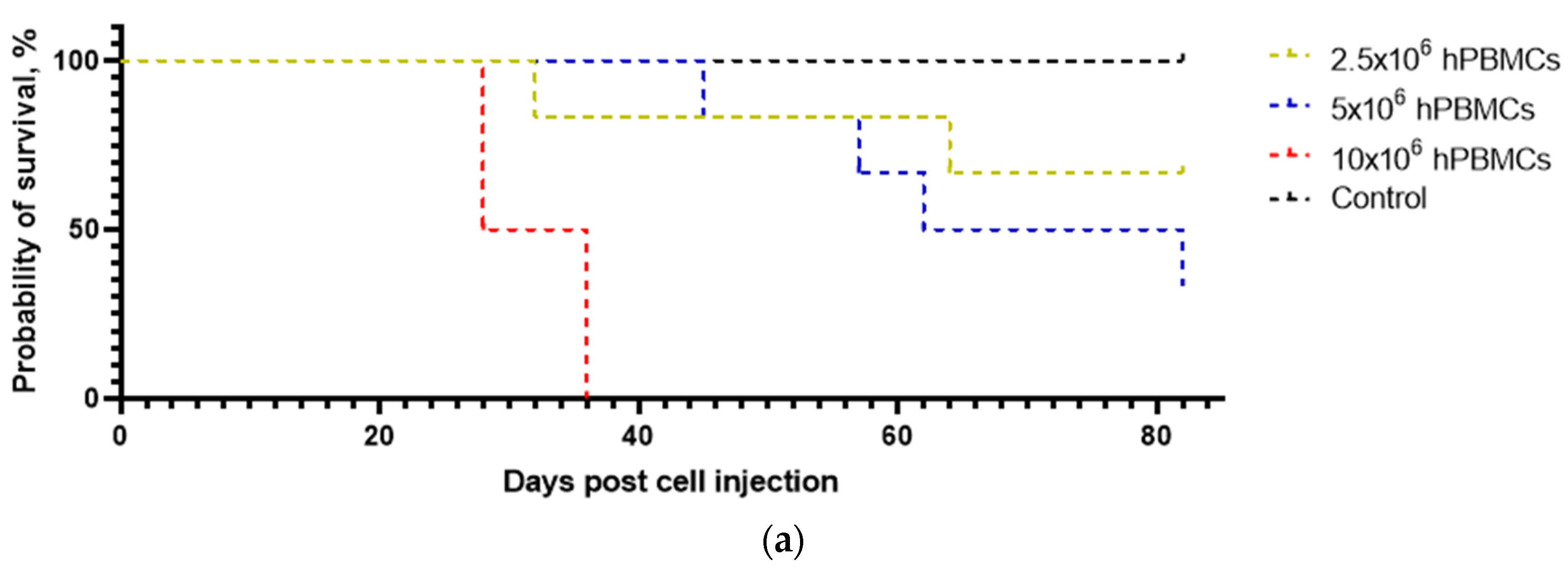
3.2. The Survival of Humanized Mice Depends on the Type and Concentration of the Graft
3.3. Analysis of the Dynamics of CBC Indices
3.3.1. Leukocyte Indices
3.3.2. Erythrocyte Indices
3.3.3. Platelet Indices
3.4. The Type and Concentration of the Graft Do Not Significantly Activate B-Cell Immunity in Mice
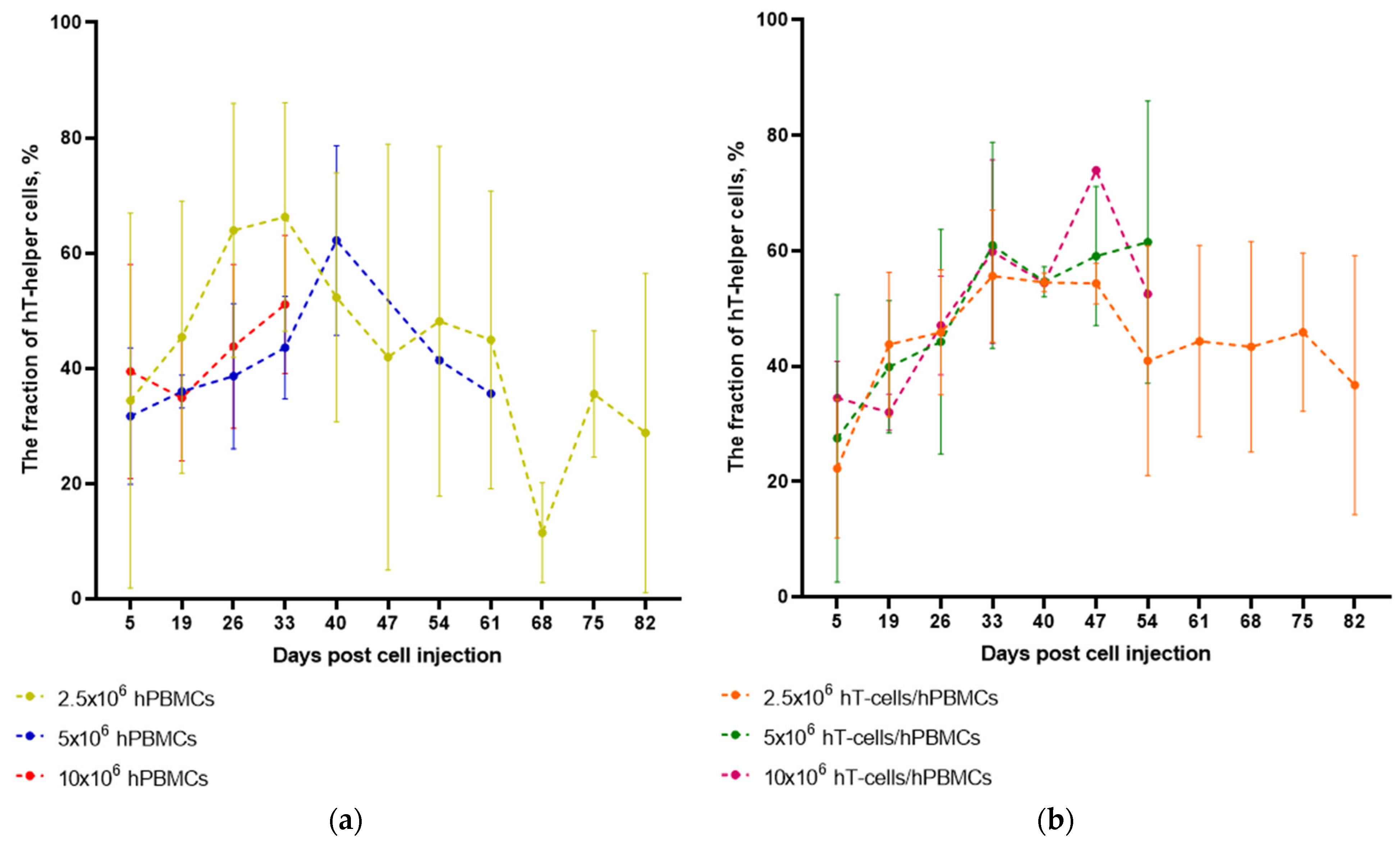
3.5. The Type of Graft Determines the Dynamics of HC Engraftment but Not the Dynamics of hT-Helper Cells Concentration After Engraftment
3.6. Definition of Linear Correlations
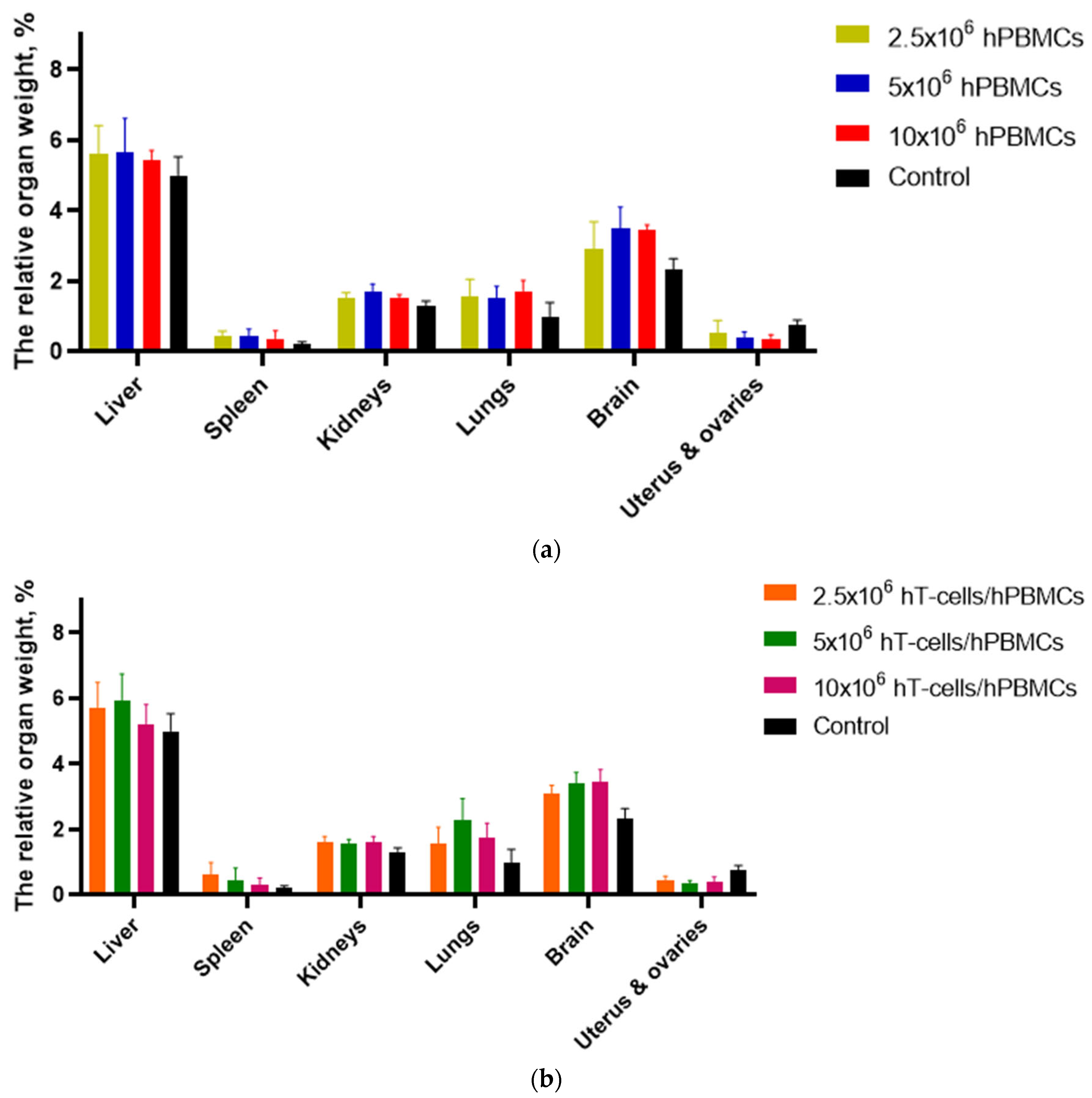
3.7. Macromorphological Analysis
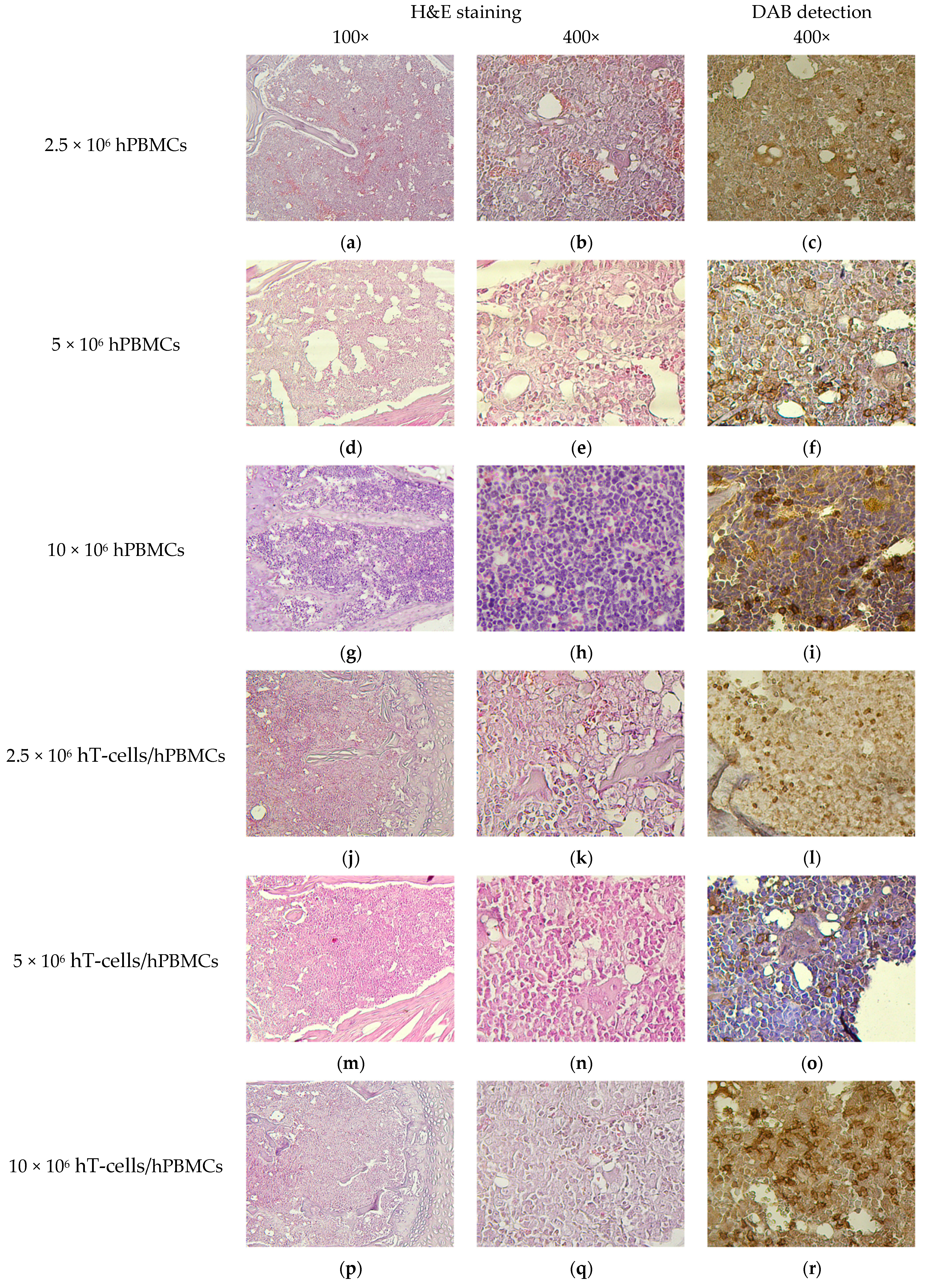
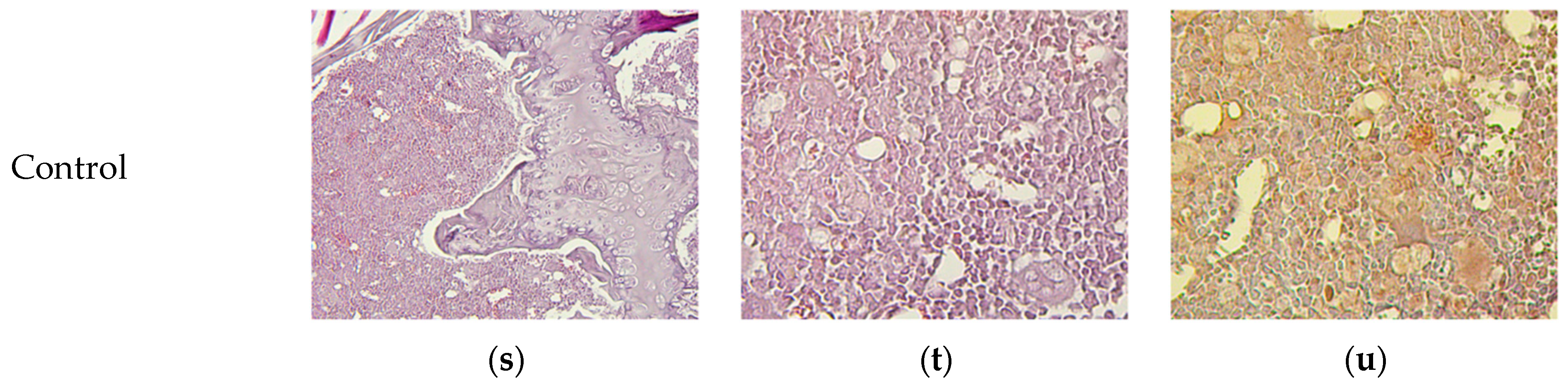
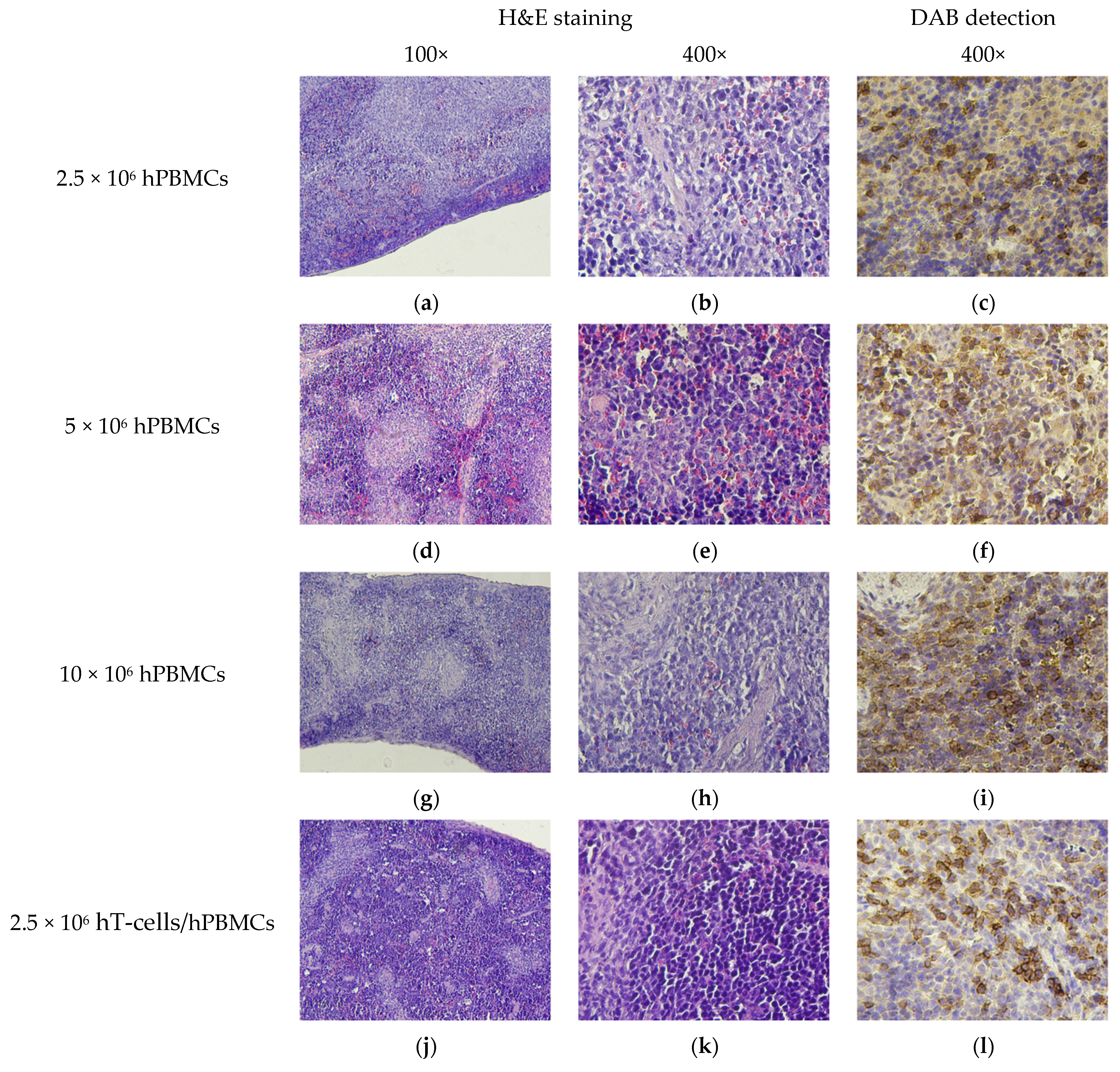
3.8. Micromorphological Examination
4. Discussion
- the degree of chimerism and GVHD progression in 2.5 × 106 hPBMCs and 2.5 × 106 hT-cells/hPBMCs mice;
- hT-helper cell concentration and GVHD progression in mice 10 × 106 hPBMCs, 5 × 106 hT-cells/hPBMCs, and 10 × 106 hT-cells/hPBMCs;
- GVHD progression and WBC including Lymph# in 10 × 106 hPBMCs mice;
- the degree of chimerism and WBC including Lymph# in 10 × 106 hPBMCs and 5 × 106 hT-cells/hPBMCs mice;
- the degree of chimerism and Mon# in hPBMCs and 5 × 106 hPBMCs mice;
- the degree of chimerism and Gran# in hPBMCs and 5 × 106 hT-cells/hPBMCs mice.
- GVHD progression and RBC in all humanized mice;
- GVHD progression and PLT in 10 × 106 hPBMCs and 10 × 106 hT-cells/hPBMCs mice;
- the degree of chimerism and RBC in 5 × 106 hPBMCs and 10 × 106 hT-cells/hPBMCs mice;
- the degree of chimerism and PLT in 10 × 106 hPBMCs and 10 × 106 hT-cells/hPBMCs mice.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, X.; Lai, Y.; Li, B.; Qin, L.; Xu, Y.; Lin, S.; Wang, S.; Wu, Q.; Liang, Q.; Huang, G.; et al. CRISPR/Cas9-Mediated Deletion of Foxn1 in NOD/SCID/IL2rg−/− Mice Results in Severe Immunodeficiency. Sci. Rep. 2017, 7, 7720. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Goodwin, N.; Ishikawa, F.; Hosur, V.; Lyons, B.L.; Greiner, D.L. Human Cancer Growth and Therapy in Immunodeficient Mouse Models. Cold Spring Harb. Protoc. 2014, 2014, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized Mice for Immune System Investigation: Progress, Promise and Challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning Regimens for Hematopoietic Cell Transplantation: One Size Does Not Fit All. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, W.; Nie, D.; Li, S.; Duan, C.; Zhou, M.; Lai, P.; Yang, S.; Ji, S.; Li, Y.; et al. Loss of PRMT7 Reprograms Glycine Metabolism to Selectively Eradicate Leukemia Stem Cells in CML. Cell Metab. 2022, 34, 818–835.e7. [Google Scholar] [CrossRef] [PubMed]
- Smutova, V.; Pará, C.; Foret, M.K.; Bennamoune, N.; Hung, S.; Spickler, C.; Riffon, R.; Rowe, J.; Festin, S.; Authier, S. Non-Clinical Cell Therapy Development Using the NCG Mouse Model as a Test System. Int. J. Toxicol. 2023, 42, 232–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lohani, S.C.; Cheng, Y.; Wang, T.; Guo, L.; Kim, W.-K.; Gorantla, S.; Li, Q. Human Microglia Extensively Reconstitute in Humanized-BLT Mice with Human Interleukin-34 Transgene and Support HIV-1 Brain Infection. Front. Immunol. 2021, 12, 672415. [Google Scholar] [CrossRef] [PubMed]
- Nagornykh, A.M.; Tyumentseva, M.A.; Tyumentsev, A.I.; Akimkin, V.G. Anatomical and Physiological Aspects of the HIV Infection Pathogenesis in Animal Models. J. Microbiol. Epidemiol. Immunobiol. 2022, 99, 587–604. [Google Scholar] [CrossRef]
- Watanabe, S.; Terashima, K.; Ohta, S.; Horibata, S.; Yajima, M.; Shiozawa, Y.; Dewan, M.Z.; Yu, Z.; Ito, M.; Morio, T.; et al. Hematopoietic Stem Cell-Engrafted NOD/SCID/IL2R Null Mice Develop Human Lymphoid Systems and Induce Long-Lasting HIV-1 Infection with Specific Humoral Immune Responses. Blood 2007, 109, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Willis, E.; Verrelle, J.; Banerjee, E.; Assenmacher, C.-A.; Tarrant, J.C.; Skuli, N.; Jacobson, M.L.; O’Rouke, D.M.; Binder, Z.A.; Radaelli, E. Humanization with CD34-Positive Hematopoietic Stem Cells in NOG-EXL Mice Results in Improved Long-Term Survival and Less Severe Myeloid Cell Hyperactivation Phenotype Relative to NSG-SGM3 Mice. Vet. Pathol. 2024, 61, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Blümich, S.; Zdimerova, H.; Münz, C.; Kipar, A.; Pellegrini, G. Human CD34+ Hematopoietic Stem Cell–Engrafted NSG Mice: Morphological and Immunophenotypic Features. Vet. Pathol. 2021, 58, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.; Wesa, A. Establishment of Humanized Mice from Peripheral Blood Mononuclear Cells or Cord Blood CD34+ Hematopoietic Stem Cells for Immune-Oncology Studies Evaluating New Therapeutic Agents. Curr. Protoc. Pharmacol. 2020, 89, e77. [Google Scholar] [CrossRef] [PubMed]
- Hasgur, S.; Aryee, K.E.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A. Generation of Immunodeficient Mice Bearing Human Immune Systems by the Engraftment of Hematopoietic Stem Cells. Methods Mol. Biol. 2016, 1438, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Lyons, B.L.; Burzenski, L.M.; Gott, B.; Chen, X.; Chaleff, S.; Kotb, M.; Gillies, S.D.; King, M.; Mangada, J.; et al. Human Lymphoid and Myeloid Cell Development in NOD/LtSz-scid IL2Rγnull Mice Engrafted with Mobilized Human Hemopoietic Stem Cells. J. Immunol. 2005, 174, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- van Eenige, R.; Verhave, P.S.; Koemans, P.J.; Tiebosch, I.A.C.W.; Rensen, P.C.N.; Kooijman, S.; Millet, O. RandoMice, a Novel, User-Friendly Randomization Tool in Animal Research. PLoS ONE 2020, 15, e0237096. [Google Scholar] [CrossRef] [PubMed]
- Seng, A.; Markiewicz, M.A. Induction and Scoring of Graft-Versus-Host Disease in a Xenogeneic Murine Model and Quantification of Human T Cells in Mouse Tissues Using Digital PCR. J. Vis. Exp. 2019, 147, e59107. [Google Scholar] [CrossRef]
- Novitzky-Basso, I.; Viswabandya, A. Biology of Graft-Versus-Host Disease. In Organ and Tissue Transplantation; Chandy, M., Radhakrishnan, Vivek, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 55–73. [Google Scholar] [CrossRef]
- Ye, J.; Liu, Q.; He, Y.; Song, Z.; Lin, B.; Hu, Z.; Hu, J.; Ning, Y.; Cai, C.; Li, Y. Combined Therapy of CAR-IL-15/IL-15Rα-T Cells and GLIPR1 Knockdown in Cancer Cells Enhanced Anti-Tumor Effect against Gastric Cancer. J. Transl. Med. 2024, 22, 171. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, T.; Zhao, S.; Lin, M. The Therapeutic Effects of MUC1-C ShRNA@Fe3O4 Magnetic Nanoparticles in Alternating Magnetic Fields on Triple-Negative Breast Cancer. Int. J. Nanomed. 2023, 18, 5651–5670. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Choi, B.-S.; Park, K.H.; Lee, H.J.; Cho, Y.K.; Kim, S.I.; Kim, S.S.; Oh, Y.-K.; Kim, Y.B. A Simple Mouse Model for the Study of Human Immunodeficiency Virus. AIDS Res. Hum. Retroviruses 2015, 32, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, J.L.; Zhou, Y.; Becker, Y.A.; Ma, S.; Kenney, S.C.; Hematti, P.; Capitini, C.M.; Burlingham, W.J.; Gendron-Fitzpatrick, A.; Gumperz, J.E. Mice Engrafted with Human Fetal Thymic Tissue and Hematopoietic Stem Cells Develop Pathology Resembling Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2013, 19, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Brehm, M.A.; Kenney, L.L.; Wiles, M.V.; Low, B.E.; Tisch, R.M.; Burzenski, L.; Mueller, C.; Greiner, D.L.; Shultz, L.D. Lack of Acute Xenogeneic Graft-Versus-Host Disease, but Retention of T-Cell Function Following Engraftment of Human Peripheral Blood Mononuclear Cells in NSG Mice Deficient in MHC Class I and II Expression. FASEB J. 2019, 33, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Morillon, Y.M.; Rumfield, C.S.; Pellom, S.T.; Sabzevari, A.; Roller, N.T.; Horn, L.A.; Jochems, C.; Palena, C.; Greiner, J.W.; Schlom, J. The Use of a Humanized NSG-Β2m−/− Model for Investigation of Immune and Anti-Tumor Effects Mediated by the Bifunctional Immunotherapeutic Bintrafusp Alfa. Front. Oncol. 2020, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Flutter, B.; Rodriguez, R.S.; Sharif-Paghaleh, E.; Barber, L.D.; Lombardi, G.; Nestle, F.O.; Stoddart, C.A. Xenogeneic Graft-Versus-Host-Disease in NOD-Scid IL-2Rγnull Mice Display a T-Effector Memory Phenotype. PLoS ONE 2012, 7, e44219. [Google Scholar] [CrossRef] [PubMed]
- Forcade, E.; Kim, H.T.; Cutler, C.; Wang, K.; Alho, A.C.; Nikiforow, S.; Ho, V.T.; Koreth, J.; Armand, P.; Alyea, E.P.; et al. Circulating T Follicular Helper Cells with Increased Function during Chronic Graft-Versus-Host Disease. Blood 2016, 127, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Choi, J.; Park, N.; Kang, J.; Kim, M.; Kim, Y.; Ju, J.H. Development of immunocompatible pluripotent stem cells via CRISPR-based human leukocyte antigen engineering. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Notta, F.; Doulatov, S.; Dick, J.E. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 2010, 115, 3704–3707. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Liao, B.; Nixon, C.C.; Cleary, R.A.; Thayer, W.O.; Birath, S.L.; Swanson, M.D.; Sheridan, P.; Zakharova, O.; Prince, F.; et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J. Clin. Investig. 2018, 128, 2862–2876. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Wahl, A.; Archin, N.; Choudhary, S.; Margolis, D.; Garcia, J.V. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology 2013, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Flerin, N.C.; Bardhi, A.; Zheng, J.H.; Korom, M.; Folkvord, J.; Kovacs, C.; Benko, E.; Truong, R.; Mota, T.; Connick, E.; et al. Establishment of a Novel Humanized Mouse Model to Investigate In Vivo Activation and Depletion of Patient-Derived HIV Latent Reservoirs. J. Virol. 2019, 93, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A. Stem Cell Research and Ethics: An Update. Oman Med. J. 2015, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Abeynaike, S.; Paust, S. Humanized Mice for the Evaluation of Novel HIV-1 Therapies. Front. Immunol. 2021, 12, 636775. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.M.; Kelleher, A.D.; Cooper, D.A. Timing of the Components of the HIV Life Cycle in Productively Infected CD4+ T Cells in a Population of HIV-Infected Individuals. J. Virol. 2011, 85, 10798–10805. [Google Scholar] [CrossRef] [PubMed]





| Type of the Graft | Animal Groups |
|---|---|
| 2.5 × 106 hPBMCs | 2.5 × 106 hPBMCs |
| 5 × 106 hPBMCs | 5 × 106 hPBMCs |
| 10 × 106 hPBMCs | 10 × 106 hPBMCs |
| 1.25 × 106 hT-cells mixed with 1.25 × 106 hPBMCs | 2.5 × 106 hT-cells/hPBMCs |
| 2.5 × 106 hT-cells mixed with 2.5 × 106 hPBMCs | 5 × 106 hT-cells/hPBMCs |
| 5 × 106 hT-cells mixed with 5 × 106 hPBMCs | 10 × 106 hT-cells/hPBMCs |
| 1× DPBS | Control |
| Dye/Antibody Conjugate | Catalogue Number |
|---|---|
| EV450 Anti-Mouse CD45 Antibody [30-F11], 500 T | E-AB-F1136Q |
| PE/TR Anti-Human CD3 Antibody [OKT-3], 200 T | E-AB-F1001P |
| PE/Cyanine7 Anti-Human CD4 Antibody [RPA-T4], 200 T | E-AB-F1109H |
| 7-AAD Staining Solution | 130-111-568 |
| r Value | Interpretation of Correlation |
|---|---|
| 0.75 to 1.00 (−0.75 to −1.00) | Very high positive (negative) |
| 0.50 to 0.75 (−0.50 to −0.75) | High positive (negative) |
| 0.25 to 0.50 (−0.25 to −0.50) | Moderate positive (negative) |
| 0.00 to 0.25 (0.00 to −0.25) | Low positive (negative) |
| Type of the Graft | % of Total Variation | p-Value |
|---|---|---|
| hPBMCs | 21.4 | 0.0009 |
| hT-cells/hPBMCs | 16.79 | 0.0206 |
| Type of the Graft | % of Total Variation | p-Value |
|---|---|---|
| hPBMCs | 46.87 | <0.0001 |
| hT-cells/hPBMCs | 21.54 | <0.0001 |
| Mouse Group | Type of the Graft | Log-Rank (Mantel–Cox) Test | Gehan–Breslow–Wilcoxon Test |
|---|---|---|---|
| hPBMCs | Chi square | 24.1 | 22.72 |
| df | 3 | 3 | |
| p-value | <0.0001 | <0.0001 | |
| hT-cells/hPBMCs | Chi square | 15.92 | 15.28 |
| df | 3 | 3 | |
| p-value | 0.0012 | 0.0016 |
| Type of the Graft | r | p-Value |
|---|---|---|
| 2.5 × 106 hPBMCs | 0.72 | 0.01 |
| 5 × 106 hPBMCs | 0.53 | 0.23 |
| 10 × 106 hPBMCs | 0.49 | 0.51 |
| 2.5 × 106 hT-cells/hPBMCs | 0.58 | 0.06 |
| 5 × 106 hT-cells/hPBMCs | 0.41 | 0.36 |
| 10 × 106 hT-cells/hPBMCs | 0.57 | 0.18 |
| Type of the Graft | r | p-Value |
|---|---|---|
| 2.5 × 106 hPBMCs | −0.59 | 0.06 |
| 5 × 106 hPBMCs | 0.24 | 0.60 |
| 10 × 106 hPBMCs | 0.95 | 0.05 |
| 2.5 × 106 hT-cells/hPBMCs | 0.03 | 0.94 |
| 5 × 106 hT-cells/hPBMCs | 0.77 | 0.04 |
| 10 × 106 hT-cells/hPBMCs | 0.73 | 0.06 |
| Type of the Graft | WBC | RBC | PLT | Lymph# | Mon# | Gran# |
|---|---|---|---|---|---|---|
| 2.5 × 106 hPBMCs | r = 0.11; p = 0.74 | r = −0.80; p = 0.003 | r = −0.54; p = 0.08 | r = −0.01; p = 0.98 | r = 0.42; p = 0.19 | r = 0.17; p = 0.62 |
| 5 × 106 hPBMCs | r = −0.21; p = 0.66 | r = −0.83; p = 0.02 | r = −0.33; p = 0.47 | r = −0.24; p = 0.61 | r = 0.04; p = 0.93 | r = 0.19; p = 0.68 |
| 10 × 106 hPBMCs | r = 0.58; p = 0.42 | r = −0.79; p = 0.21 | r = −0.66; p = 0.34 | r = 0.79; p = 0.21 | r = 0.12; p = 0.88 | r = 0.44; p = 0.56 |
| 2.5 × 106 hT-cells/hPBMCs | r = −0.19; p = 0.57 | r = −0.95; p = 0.00001 | r = −0.73; p = 0.01 | r = 0.01; p = 0.99 | r = −0.14; p = 0.67 | r = −0.29; p = 0.39 |
| 5 × 106 hT-cells/hPBMCs | r = 0.12; p = 0.80 | r = −0.91; p = 0.005 | r = −0.12; p = 0.80 | r = 0.13; p = 0.78 | r = 0.18; p = 0.70 | r = 0.12; p = 0.80 |
| 10 × 106 hT-cells/hPBMCs | r = 0.07; p = 0.89 | r = −0.83; p = 0.02 | r = −0.72; p = 0.07 | r = 0.26; p = 0.58 | r = −0.17; p = 0.71 | r = −0.15; p = 0.75 |
| Type of the Graft | WBC | RBC | PLT | Lymph# | Mon# | Gran# |
|---|---|---|---|---|---|---|
| 2.5 × 106 hPBMCs | r = 0.45; p = 0.17 | r = −0.68; p = 0.02 | r = −0.46; p = 0.15 | r = 0.06; p = 0.85 | r = 0.69; p = 0.02 | r = 0.67; p = 0.03 |
| 5 × 106 hPBMCs | r = 0.57; p = 0.18 | r = −0.70; p = 0.08 | r = −0.11; p = 0.81 | r = 0.30; p = 0.52 | r = 0.73; p = 0.06 | r = 0.82; p = 0.02 |
| 10 × 106 hPBMCs | r = 0.97; p = 0.03 | r = −0.55; p = 0.45 | r = −0.69; p = 0.31 | r = 0.84; p = 0.16 | r = 0.89; p = 0.11 | r = 1.00; p = 0.004 |
| 2.5 × 106 hT-cells/hPBMCs | r = 0.52; p = 0.10 | r = −0.64; p = 0.03 | r = −0.08; p = 0.81 | r = 0.52; p = 0.10 | r = 0.38; p = 0.25 | r = 0.36; p = 0.27 |
| 5 × 106 hT-cells/hPBMCs | r = 0.80; p = 0.03 | r = −0.26; p = 0.57 | r = 0.05; p = 0.91 | r = 0.84; p = 0.02 | r = 0.65; p = 0.11 | r = 0.77; p = 0.04 |
| 10 × 106 hT-cells/hPBMCs | r = 0.53; p = 0.22 | r = −0.74; p = 0.06 | r = −0.89; p = 0.01 | r = 0.46; p = 0.30 | r = 0.43; p = 0.33 | r = 0.49; p = 0.26 |
| Pathology | Mice Count |
|---|---|
| Anemia of the spleen | 17 |
| Anemia of the liver | 15 |
| Myocardial anemia | 14 |
| Anemia of the kidneys | 12 |
| Uterine and ovarian atrophy | 10 |
| Atrophy of the spleen | 9 |
| Liver atrophy | 7 |
| Hyperplasia of the spleen | 5 |
| Type of the Graft | BM | Spleen | Kidneys | Liver | Lungs | Small Intestine | Uterus | Brain |
|---|---|---|---|---|---|---|---|---|
| hPBMCs | rs = 0.5 p = 1.0 | rs = 0.5 p = 1.0 | rs = −1.0 p = 0.3 | rs = 0 p = 1.0 | rs = 0.5 p = 1.0 | rs = 0.5 p = 1.0 | rs = −0.5 p = 1.0 | rs = −1.0 p = 0.3 |
| hT-cells/PBMCs | rs = 0.5 p = 1.0 | rs = −1.0 p = 0.3 | rs = −0.5 p = 1.0 | rs = 0.9 p = 0.7 | rs = 0.5 p = 1.0 | rs = 0 p = 1.0 | rs = −1.0 p = 0.3 | rs = 0 p = 1.0 |
| Concentration of hPBMCs and Route of Administration | hCD3+ T-Cells Degree of Engraftment, % | Duration of Observation, d.p.i. | Graft Localization | References |
|---|---|---|---|---|
| 10 × 106, intraperitoneally | ~62–79 | 112 | Peripheral blood, lymph nodes, spleen, liver | [20] |
| 10 × 106, intravenously | No data | 42 | Peripheral blood, skin, lungs, liver, eyes, nose, ear, tongue, salivary glands, brain | [21] |
| 10 × 106, intravenously | ~80–99 | ~84–119 | Peripheral blood, spleen | [22] |
| 10 × 106, intravenously | ~85 | 35 | Peripheral blood | [23] |
| 10 × 106, intravenously | ~95–98 | 49 | Peripheral blood, spleen, lymph node, bone marrow | [24] |
| CBC Index | The Type of the Graft | |
|---|---|---|
| 2.5 × 106 hPBMCs | 2.5 × 106 hT-Cells/hPBMCs | |
| WBC | No | No |
| RBC | No | Yes |
| HGB | Yes | Yes |
| HCT | No | Yes |
| MCV | Yes | Yes |
| MCH | No | No |
| MCHC | Yes | Yes |
| RDW | Yes | Yes |
| PLT | Yes | Yes |
| MPV | Yes | Yes |
| PDW | No | No |
| PCT | Yes | Yes |
| Lymph % | No | No |
| Mon % | Yes | Yes |
| Gran % | No | No |
| Lymph # | No | No |
| Mon # | Yes | No |
| Gran # | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagornykh, A.M.; Tyumentseva, M.A.; Tyumentsev, A.I.; Fedotov, L.A.; Karbyshev, K.S.; Orlova, E.A.; Volchkova, L.N.; Danilova, L.S.; Akinin, A.S.; Akimkin, V.G. Human Immune System Reconstitution in NOD/Shi-Prkdcscid Il2rgem1/Cyagen Mice to Study HIV Infection: Challenges and Pitfalls. Life 2025, 15, 1129. https://doi.org/10.3390/life15071129
Nagornykh AM, Tyumentseva MA, Tyumentsev AI, Fedotov LA, Karbyshev KS, Orlova EA, Volchkova LN, Danilova LS, Akinin AS, Akimkin VG. Human Immune System Reconstitution in NOD/Shi-Prkdcscid Il2rgem1/Cyagen Mice to Study HIV Infection: Challenges and Pitfalls. Life. 2025; 15(7):1129. https://doi.org/10.3390/life15071129
Chicago/Turabian StyleNagornykh, Aleksey M., Marina A. Tyumentseva, Aleksandr I. Tyumentsev, Leonid A. Fedotov, Konstantin S. Karbyshev, Evgeniya A. Orlova, Liliia N. Volchkova, Lubov S. Danilova, Andrey S. Akinin, and Vasiliy G. Akimkin. 2025. "Human Immune System Reconstitution in NOD/Shi-Prkdcscid Il2rgem1/Cyagen Mice to Study HIV Infection: Challenges and Pitfalls" Life 15, no. 7: 1129. https://doi.org/10.3390/life15071129
APA StyleNagornykh, A. M., Tyumentseva, M. A., Tyumentsev, A. I., Fedotov, L. A., Karbyshev, K. S., Orlova, E. A., Volchkova, L. N., Danilova, L. S., Akinin, A. S., & Akimkin, V. G. (2025). Human Immune System Reconstitution in NOD/Shi-Prkdcscid Il2rgem1/Cyagen Mice to Study HIV Infection: Challenges and Pitfalls. Life, 15(7), 1129. https://doi.org/10.3390/life15071129








