One-Year Comparative Evaluation of Highly Aspherical Lenslets and Horizontally Asymmetric Peripheral Defocus Lenses for Myopia Control in School-Aged Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Participants
2.3. Clinical Examinations
2.4. Cycloplegia Protocol
2.5. Spectacle Lens Characteristics
2.6. Data Management
2.7. Outcome Measures
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudnicka, A.R.; Kapetanakis, V.V.; Wathern, A.K.; Logan, N.S.; Gilmartin, B.; Whincup, P.H.; Cook, D.G.; Owen, C.G. Global variations and time trends in the prevalence of childhood myopia: A systematic review and quantitative meta-analysis. Br. J. Ophthalmol. 2016, 100, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Pu, Y.; Chen, J.; Liu, M.; Ouyang, B.; Jin, Z.; Ge, W.; Wu, Z.; Yang, X.; Qin, C.; et al. Global prevalence, trend and projection of myopia in children and adolescents from 1990 to 2050: A comprehensive systematic review and meta-analysis. Br. J. Ophthalmol. 2025, 109, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.X. Prevention of myopia in children. JAMA 2015, 314, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, Y.; Li, Z.; Wang, W.; Xuan, M.; Zhang, J.; Hu, Y.; Chen, Y.; Xiao, O.; Yin, Q.; et al. Axial elongation trajectories in Chinese children and adults with high myopia. JAMA Ophthalmol. 2024, 142, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F.; Chia, A.; Cho, P.; Guggenheim, J.A.; Polling, J.R.; Read, S.; Sankaridurg, P.; Saw, S.-M.; Trier, K.; Walline, J.J.; et al. IMI—Interventions for controlling myopia onset and progression report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M106–M131. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, A.; Huang, Y.; Li, X.; Pan, Y.; Ding, C.; Lim, E.W.; Zheng, J.; Spiegel, D.P.; Drobe, B.; et al. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. Br. J. Ophthalmol. 2022, 106, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Rodenstock GmbH. Efficacy and Satisfaction in Everyday Use: MyCon Lenses with HAPD™ Technology [Internet]; Rodenstock: Munich, Germany, 2023; Available online: https://www.rodenstock-pro.com/dam/jcr%3A3127ee0d-7c35-4818-ba6d-7e86eb2886e2/ROD_RZSP_A4%20Brosch%20B2B%20Whitepaper_MyCon_Teil3_Efficacy_02_IsoCv2_X4_250513.pdf (accessed on 2 June 2025).
- Tarutta, E.P.; Proskurina, O.V.; Tarasova, N.A.; Milash, S.V.; Markosyan, G.A. Long-term results of perifocal defocus spectacle lens correction in children with progressive myopia. Vestn. Oftalmol. 2019, 135, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed]
- Myopia Profile. Essilor Stellest® [Internet]; Myopia Profile Pty Ltd.: Brisbane, Australia, 2025; Available online: https://www.myopiaprofile.com/product/essilor-stellest (accessed on 7 July 2025).
- Essilor International. Stellest™ Lenses: Essilor’s Solution to Slow Down Myopia Progression in Children [Internet]; Essilor: Charenton-le-Pont, France, 2021; Available online: https://d15k2d11r6t6rl.cloudfront.net/public/users/Integrators/BeeProAgency/20036_8016/2021%20Stellest_Brochure_A4%20Short%20Version_New%20%28final%2020211103%29.pdf (accessed on 7 July 2025).
- Gifford, K.L. Essilor Stellest® Glasses and Lenses—A Guide for Parents [Internet]; My Kids Vision: Brisbane, Australia, 2025; Available online: https://www.mykidsvision.org/knowledge-centre/essilor-stellest-glasses-lenses-parents-guide (accessed on 7 July 2025).
- Rodenstock GmbH. Children’s Lenses—Rodenstock MyCon™ [Internet]; Rodenstock: Munich, Germany, 2025; Available online: https://www.rodenstock.com/lenses/childrens-lenses (accessed on 7 July 2025).
- Rodenstock. Rodenstock MyCon [Internet]; Rodenstock Hrvatska: Zagreb, Croatia, 2025; Available online: https://rodenstock.hr/rodenstock-mycon/ (accessed on 7 July 2025).
- Rodenstock GmbH. Instructions for Use: Myopia Management Lenses (MyCon™) [Internet], Version 3; Rodenstock: Munich, Germany, 2024; Available online: https://www.rodenstock-pro.com/dam/jcr%3A00c9948d-8ed1-427c-92e5-1f126273c10a/instruction_for_use_rodenstock_mycon_lenses.pdf (accessed on 7 July 2025).
- Lembo, A.; Schiavetti, I.; Serafino, M.; Caputo, R.; Nucci, P. Comparison of the performance of myopia control in European children and adolescents with defocus incorporated multiple segments (DIMS) and highly aspherical lenslets (HAL) spectacles. BMJ Paediatr. Open 2024, 8, e003187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva-Leite, S.; Amorim-de-Sousa, A.; Queirós, A.; González-Méijome, J.M.; Fernandes, P. Peripheral refraction and visual function of novel perifocal ophthalmic lens for the control of myopia progression. J. Clin. Med. 2023, 12, 1435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donovan, L.; Sankaridurg, P.; Ho, A.; Naduvilath, T.; Smith, E.L., 3rd; Holden, B.A. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis. Sci. 2012, 89, 27–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Huang, Y.; Liu, C.; Chang, X.; Cui, Z.; Yang, Q.; Drobe, B.; Bullimore, M.A.; Chen, H.; Bao, J. Myopia control efficacy of spectacle lenses with highly aspherical lenslets: Results of a 5-year follow-up study. Eye Vis. 2025, 12, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bao, J.; Huang, Y.; Li, X.; Yang, A.; Zhou, F.; Wu, J.; Wang, C.; Li, Y.; Lim, E.W.; Spiegel, D.P.; et al. Spectacle lenses with aspherical lenslets for myopia control vs. single-vision spectacle lenses: A randomized clinical trial. JAMA Ophthalmol. 2022, 140, 472–478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Total (n = 57) | SVL (n = 20) | HAPD (n = 21) | HAL (n = 16) | |
|---|---|---|---|---|
| Gender | ||||
| F | 28 (49.1%) | 11 (55%) | 13 (61.9%) | 4 (25%) |
| M | 29 (50.9%) | 9 (45%) | 8 (38.1%) | 12 (75%) |
| Age, median (IQR) | 12 (10–14) | 12.5 (11–14.5) | 12 (10.8–13.3) | 11 (9.5–14) |
| Age group | ||||
| 8−12 | 31 (54.4%) | 10 (50%) | 11 (52.4%) | 10 (62.5%) |
| 13–17 | 26 (45.6%) | 10 (50%) | 10 (47.6%) | 6 (37.5%) |
| Right eye baseline SER, median (IQR) | −3.5 (−4.5–−2.6) | −2.7 (−3.9–−1.7) | −3.8 (−4.7–−3.0) | −3.9 (−4.6–−3.4) |
| Miopia severity group, right eye | ||||
| −1 to −4 | 39 (68.4%) | 17 (85%) | 11 (52.4%) | 11 (68.8%) |
| −4.1 to −6 | 18 (31.6%) | 3 (15%) | 10 (47.6%) | 5 (31.3%) |
| Right eye baseline AL, median (IQR) | 25.1 (24.7–25.8) | 25.0 (24.3–25.5) | 25.1 (24.8–25.9) | 25.4 (24.7–26.3) |
| Axial length group, right eye | ||||
| <25 mm | 28 (49.1%) | 11 (55%) | 10 (47.6%) | 7 (43.8%) |
| >25 mm | 29 (50.9%) | 9 (45%) | 11 (52.4%) | 9 (56.3%) |
| Left eye baseline SER, median (IQR) | −3.3 (−4.1–−2.3) | −2.4 (−3.2–−2) | −3.5 (−4.6–−3.1) | −3.8 (−4.5–−2.9) |
| Left eye baseline AL, median (IQR) | 25.1 (24.7–25.6) | 24.9 (24.5–25.3) | 25.2 (24.8–25.7) | 25.2 (24.7–26.4) |
| RE | Total (n = 57) | SVL (n = 20) | HAPD (n = 21) | HAL (n = 16) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min−Max | Mean ± SD | Median (IQR) | Min−Max | Mean ± SD | Median (IQR) | Min−Max | Mean ± SD | Median (IQR) | Min−Max | Mean ± SD | Median (IQR) | |
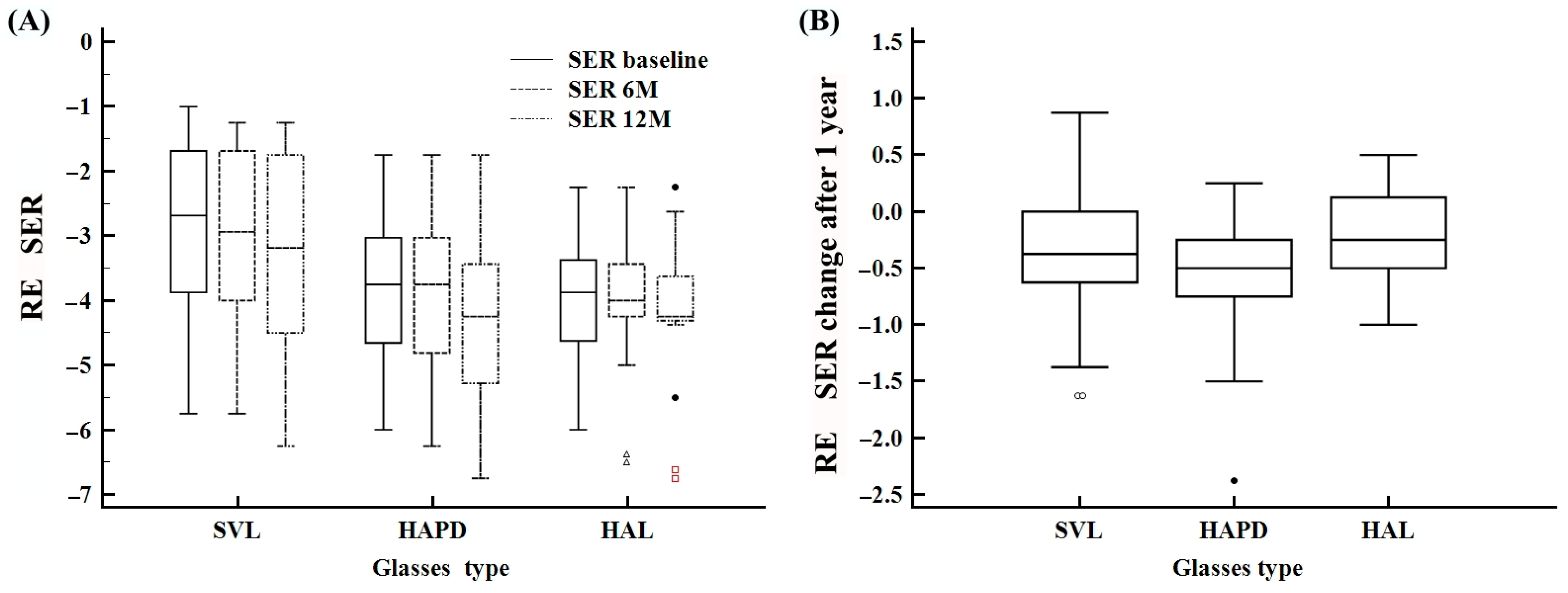
| SER baseline | −6.0–−1.0 | −3.5 ± 1.3 | −3.5 (−4.5–−2.5) | −5.7–−1.0 | −2.8 ± 1.3 | −2.6 (−3.8–−1.6) | −6.0–−1.7 | −3.7 ± 1.2 | −3.7 (−4.6–−3.0) | −6.0–−2.2 | −4.0 ± 1.1 | −3.8 (−4.6–−3.3) |
| SER 6 M | −6.5–−1.2 | −3.6 ± 1.3 | −3.7 (−4.3–−2.6) | −5.7–−1.2 | −2.9 ± 1.3 | −2.9 (−4.0–−1.6) | −6.2–−1.7 | −3.9 ± 1.3 | −3.7 (−4.8–−3.0) | −6.5–−2.2 | −4.0 ± 1.1 | −4.0 (−4.2–−3.4) |
| SER 12 M | −6.7–−1.2 | −3.9 ± 1.4 | −4.0 (−5.0–−2.9) | −6.2–−1.2 | −3.2 ± 1.5 | −3.1 (−4.5–−1.7) | −6.7–−1.7 | −4.2 ± 1.4 | −4.2 (−5.2–−3.4) | −6.7–−2.2 | −4.2 ± 1.2 | −4.2 (−4.3–−3.6) |
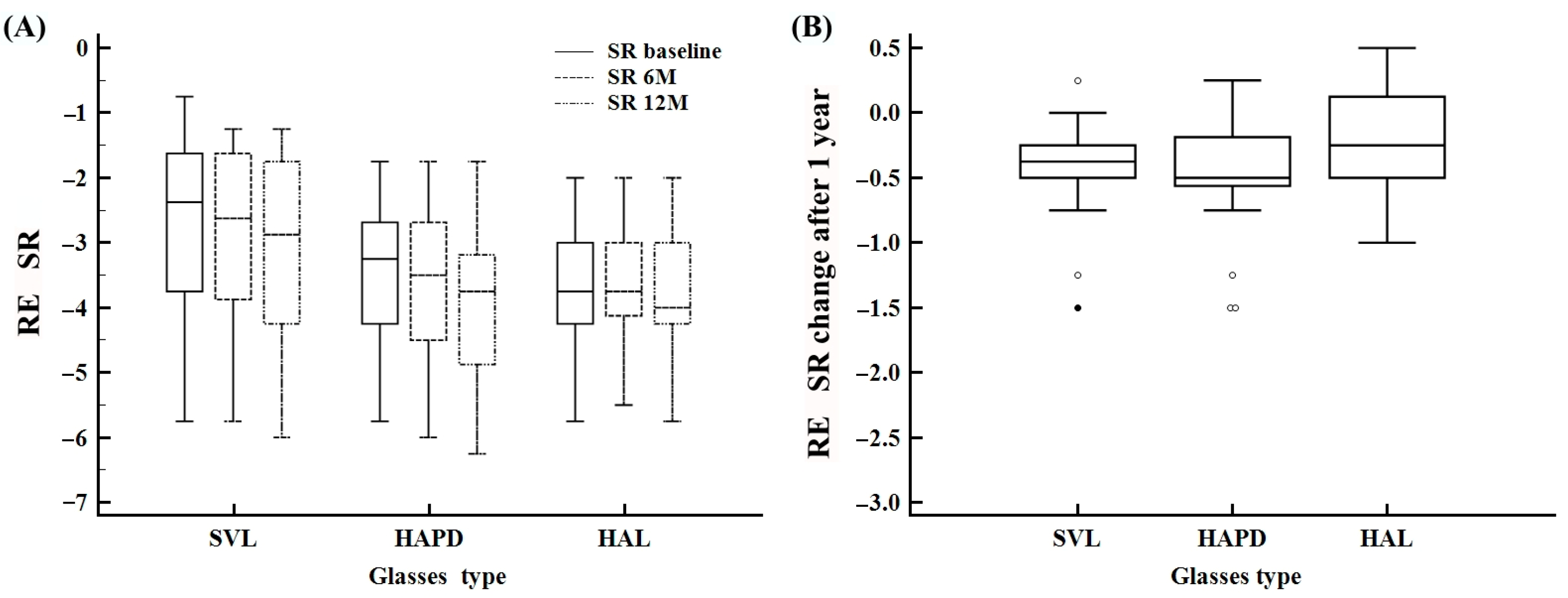
| SR baseline | −5.7–−0.7 | −3.2 ± 1.2 | −3.2 (−4.0–−2.1) | −5.7–−0.7 | −2.6 ± 1.3 | −2.3 (−3.7–−1.6) | −5.7–−1.7 | −3.4 ± 1.0 | −3.2 (−4.2–−2.6) | −5.7–−2.0 | −3.6 ± 1.0 | −3.7 (−4.2–−3.0) |
| SR 6 M sfera | −6.0–−1.2 | −3.3 ± 1.2 | −3.2 (−4.2–−2.5) | −5.7–−1.2 | −2.7 ± 1.3 | −2.6 (−3.8–−1.6) | −6.0–−1.7 | −3.6 ± 1.2 | −3.5 (−4.5–−2.6) | −5.5–−2.0 | −3.7 ± 1.0 | −3.7 (−4.1–−3.0) |
| SR 12 M sfera | −6.2–−1.2 | −3.6 ± 1.3 | −3.5 (−4.5–−2.6) | −6.0–−1.2 | −3.0 ± 1.4 | −2.8 (−4.2–−1.7) | −6.2–−1.7 | −3.9 ± 1.2 | −3.7 (−4.8–−3.1) | −5.7–−2.0 | −3.8 ± 1.1 | −4.0 (−4.2–−3.0) |
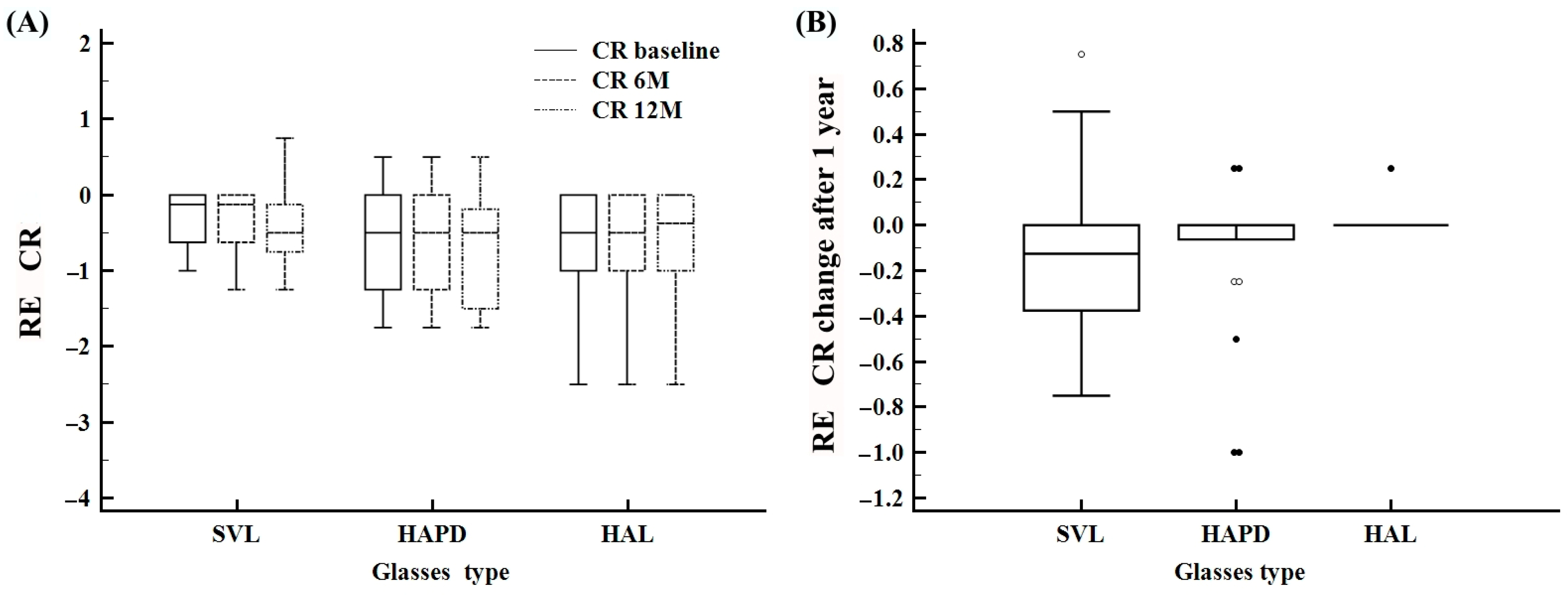
| CR baseline | −2.0–0.50 | −0.5 ± 0.5 | −0.5 (−0.8–0.0) | −1.0–0.0 | −0.3 ± 0.3 | −0.1 (−0.6–0.0) | −1.7–0.50 | −0.6 ± 0.6 | −0.5 (−1.2–0.0) | −2.0–0.0 | −0.5 ± 0.6 | −0.5 (−0.7–0.0) |
| CR 6 M cilindar | −2.0–0.50 | −0.5 ± 0.6 | −0.5 (−1.0–0.0) | −1.2–0.0 | −0.3 ± 0.4 | −0.1 (−0.6–0.0) | −1.7–0.50 | −0.6 ± 0.6 | −0.5 (−1.2–0.0) | −2.0–0.0 | −0.5 ± 0.6 | −0.5 (−0.6–0.0) |
| CR 12 M cilindar | −2.0–0.75 | −0.5 ± 0.6 | −0.5 (−1.0–0.0) | −1.2–0.75 | −0.4 ± 0.4 | −0.5 (−0.7–−0.1) | −1.7–0.50 | −0.7 ± 0.6 | −0.5 (−1.5–−0.1) | −2.0–0.0 | −0.5 ± 0.6 | −0.2 (−0.7–0.0) |
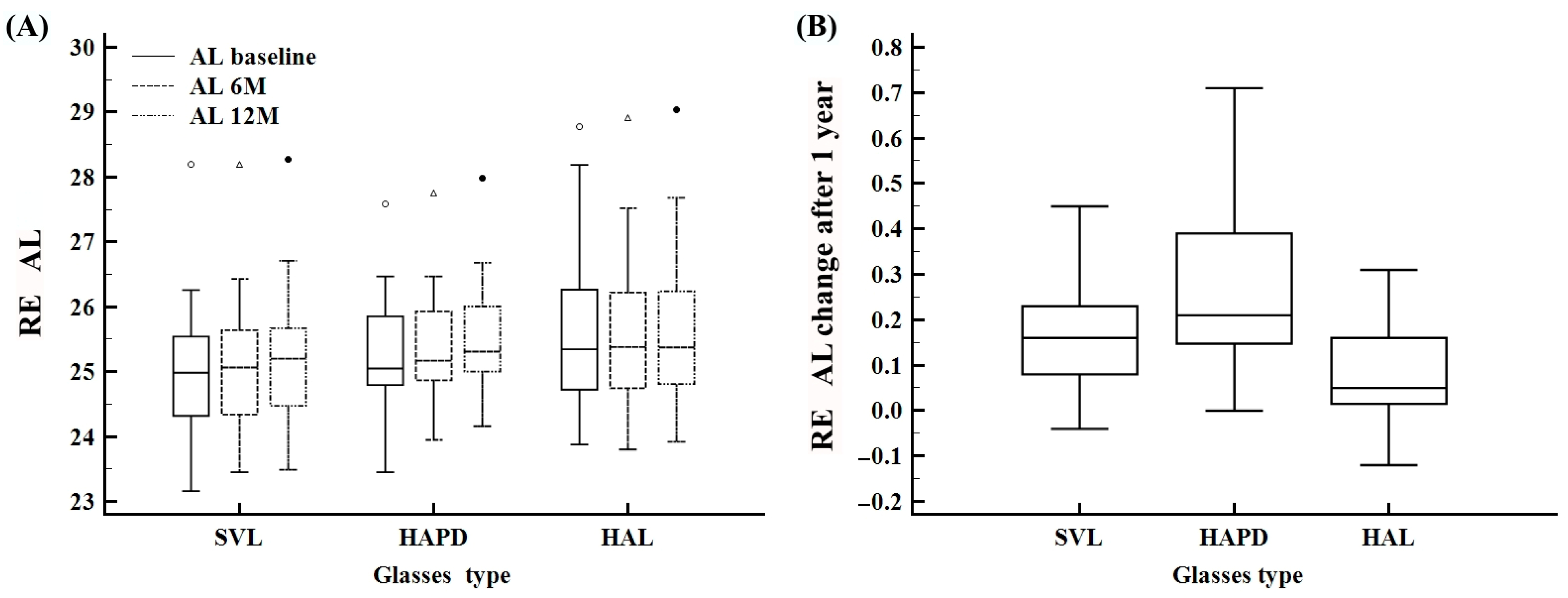
| AL baseline | 23.1–28.7 | 25.2 ± 1.1 | 25.0 (24.6–25.8) | 23.1–28.1 | 25.0 ± 1.0 | 24.9 (24.3–25.5) | 23.4–27.5 | 25.2 ± 0.9 | 25.0 (24.7–25.8) | 23.8–28.7 | 25.6 ± 1.3 | 25.3 (24.7–26.2) |
| AL 6 M | 23.4–28.9 | 25.3 ± 1.0 | 25.1 (24.7–25.8) | 23.4–28.1 | 25.1 ± 1.0 | 25.0 (24.3–25.6) | 23.9–27.7 | 25.4 ± 0.8 | 25.1 (24.8–25.9) | 23.8–28.9 | 25.5 ± 1.3 | 25.3 (24.7–26.2) |
| AL 12 M | 23.4–29.0 | 25.4 ± 1.0 | 25.2 (24.8–25.9) | 23.4–28.2 | 25.2 ± 1.0 | 25.2 (24.4–25.6) | 24.1–27.9 | 25.5 ± 0.8 | 25.3 (25.0–26.0) | 23.9–29.0 | 25.6 ± 1.3 | 25.3 (24.8–26.2) |
| SER difference 12 M | −1.6–0.50 | −0.4 ± 0.4 | −0.3 (−0.7–0.0) | −1.6–0.25 | −0.4 ± 0.4 | −0.4 (−0.7–−0.1) | −1.5–0.12 | −0.5 ± 0.4 | −0.5 (−0.7–−0.1) | −1.0–0.50 | −0.1 ± 0.4 | −0.3 (−0.5–0.12) |
| SR difference 12 M | −1.5–0.50 | −0.3 ± 0.4 | −0.2 (−0.5–0.0) | −1.5–0.25 | −0.4 ± 0.4 | −0.3 (−0.5–−0.2) | −1.5–0.25 | −0.4 ± 0.4 | −0.5 (−0.5–−0.1) | −1.0–0.50 | −0.2 ± 0.4 | −0.3 (−0.5–0.12) |
| CR difference 12 M | −1.0–0.75 | −0.0 ± 0.2 | 0.0 (−0.2–0.0) | −0.7–0.75 | −0.1 ± 0.3 | −0.1 (−0.3–0.0) | −1.0–0.25 | −0.1 ± 0.3 | 0.0 (−0.0–0.0) | 0.0–0.25 | 0.01 ± 0.0 | 0.0 (0.0–0.0) |
| AL difference 12 M | −0.1–0.71 | 0.17 ± 0.1 | 0.16 (0.05–0.26) | −0.0–0.45 | 0.17 ± 0.1 | 0.16 (0.08–0.23) | 0.0–0.71 | 0.25 ± 0.1 | 0.21 (0.14–0.39) | −0.1–0.31 | 0.07 ± 0.1 | 0.1 (0.0–0.2) |
| RE | SVL (n = 20) | HAPD (n = 21) | HAL (n = 16) | Total | Chi-Square |
|---|---|---|---|---|---|
| AL decrease | 2 (10%) | 0 (0%) | 4 (25%) | 6 (10.5%) | p = 0.0424 |
| AL increase | 7 (35%) | 11 (52.4%) | 2 (12.5%) | 20 (35.1%) | |
| Al stable | 11 (55%) | 10 (47.6%) | 10 (62.5%) | 31 (54.4%) | |
| Myopia improve | 5 (25%) | 5 (23.8%) | 7 (43.8%) | 17 (29.8%) | p = 0.5272 |
| Myopia increase | 7 (35%) | 9 (42.9%) | 3 (18.8%) | 19 (33.3%) | |
| Myopia stable | 8 (40%) | 7 (33.3%) | 6 (37.5%) | 21 (36.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orešković, I.; Malenica Ravlić, M.; Knežević, L.; Doko Mandić, B.; Marić, G.; Vukojević, A.; Zorić Geber, M.; Vatavuk, Z.; Sabol, I.; Škunca Herman, J. One-Year Comparative Evaluation of Highly Aspherical Lenslets and Horizontally Asymmetric Peripheral Defocus Lenses for Myopia Control in School-Aged Children. Life 2025, 15, 1119. https://doi.org/10.3390/life15071119
Orešković I, Malenica Ravlić M, Knežević L, Doko Mandić B, Marić G, Vukojević A, Zorić Geber M, Vatavuk Z, Sabol I, Škunca Herman J. One-Year Comparative Evaluation of Highly Aspherical Lenslets and Horizontally Asymmetric Peripheral Defocus Lenses for Myopia Control in School-Aged Children. Life. 2025; 15(7):1119. https://doi.org/10.3390/life15071119
Chicago/Turabian StyleOrešković, Ivana, Maja Malenica Ravlić, Lana Knežević, Blanka Doko Mandić, Goran Marić, Ante Vukojević, Mia Zorić Geber, Zoran Vatavuk, Ivan Sabol, and Jelena Škunca Herman. 2025. "One-Year Comparative Evaluation of Highly Aspherical Lenslets and Horizontally Asymmetric Peripheral Defocus Lenses for Myopia Control in School-Aged Children" Life 15, no. 7: 1119. https://doi.org/10.3390/life15071119
APA StyleOrešković, I., Malenica Ravlić, M., Knežević, L., Doko Mandić, B., Marić, G., Vukojević, A., Zorić Geber, M., Vatavuk, Z., Sabol, I., & Škunca Herman, J. (2025). One-Year Comparative Evaluation of Highly Aspherical Lenslets and Horizontally Asymmetric Peripheral Defocus Lenses for Myopia Control in School-Aged Children. Life, 15(7), 1119. https://doi.org/10.3390/life15071119






