Postbiotic Intervention in Sarcopenia: The Role of Lactiplantibacillus plantarum HY7715 and Its Extracellular Vesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Heat-Killed Probiotic
2.2. Preparation of HY7715-Derived EVs
2.3. Analysis of the EVs
2.4. Cell Culture and EVs
2.5. Western Blot Analysis
2.6. RNA Isolation, cDNA Synthesis, and Gene Expression Analysis
2.7. Mitochondrial Staining
2.8. Statistical Analysis
3. Results
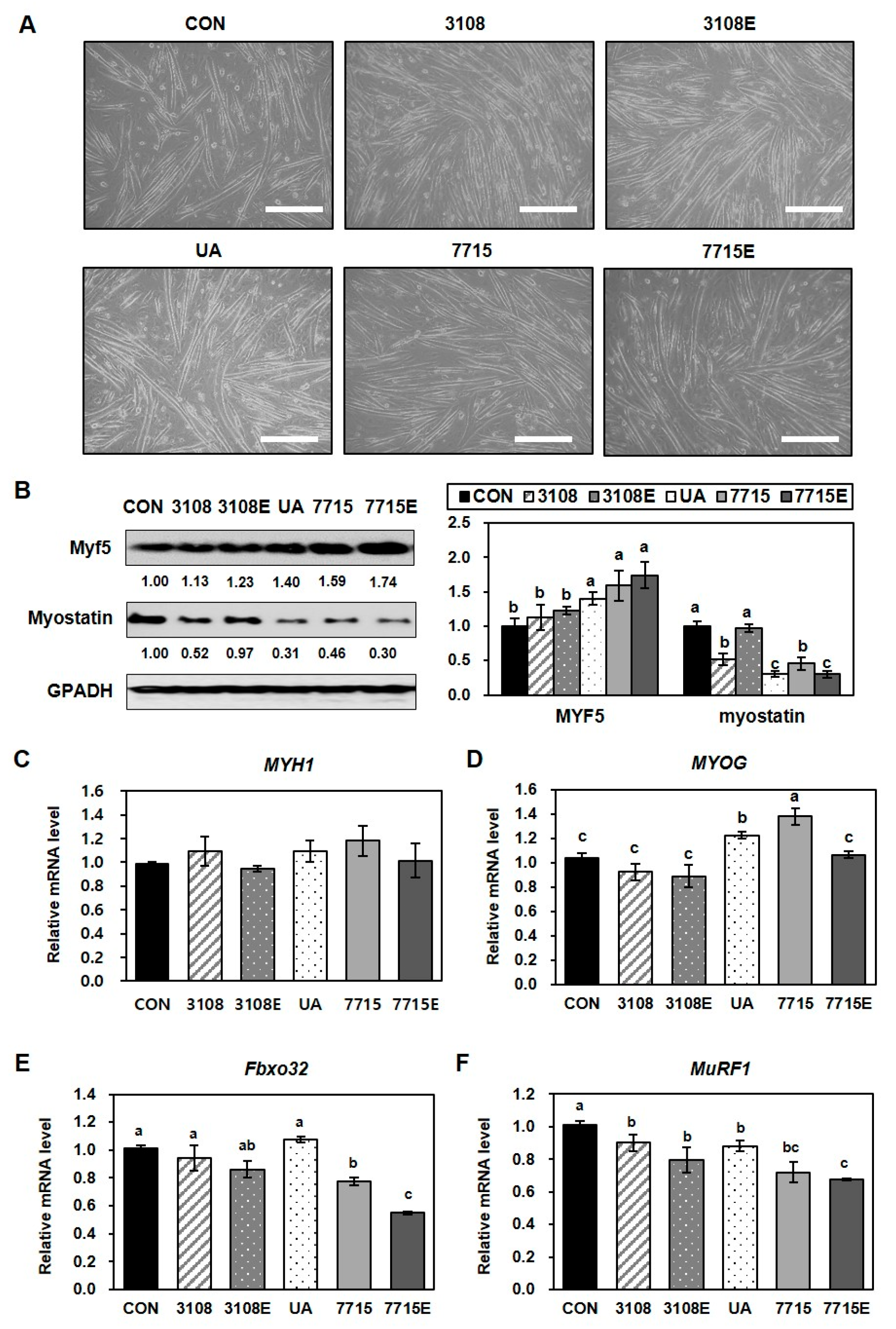
3.1. Analysis of Lactiplantibacillus plantarum HY7715-Derived EVs
3.2. Heat-Killed HY7715 Enhances Myoblast Differentiation and Suppresses Atrophy in C2C12 Myotubes
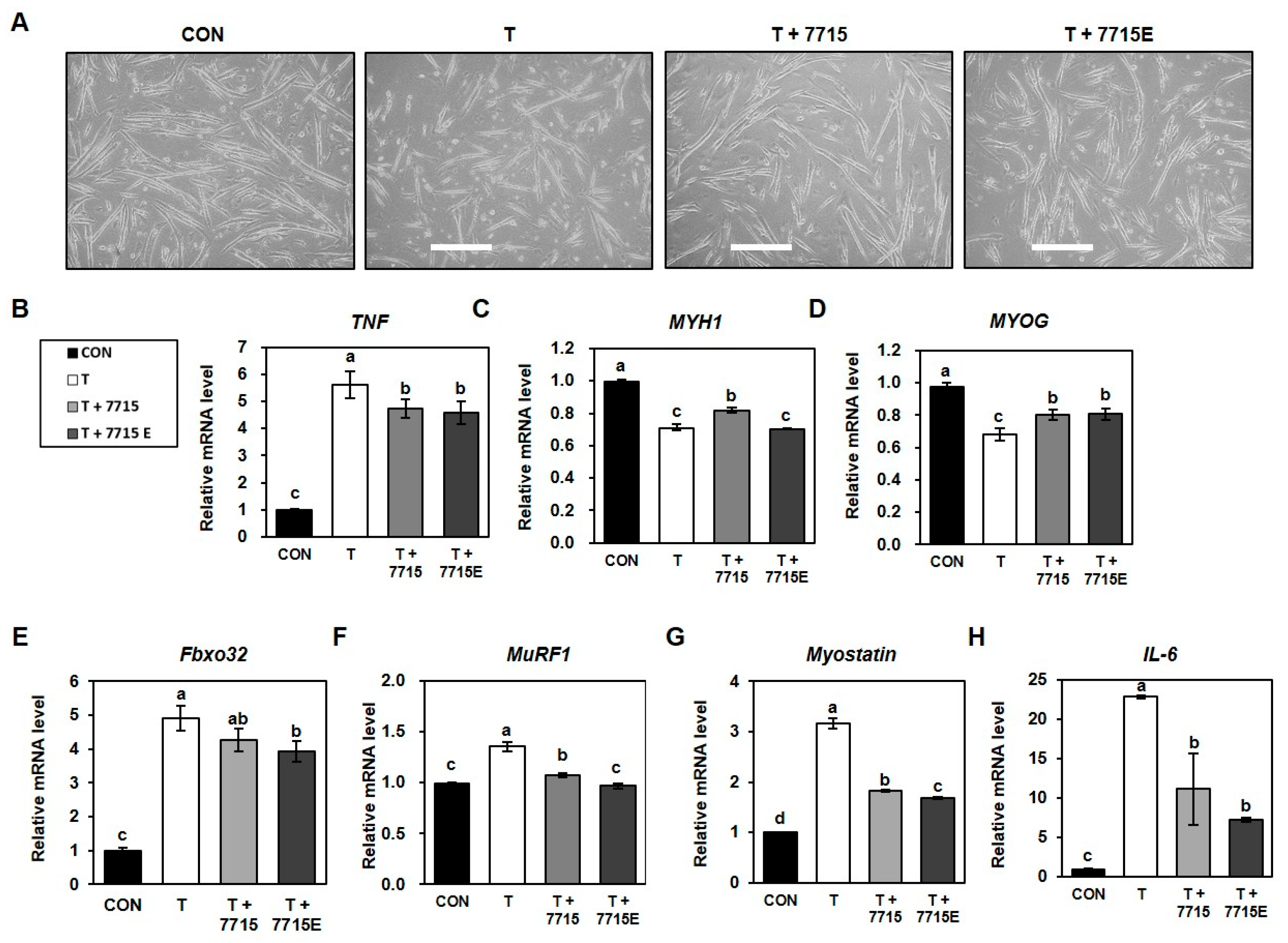
3.3. Heat-Killed HY7715 and HY7715-Derived EVs Suppress Protein Degradation and Promote Myotube Formation in TNFα-Treated C2C12 Cells
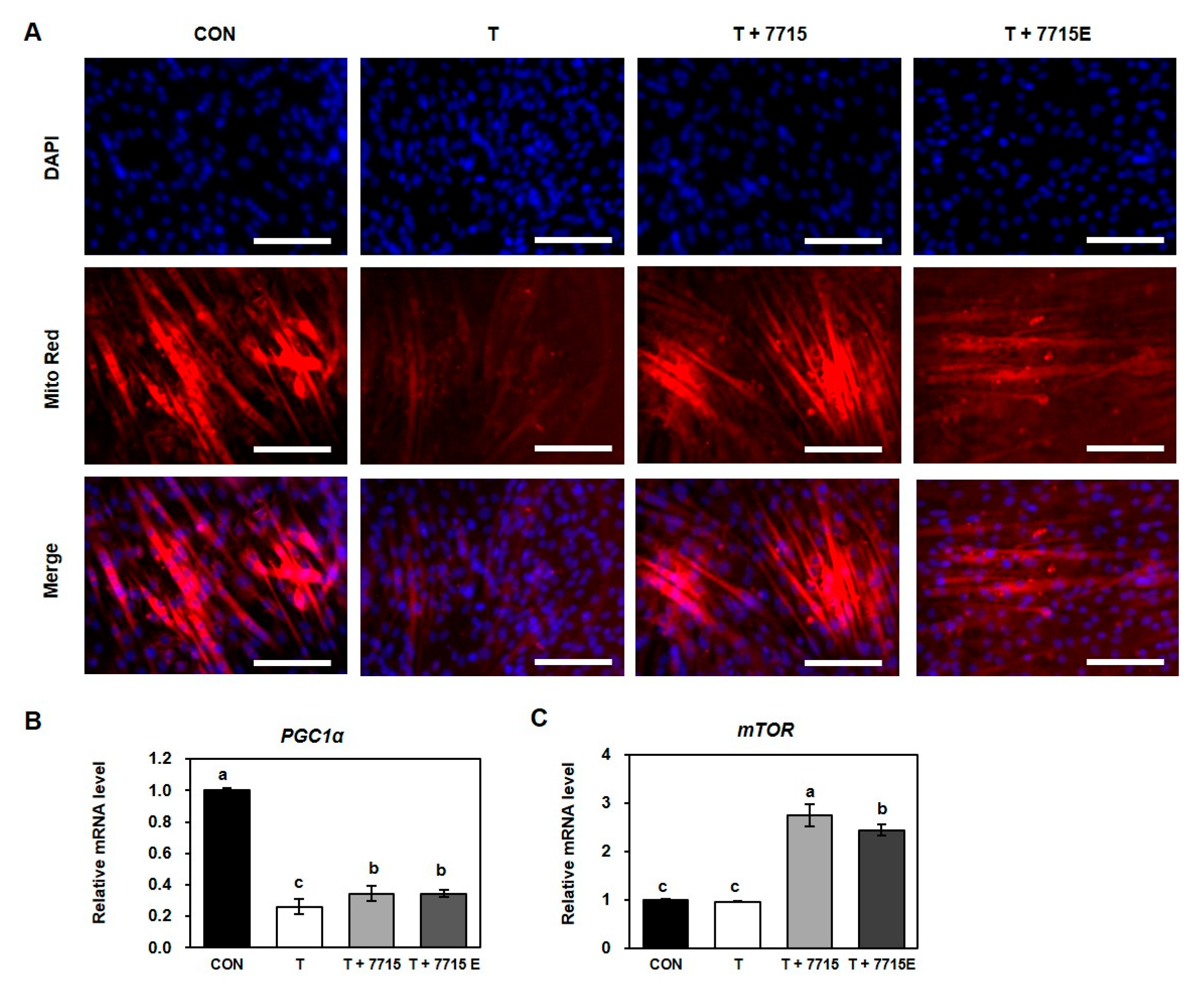
3.4. Heat-Killed HY7715 and HY7715-Derived EVs Increase Mitochondrial Biogenesis in TNFα-Treated C2C12 Myotubes
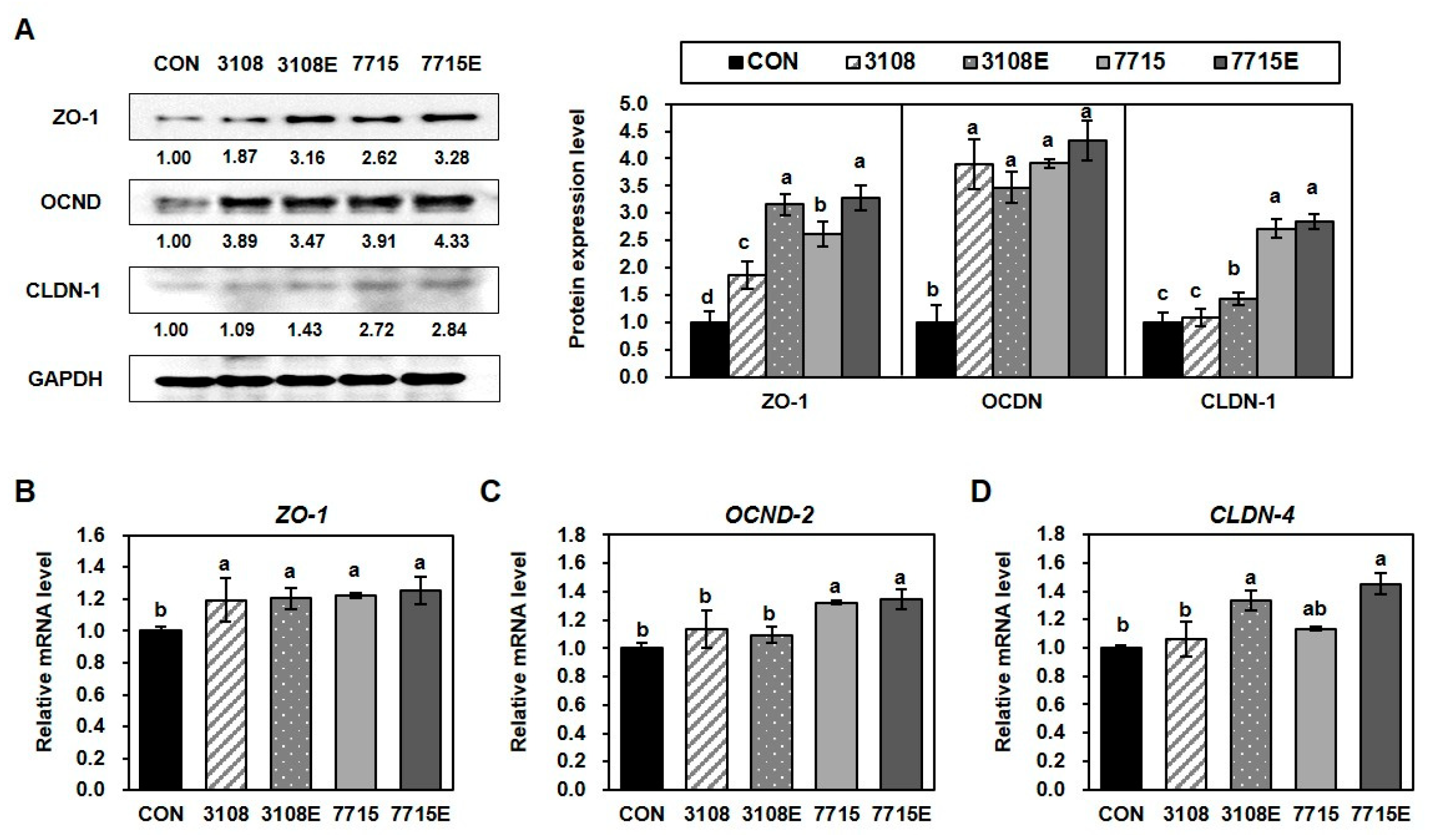
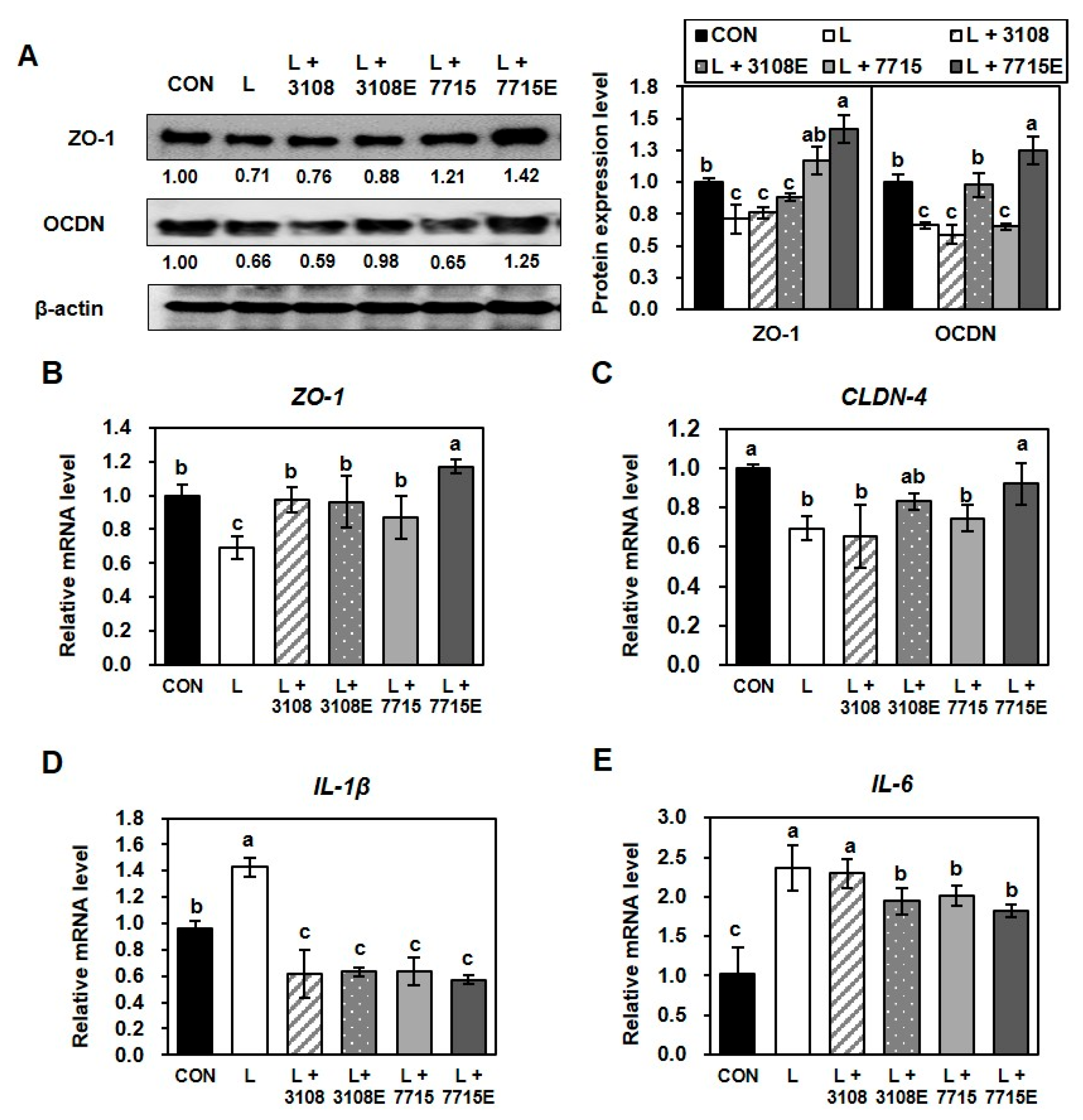
3.5. Heat-Killed HY7715 and HY7715-Derived EVs Improve the Tight Junctions Between Intestinal Cells
3.6. Heat-Killed HY7715 and HY7715-Derived EVs Ameliorate LPS-Induced Disruption of Tight Junctions in Intestinal Epithelial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HY7715 | Lactiplantibacillus plantarum HY7715 |
| EVs | Extracellular vesicles |
| LAB | Lactic Acid Bacteria |
| KCTC3108 | Lactiplantibacillus plantarum KCTC3108 |
| UA | Ursolic acid |
| PGC-1α | Peroxisome proliferator-activated receptor coactivator 1 alpha |
| mTOR | Mechanistic target of rapamycin |
| MYF5 | Myogenic factor 5 |
| MYH1 | Myosin heavy chain 1 |
| MYOG | Myogenin |
| Fbox32 | F-Box Protein 32, atrogin-1 |
| MuRF1 | Muscle ring-finger protein-1, TRIM63 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| TNFα | Tumor necrosis factor alpha |
| LPS | Lipopolysaccharide |
| ZO-1 | Human zonula occludens-1 |
| OCDN | Occludin |
| CLDN-1 | Claudin-1 |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
References
- Feng, L.T.; Chen, Z.N.; Bian, H. Skeletal muscle: Molecular structure, myogenesis, biological functions, and diseases. MedComm 2024, 5, e649. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Martins, J.P.; Carvalho, F.A.; Voituriez, R.; Abella, J.V.G.; Santos, N.C.; Cadot, B.; Way, M.; Gomes, E.R. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J.; Ryan, E.D.; Herda, T.J.; Costa, P.B.; Herda, A.A.; Cramer, J.T. Age-related changes in the rate of muscle activation and rapid force characteristics. Age 2014, 36, 839–849. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of age-related mitochondrial dysfunction in sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Fan, Y.-B.; Tao, X.-H.; Pan, W.-L.; Wu, Y.-X.; Wang, X.-H.; He, Y.-Q.; Xiao, W.-F.; Li, Y.-S. Mitochondrial quality control in sarcopenia: Updated overview of mechanisms and interventions. Aging Dis. 2021, 12, 2016–2030. [Google Scholar] [CrossRef]
- Kim, M.-J.; Sinam, I.S.; Siddique, Z.; Jeon, J.-H.; Lee, I.-K. The link between mitochondrial dysfunction and sarcopenia: An update focusing on the role of pyruvate dehydrogenase kinase 4. Diabetes Metab. J. 2023, 47, 153–163. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Yamada, T.; Ivarsson, N.; Hernández, A.; Fahlström, A.; Cheng, A.J.; Zhang, S.J.; Bruton, J.D.; Ulfhake, B.; Westerblad, H. Impaired mitochondrial respiration and decreased fatigue resistance followed by severe muscle weakness in skeletal muscle of mitochondrial DNA mutator mice. J. Physiol. 2012, 590, 6187–6197. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial quality control and muscle mass maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Grevendonk, L.; Connell, N.J.; McCrum, C.; Fealy, C.E.; Bilet, L.; Bruls, Y.M.H.; Mevenkamp, J.; Schrauwen-Hinderling, V.B.; Jörgensen, J.A.; Moonen-Kornips, E.; et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat. Commun. 2021, 12, 4773. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Chang, S.S.; Chang, H.Y.; Wu, C.H.; Pan, C.H.; Chang, C.C.; Chan, C.H.; Huang, H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut–muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 2022, 13, 515–531. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef]
- Park, J.E.; Lee, S.; Kim, K. The effect of combining nutrient intake and physical activity levels on central obesity, sarcopenia, and sarcopenic obesity: A population-based cross-sectional study in South Korea. BMC Geriatr. 2023, 23, 119. [Google Scholar] [CrossRef]
- Wang, L.; Meng, Q.; Su, C.-H. From Food Supplements to Functional Foods: Emerging Perspectives on Post-Exercise Recovery Nutrition. Nutrients 2024, 16, 4081. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, J.-K.; Hu, Y.-M. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res. Rev. 2022, 81, 101739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, M.; Yoo, J.; Lee, S.; Kang, M.; Yun, B.; Kim, J.N.; Moon, H.; Chung, Y.; Oh, S. Lactobacillus rhamnosus JY02 ameliorates sarcopenia by anti-atrophic effects in a dexamethasone-induced cellular and murine model. J. Microbiol. Biotechnol. 2023, 33, 915–925. [Google Scholar] [CrossRef]
- Lee, K.; Jin, H.; Chei, S.; Oh, H.-J.; Lee, J.-Y.; Lee, B.-Y. Effect of dietary silk peptide on obesity, hyperglycemia, and skeletal muscle regeneration in high-fat diet-fed mice. Cells 2020, 9, 377. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, E.-J.; Lee, J.-H.; Yoo, M.-S.; Heo, K.; Shim, J.-J.; Lee, J.-L. Probiotic potential of a novel vitamin B2-overproducing Lactobacillus plantarum strain, HY7715, isolated from Kimchi. Appl. Sci. 2021, 11, 5765. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Lactobacillus plantarum HY7715 ameliorates sarcopenia by improving skeletal muscle mass and function in aged Balb/c mice. Int. J. Mol. Sci. 2021, 22, 10023. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef] [PubMed]
- Franco, W. Postbiotics and parabiotics derived from bacteria and yeast: Current trends and future perspectives. CyTA-J. Food 2024, 22, 2425838. [Google Scholar] [CrossRef]
- You, C.-B.; Lee, E.-S.; Lee, M.-K.; Lee, G.-Y.; Park, H. Antioxidant and anti-inflammatory activities of heat-killed Lactiplantibacillus plantarum isolated from kimchi. Curr. Top. Lact. Acid Bact. Probiotics 2022, 8, 66–78. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakai, H.; Ohara, T.; Kawasaki, K.; Murosaki, S.; Hirose, Y. Beneficial effect of heat-killed Lactiplantibacillus plantarum L-137 on intestinal barrier function of rat small intestinal epithelial cells. Sci. Rep. 2024, 14, 12319. [Google Scholar] [CrossRef] [PubMed]
- Palomino, R.A.Ñ.; Vanpouille, C.; Costantini, P.E.; Margolis, L.; Oh, J. Microbiota–host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014, 47, 531–539. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Kubat, G.B.; Bouhamida, E.; Ulger, O.; Turkel, I.; Pedriali, G.; Ramaccini, D.; Ekinci, O.; Ozerklig, B.; Atalay, O.; Patergnani, S.; et al. Mitochondrial dysfunction and skeletal muscle atrophy: Causes, mechanisms, and treatment strategies. Mitochondrion 2023, 72, 33–58. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and biogenesis of lipopolysaccharides. Ecosal Plus 2018, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Ogawa, S.; Yakabe, M.; Akishita, M. Age-related sarcopenia and its pathophysiological bases. Inflamm. Regen. 2016, 36, 17. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Hur, K.Y.; Lee, M.-S. Gut microbiota and metabolic disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Krzyżek, P.; Marinacci, B.; Vitale, I.; Grande, R. Extracellular vesicles of probiotics: Shedding light on the biological activity and future applications. Pharmaceutics 2023, 15, 522. [Google Scholar] [CrossRef]
- Li, M.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Lactic acid bacteria derived extracellular vesicles: Emerging bioactive nanoparticles in modulating host health. Gut Microbes 2024, 16, 2427311. [Google Scholar] [CrossRef]
- Lee, K.; Gwon, H.; Kim, J.Y.; Shim, J.J.; Lee, J.H. Exosomes from Limosilactobacillus fermentum Ameliorate Benzalkonium Chloride-Induced Inflammation in Conjunctival Cells. Int. J. Mol. Sci. 2024, 25, 12282. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.; Sun, P.Y.; Davies, K.J.; Grune, T. Sarcopenia–Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulos, A.; Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Han, Y.; Hu, Y.; Geng, Z.; Su, J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics 2022, 12, 6576–6594. [Google Scholar] [CrossRef]
- Liu, C.; Yazdani, N.; Moran, C.S.; Salomon, C.; Seneviratne, C.J.; Ivanovski, S.; Han, P. Unveiling clinical applications of bacterial extracellular vesicles as natural nanomaterials in disease diagnosis and therapeutics. Acta Biomater. 2024, 180, 18–45. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Park, S.D.; Kim, J.Y.; Shim, J.J.; Lee, J.H. Postbiotic Intervention in Sarcopenia: The Role of Lactiplantibacillus plantarum HY7715 and Its Extracellular Vesicles. Life 2025, 15, 1101. https://doi.org/10.3390/life15071101
Lee K, Park SD, Kim JY, Shim JJ, Lee JH. Postbiotic Intervention in Sarcopenia: The Role of Lactiplantibacillus plantarum HY7715 and Its Extracellular Vesicles. Life. 2025; 15(7):1101. https://doi.org/10.3390/life15071101
Chicago/Turabian StyleLee, Kippeum, Soo Dong Park, Joo Yun Kim, Jae Jung Shim, and Jae Hwan Lee. 2025. "Postbiotic Intervention in Sarcopenia: The Role of Lactiplantibacillus plantarum HY7715 and Its Extracellular Vesicles" Life 15, no. 7: 1101. https://doi.org/10.3390/life15071101
APA StyleLee, K., Park, S. D., Kim, J. Y., Shim, J. J., & Lee, J. H. (2025). Postbiotic Intervention in Sarcopenia: The Role of Lactiplantibacillus plantarum HY7715 and Its Extracellular Vesicles. Life, 15(7), 1101. https://doi.org/10.3390/life15071101





