Novel Acylated Naringin Enhances Propionate Release and Stimulates the Growth of Flavanone-Metabolizing Bacteria in an In Vitro Batch Fermentation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. SCFA-Acylated Naringin Derivatives
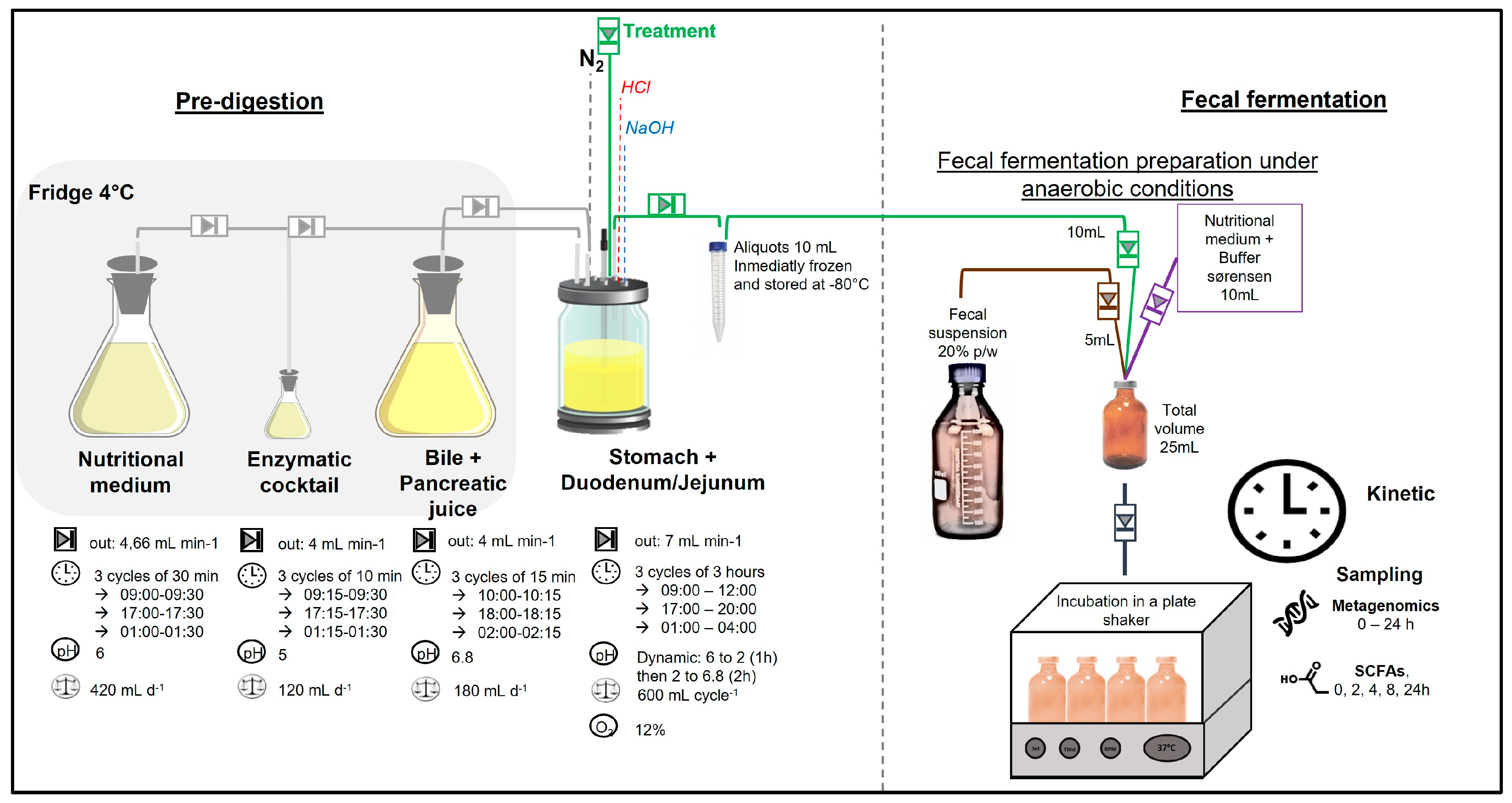
2.2. Fecal Fermentation
- Pre-digestion: To simulate gastric and intestinal phases, treatments were pre-digested in the stomach and small intestine of a simulated human intestinal model (SHIME®) as reported by Van de Wiele et al. [13]. Figure 2 illustrates the experimental setup, where the first vessel simulates the stomach and small intestine using a fill-and-draw method. This vessel incorporates a nutritional medium, pancreatic enzymes, and bile juice to mimic the digestive processes. Upon completion of the upper digestion phase, the vessel contents were aliquoted into 15 mL sterile tubes (10 mL) and stored at −80 °C until the fecal fermentation.
- Fecal fermentation: A fecal suspension was prepared by mixing fresh fecal matter from each donor individually with an anaerobic phosphate buffer at a 20% (w/v) concentration, as reported by Van den Abbeele et al. [14]. In duplicate, sterile 50 mL penicillin bottles were inoculated with 5 mL of the fecal solution, 10 mL of a nutritional medium containing Sørensen buffer, and 10 mL of pre-digested aliquots. All procedures were conducted under strict anaerobic conditions. The bottles were securely sealed and maintained at a temperature of 37 ± 2 °C while shaking at 80 rpm for a duration of 24 h. This in vitro fecal batch fermentation method was standardized and validated by Lessard, et al. [15,16], to assess interindividual variability and the capacity of the GM to metabolize (poly)phenols. Time 0 samples were collected using a sterile syringe immediately after sealing the bottles. A detailed sampling schedule is provided in the Supplementary Materials.
2.3. Microbial Population Analysis
2.4. Short-Chain Fatty Acids
2.5. Statistical Analysis
3. Results
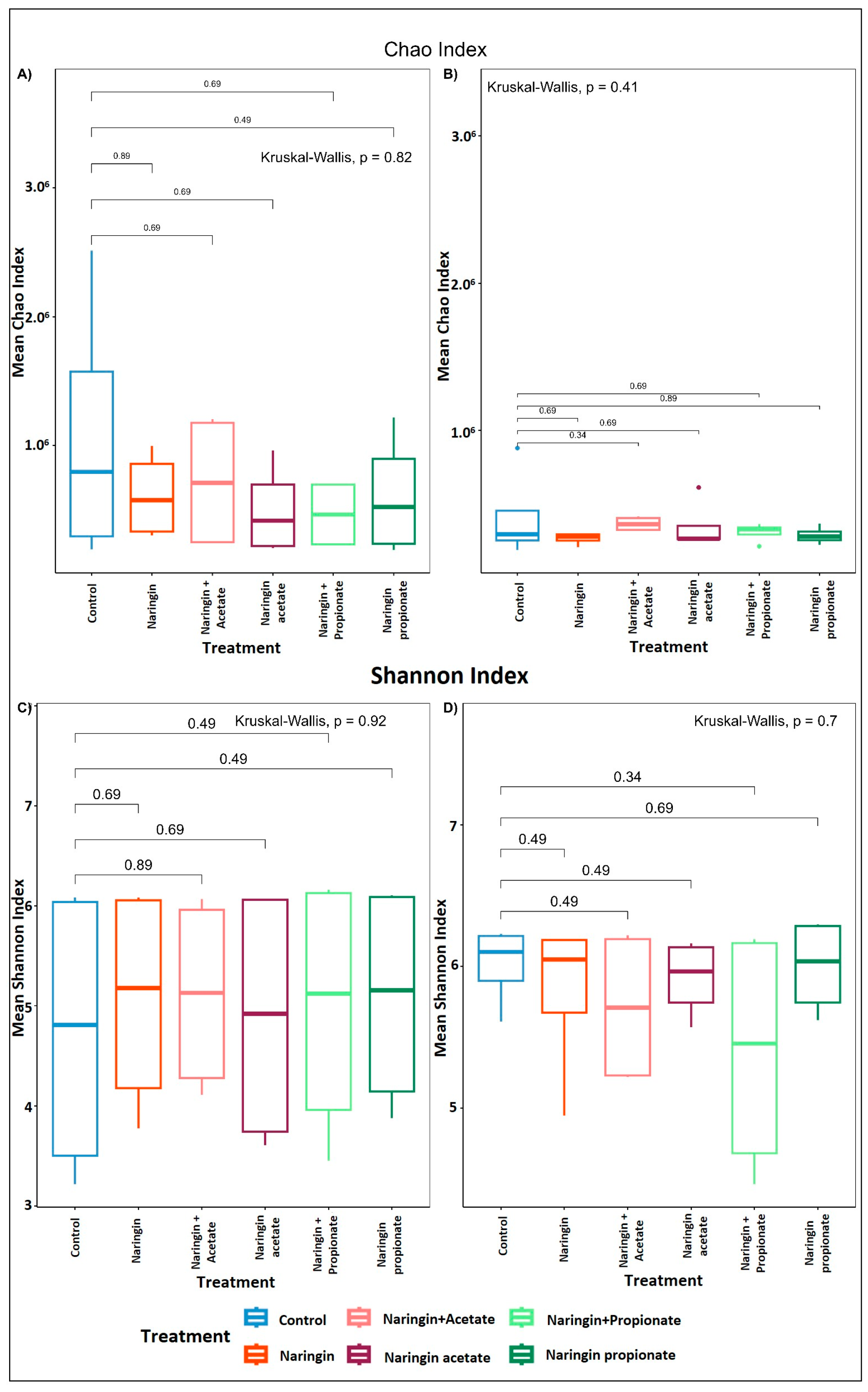
3.1. Donors’ Colonic Microbiota Composition and Their Variability
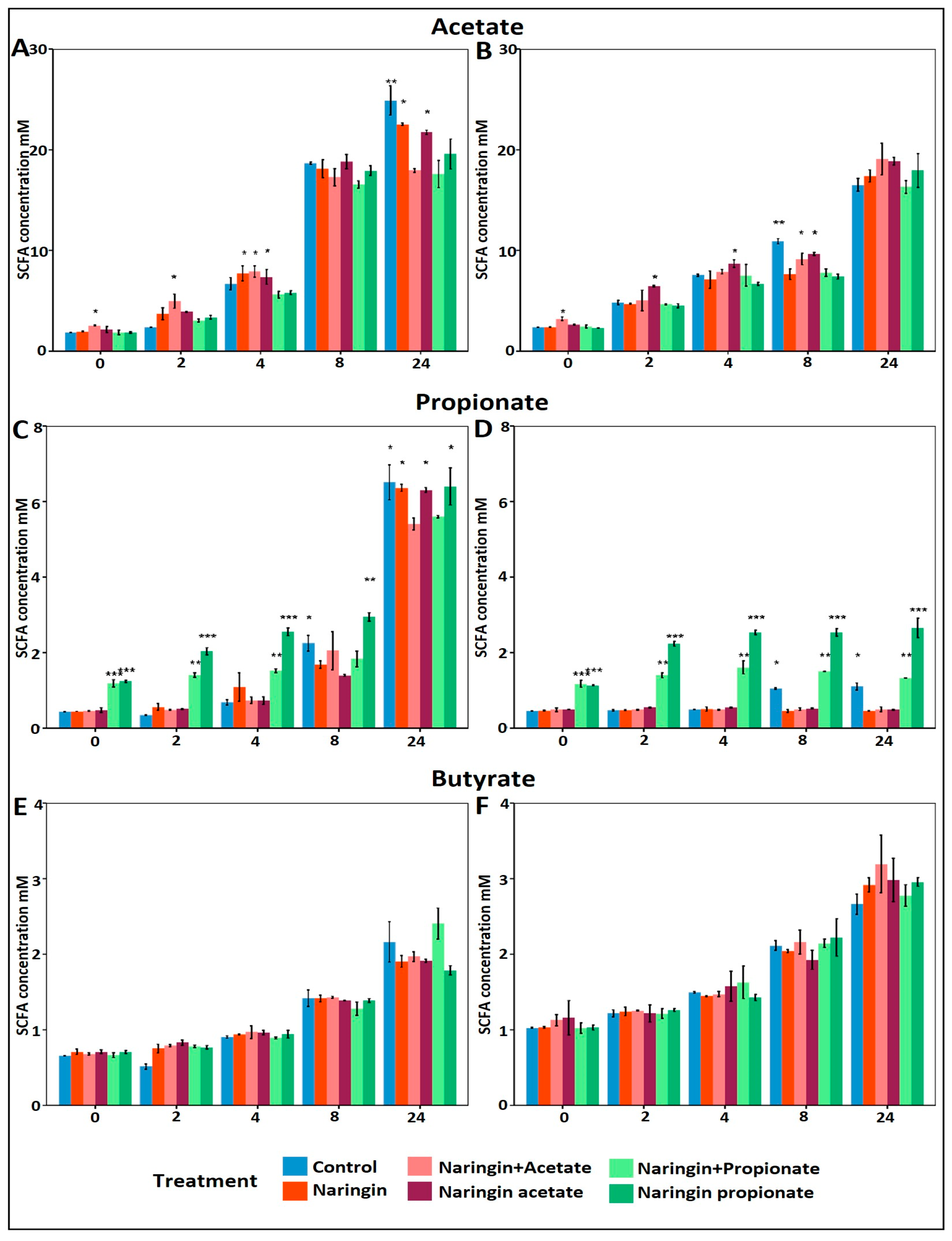
3.2. Increased Propionate Production with Naringin Propionate
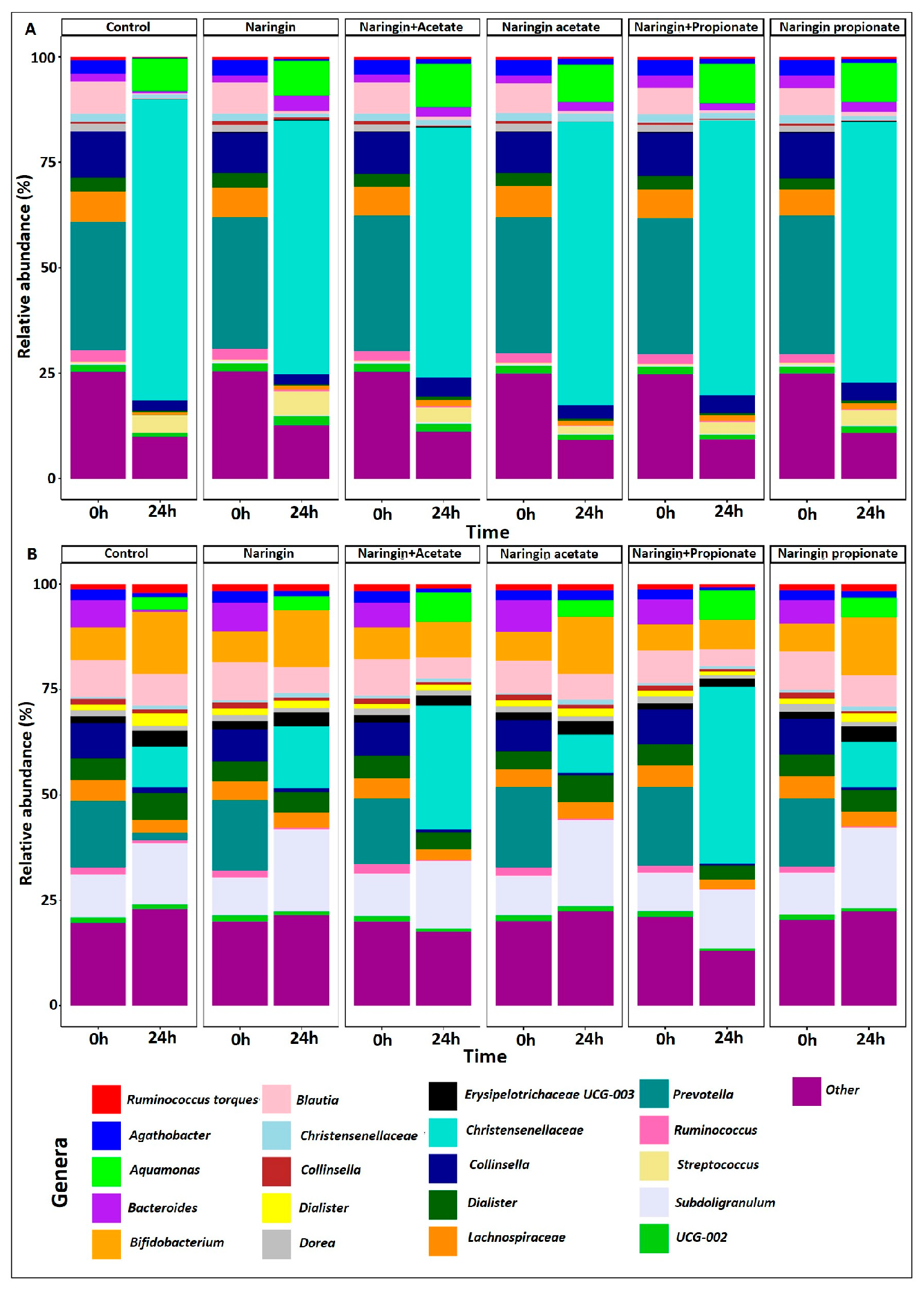
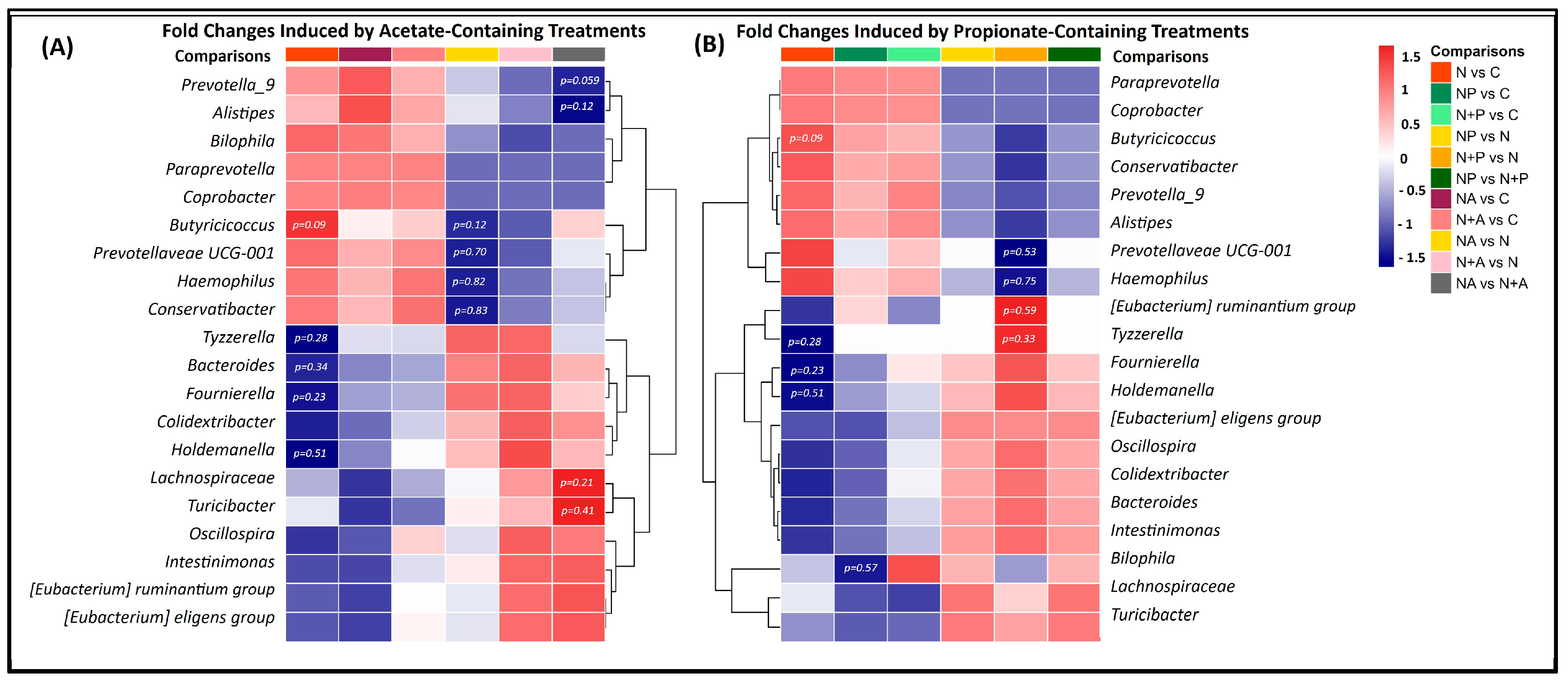
3.3. Distinct Microbial Shifts Induced by Naringin Treatments Highlight Limited but Specific Changes in GM Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NCDs | Non-communicable diseases |
| GM | Gut microbiota |
| SCFAs | Short-chain fatty acids |
| IBD | Inflammatory bowel disease |
| SHIME | Simulator of the Human Intestinal Microbial Ecosystem |
| g | Gram |
| M | Molar |
| mL | Milliliter |
| w/v | Weight per volume |
| °C | Degrees Celsius |
| rpm | Revolutions per minute |
| DNA | Deoxyribonucleic acid |
| ng | Nanogram |
| µL | Microliter |
| rRNA | Ribosomal ribonucleic acid |
| ASVs | Amplicon sequence variants |
| db-RDA | Distance-based redundancy analysis |
| DESeq | Differential expression analysis based on Negative Binomial distribution |
| Log | Logarithm |
| TCA | Tricarboxylic acid |
| RDA | ReDundandy Analysis |
| SD | Standard deviation |
References
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The Global Burden of Metabolic Disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The Gut Microbiota at the Intersection of Diet and Human Health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Roelofsen, H.; Priebe, M.; Weening, D.; Dijkstra, M.; Hoek, A.; Rezaee, F.; Venema, K.; Vonk, R.J. Regulation of Adipokine Production in Human Adipose Tissue by Propionic Acid. Eur. J. Clin. Investig. 2010, 40, 401–407. [Google Scholar] [CrossRef]
- Grüter, T.; Mohamad, N.; Rilke, N.; Blusch, A.; Sgodzai, M.; Demir, S.; Pedreiturria, X.; Lemhoefer, K.; Gisevius, B.; Haghikia, A.; et al. Propionate Exerts Neuroprotective and Neuroregenerative Effects in the Peripheral Nervous System. Proc. Natl. Acad. Sci. USA. 2023, 120, e2216941120. [Google Scholar] [CrossRef]
- Canani, R.B. Potential Beneficial Effects of Butyrate in Intestinal and Extraintestinal Diseases. World J. Gastroenterol. 2011, 17, 1519. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- De Preter, V.; Arijs, I.; Windey, K.; Vanhove, W.; Vermeire, S.; Schuit, F.; Rutgeerts, P.; Verbeke, K. Impaired Butyrate Oxidation in Ulcerative Colitis Is Due to Decreased Butyrate Uptake and a Defect in the Oxidation Pathway. Inflamm. Bowel Dis. 2012, 18, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. IJMS 2022, 23, 1105. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Gutiérrez-Navarro, E.; Padilla-de La Rosa, J.D.; Macías, A.; Solís, J.; Sandoval, G. Enzymatically Acylated Naringin with Gut Modulation Potential. Electron. J. Biotechnol. 2024, 68, 47–56. [Google Scholar] [CrossRef]
- Van de Wiele, T.; Van den Abbeele, P.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). In The Impact of Food Bioactives on Health; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Germany, 2015; pp. 305–317. ISBN 978-3-319-15791-7. [Google Scholar]
- Van den Abbeele, P.; Duysburgh, C.; Cleenwerck, I.; Albers, R.; Marzorati, M.; Mercenier, A. Consistent Prebiotic Effects of Carrot RG-I on the Gut Microbiota of Four Human Adult Donors in the SHIME® Model despite Baseline Individual Variability. Microorganisms 2021, 9, 2142. [Google Scholar] [CrossRef] [PubMed]
- Lessard-Lord, J.; Roussel, C.; Guay, V.; Desjardins, Y. Characterization of the Interindividual Variability Associated with the Microbial Metabolism of (−)-Epicatechin. J. Agric. Food Chem. 2023, 71, 13814–13827. [Google Scholar] [CrossRef] [PubMed]
- Lessard-Lord, J.; Roussel, C.; Guay, V.; Desjardins, Y. Assessing the Gut Microbiota’s Ability to Metabolize Oligomeric and Polymeric Flavan-3-ols from Aronia and Cranberry. Mol. Nutr. Food Res. 2024, 68, 2300641. [Google Scholar] [CrossRef]
- Cattero, V.; Roussel, C.; Lessard-Lord, J.; Roy, D.; Desjardins, Y. Supplementation with a Cranberry Extract Favors the Establishment of Butyrogenic Guilds in the Human Fermentation SHIME System. Microbiome Res. Rep. 2024, 3, 34. [Google Scholar] [CrossRef]
- Geirnaert, A.; Wang, J.; Tinck, M.; Steyaert, A.; Van den Abbeele, P.; Eeckhaut, V.; Vilchez-Vargas, R.; Falony, G.; Laukens, D.; De Vos, M.; et al. Interindividual Differences in Response to Treatment with Butyrate-Producing Butyricicoccus Pullicaecorum 25–3T Studied in an in Vitro Gut Model. FEMS Microbiol. Ecol. 2015, 91, fiv054. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Roussel, C.; Chabaud, S.; Lessard-Lord, J.; Cattero, V.; Pellerin, F.-A.; Feutry, P.; Bochard, V.; Bolduc, S.; Desjardins, Y. UPEC Colonic-Virulence and Urovirulence Are Blunted by Proanthocyanidins-Rich Cranberry Extract Microbial Metabolites in a Gut Model and a 3D Tissue-Engineered Urothelium. Microbiol. Spectr. 2022, 10, e02432-21. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M. False Discovery Rates: A New Deal. Biostat. 2016, 18, kxw041. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting Whole Grains for Refined Grains in a 6-Wk Randomized Trial Has a Modest Effect on Gut Microbiota and Immune and Inflammatory Markers of Healthy Adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef]
- Fechner, A.; Kiehntopf, M.; Jahreis, G. The Formation of Short-Chain Fatty Acids Is Positively Associated with the Blood Lipid–Lowering Effect of Lupin Kernel Fiber in Moderately Hypercholesterolemic Adults. J. Nutr. 2014, 144, 599–607. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Van Der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dilixiati, Y.; Xiao, L.; Yang, H.; Zhang, Z. Different Short-Chain Fatty Acids Unequally Modulate Intestinal Homeostasis and Reverse Obesity-Related Symptoms in Lead-Exposed High-Fat Diet Mice. J. Agric. Food Chem. 2024, 72, 18971–18985. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Chang, S.K.C.; Liao, J.-X.; Chen, Y.-W.; Huang, H.-T.; Li, Y.-L.; Hou, C.-Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Fievez, V.; De Buck, J.; Pasmans, F.; Martel, A.; Haesebrouck, F.; Ducatelle, R. Microencapsulated Short-Chain Fatty Acids in Feed Modify Colonization and Invasion Early After Infection with Salmonella Enteritidis in Young Chickens. Poult. Sci. 2004, 83, 69–74. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Cai, D.; Guo, X.; Tong, P.; Yin, F.; Liu, X.; Zhou, D. In Vitro Gastrointestinal Digestion and Microbial Hydrolysis of Hydroxytyrosol-SCFA and Tyrosol-SCFA Acyl Esters: Controlled-Release of SCFAs and Polyphenols. J. Agric. Food Chem. 2023, 71, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J. The Acetate Switch. Microbiol. Mol. Biol. Rev. 2005, 69, 12–50. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, J.; Cockburn, D.W. In Vitro Fermentation Reveals Changes in Butyrate Production Dependent on Resistant Starch Source and Microbiome Composition. Front. Microbiol. 2021, 12, 640253. [Google Scholar] [CrossRef]
- Arhan, P.; Devroede, G.; Jehannin, B.; Lanza, M.; Faverdin, C.; Dornic, C.; Persoz, B.; Tétreault, L.; Perey, B.; Pellerin, D. Segmental Colonic Transit Time. Dis. Colon Rectum 1981, 24, 625–629. [Google Scholar] [CrossRef]
- Tomita, R.; Igarashi, S.; Ikeda, T.; Jiang, K.; Sugito, J.; Sakurai, K.; Fujisaki, S.; Koshinaga, T.; Shibata, M. Study of Segmental Colonic Transit Time in Healthy Men. Hepatogastroenterology 2011, 58, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial Activity of Naringin Derivatives against Pathogenic Strains: Naringin Derivatives as Antibacterial Agents. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef]
- Celis, A.I.; Relman, D.A.; Huang, K.C. The Impact of Iron and Heme Availability on the Healthy Human Gut Microbiome in Vivo and in Vitro. Cell Chem. Biol. 2023, 30, 110–126.e3. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Khalili, M.; Margedari, A. Naringin Chelates Excessive Iron and Prevents the Formation of Amyloid-Beta Plaques in the Hippocampus of Iron-Overloaded Mice. Front. Pharmacol. 2021, 12, 651156. [Google Scholar] [CrossRef]
- Dostal, A.; Lacroix, C.; Bircher, L.; Pham, V.T.; Follador, R.; Zimmermann, M.B.; Chassard, C. Iron Modulates Butyrate Production by a Child Gut Microbiota In Vitro. mBio 2015, 6, e01453-15. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. Tuning Expectations to Reality: Don’t Expect Increased Gut Microbiota Diversity with Dietary Fiber. J. Nutr. 2023, 153, 3156–3163. [Google Scholar] [CrossRef] [PubMed]
- Zajac, D.J.; Shaw, B.C.; Braun, D.J.; Green, S.J.; Morganti, J.M.; Estus, S. Exogenous Short Chain Fatty Acid Effects in APP/PS1 Mice. Front. Neurosci. 2022, 16, 873549. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, J.; Lee, A.; Jo, S.V.; Park, C.H.; Han, D.S.; Eun, C.S. Impact of Short-Chain Fatty Acid Supplementation on Gut Inflammation and Microbiota Composition in a Murine Colitis Model. J. Nutr. Biochem. 2022, 101, 108926. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Saad, S.; Tillett, B.J.; McGuire, H.M.; Bordbar, S.; Yap, Y.A.; Nguyen, L.T.; Wilkins, M.R.; Corley, S.; Brodie, S.; et al. Metabolite-Based Dietary Supplementation in Human Type 1 Diabetes Is Associated with Microbiota and Immune Modulation. Microbiome 2022, 10, 9. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat Phenolic Compounds Regulate Metabolic Syndrome in High Fat Diet-Fed Mice via Gut Microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Cao, R.; Wu, X.; Guo, H.; Pan, X.; Huang, R.; Wang, G.; Liu, J. Naringin Exhibited Therapeutic Effects against DSS-Induced Mice Ulcerative Colitis in Intestinal Barrier–Dependent Manner. Molecules 2021, 26, 6604. [Google Scholar] [CrossRef] [PubMed]
- Van Rymenant, E.; Salden, B.; Voorspoels, S.; Jacobs, G.; Noten, B.; Pitart, J.; Possemiers, S.; Smagghe, G.; Grootaert, C.; Van Camp, J. A Critical Evaluation of In Vitro Hesperidin 2S Bioavailability in a Model Combining Luminal (Microbial) Digestion and Caco-2 Cell Absorption in Comparison to a Randomized Controlled Human Trial. Mol. Nutr. Food Res. 2018, 62, 1700881. [Google Scholar] [CrossRef]
- Fisher, C.K.; Mehta, P. Identifying Keystone Species in the Human Gut Microbiome from Metagenomic Timeseries Using Sparse Linear Regression. PLoS ONE 2014, 9, e102451. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and Related Species: The Keystone Taxa of the Human Gut Microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef]
- Russell, A.B.; Wexler, A.G.; Harding, B.N.; Whitney, J.C.; Bohn, A.J.; Goo, Y.A.; Tran, B.Q.; Barry, N.A.; Zheng, H.; Peterson, S.B.; et al. A Type VI Secretion-Related Pathway in Bacteroidetes Mediates Interbacterial Antagonism. Cell Host Microbe 2014, 16, 227–236. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Aakko, J.; Pietilä, S.; Toivonen, R.; Rokka, A.; Mokkala, K.; Laitinen, K.; Elo, L.; Hänninen, A. A Carbohydrate-Active Enzyme (CAZy) Profile Links Successful Metabolic Specialization of Prevotella to Its Abundance in Gut Microbiota. Sci. Rep. 2020, 10, 12411. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Xu, F.; Zhao, S.; Wu, X.; Wang, Y.; Xie, J. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-κB Axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, M.; Wang, Y.; Gong, S.; Yao, W.; Li, W.; Gao, H.; Wei, M. Puerarin Improves the Bone Micro-Environment to Inhibit OVX-Induced Osteoporosis via Modulating SCFAs Released by the Gut Microbiota and Repairing Intestinal Mucosal Integrity. Biomed. Pharmacother. 2020, 132, 110923. [Google Scholar] [CrossRef]
- Pan, L.; Ye, H.; Pi, X.; Liu, W.; Wang, Z.; Zhang, Y.; Zheng, J. Effects of Several Flavonoids on Human Gut Microbiota and Its Metabolism by in Vitro Simulated Fermentation. Front. Microbiol. 2023, 14, 1092729. [Google Scholar] [CrossRef]
- Harber, K.J.; De Goede, K.E.; Verberk, S.G.S.; Meinster, E.; De Vries, H.E.; Van Weeghel, M.; De Winther, M.P.J.; Van Den Bossche, J. Succinate Is an Inflammation-Induced Immunoregulatory Metabolite in Macrophages. Metabolites 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zuo, Q.; Hai, Y.; Sun, X.J. Lactulose: An Indirect Antioxidant Ameliorating Inflammatory Bowel Disease by Increasing Hydrogen Production. Med. Hypotheses 2011, 76, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wexler, A.G.; Goodman, A.L. An Insider’s Perspective: Bacteroides as a Window into the Microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kwon, Y.M.; Kim, I.-S.; Kim, J.-A.; Yu, D.-Y.; Adhikari, B.; Lee, S.-S.; Choi, I.-S.; Cho, K.-K. Effects of the Brown Seaweed Laminaria Japonica Supplementation on Serum Concentrations of IgG, Triglycerides, and Cholesterol, and Intestinal Microbiota Composition in Rats. Front. Nutr. 2018, 5, 23. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Dong, W.; Huang, K.; Ran, L.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of Long-Term Intake of Anthocyanins from Lycium Ruthenicum Murray on the Organism Health and Gut Microbiota in Vivo. Food Res. Int. 2020, 130, 108952. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut Microbiota-Derived Short Chain Fatty Acids Are Potential Mediators in Gut Inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted Distribution of the Butyrate Kinase Pathway among Butyrate-Producing Bacteria from the Human Colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The Potential Influence of Fruit Polyphenols on Colonic Microflora and Human Gut Health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.H.; Durand, G.; Khelaifia, S.; Armstrong, N.; Robert, C.; Cadoret, F.; Di Pinto, F.; Delerce, J.; Levasseur, A.; Raoult, D.; et al. Fournierella Massiliensis Gen. Nov., sp. Nov., a New Human-Associated Member of the Family Ruminococcaceae: The Microbiology Society Has Been Notified That This Paper Is Being Reviewed as Part of a “Scientific Misconduct Investigation” by the University of Aix-Marseille and the French Authorities. This Editor’s Note Is Issued until the Findings of That Investigation Are Published, and Any Further Action Will Be Agreed with the Microbiology Society’s Publishing Panel. Int. J. Syst. Evol. Microbiol. 2017, 67, 1393–1399. [Google Scholar] [CrossRef]
- Romaní-Pérez, M.; López-Almela, I.; Bullich-Vilarrubias, C.; Rueda-Ruzafa, L.; Gómez Del Pulgar, E.M.; Benítez-Páez, A.; Liebisch, G.; Lamas, J.A.; Sanz, Y. Holdemanella Biformis Improves Glucose Tolerance and Regulates GLP-1 Signaling in Obese Mice. FASEB J. 2021, 35, e21734. [Google Scholar] [CrossRef] [PubMed]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; Da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of Bacterial Genera Associated with Therapeutic Response to Immune Checkpoint PD-1 Blockade in a United States Cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Fosso, B.; Melocchi, L.; Nizzoli, G.; Troisi, J.; et al. Endogenous Murine Microbiota Member Faecalibaculum Rodentium and Its Human Homologue Protect from Intestinal Tumour Growth. Nat. Microbiol. 2020, 5, 511–524. [Google Scholar] [CrossRef]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut Microbiota Turicibacter Strains Differentially Modify Bile Acids and Host Lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Chen, L.-W.; Xu, J.; Soh, S.E.; Aris, I.M.; Tint, M.-T.; Gluckman, P.D.; Tan, K.H.; Shek, L.P.-C.; Chong, Y.-S.; Yap, F.; et al. Implication of Gut Microbiota in the Association between Infant Antibiotic Exposure and Childhood Obesity and Adiposity Accumulation. Int. J. Obes. 2020, 44, 1508–1520. [Google Scholar] [CrossRef]
- Chang, S.-C.; Shen, M.-H.; Liu, C.-Y.; Pu, C.-M.; Hu, J.-M.; Huang, C.-J. A Gut Butyrate-producing Bacterium Butyricicoccus Pullicaecorum Regulates Short-chain Fatty Acid Transporter and Receptor to Reduce the Progression of 1,2-dimethylhydrazine-associated Colorectal Cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Kingkaw, A.; Raethong, N.; Patumcharoenpol, P.; Suratannon, N.; Nakphaichit, M.; Keawsompong, S.; Roytrakul, S.; Vongsangnak, W. Analyzing Predominant Bacterial Species and Potential Short-Chain Fatty Acid-Associated Metabolic Routes in Human Gut Microbiome Using Integrative Metagenomics. Biology 2022, 12, 21. [Google Scholar] [CrossRef]
- Sohn, J.; Li, L.; Zhang, L.; Genco, R.J.; Falkner, K.L.; Tettelin, H.; Rowsam, A.M.; Smiraglia, D.J.; Novak, J.M.; Diaz, P.I.; et al. Periodontal Disease Is Associated with Increased Gut Colonization of Pathogenic Haemophilus Parainfluenzae in Patients with Crohn’s Disease. Cell Rep. 2023, 42, 112120. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.S.; Briggs, J.; Zhang, X.; Farnell, B.; Ndeh, D.; Labourel, A.; Baslé, A.; Cartmell, A.; Terrapon, N.; Stott, K.; et al. Dietary Pectic Glycans Are Degraded by Coordinated Enzyme Pathways in Human Colonic Bacteroides. Nat. Microbiol. 2017, 3, 210–219. [Google Scholar] [CrossRef]
- Chung, W.S.F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A.S.; Wells, J.M.; Flint, H.J.; Duncan, S.H. Prebiotic Potential of Pectin and Pectic Oligosaccharides to Promote Anti-Inflammatory Commensal Bacteria in the Human Colon. FEMS Microbiol. Ecol. 2017, 93, fix127. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Liu, S.; Zhao, L.; Pei, J. Screening β-Glucosidase and α-Rhamnosidase for Biotransformation of Naringin to Naringenin by the One-Pot Enzymatic Cascade. Enzym. Microb. Technol. 2023, 167, 110239. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Yousi, F.; Kainan, C.; Junnan, Z.; Chuanxing, X.; Lina, F.; Bangzhou, Z.; Jianlin, R.; Baishan, F. Evaluation of the Effects of Four Media on Human Intestinal Microbiota Culture in Vitro. AMB Expr. 2019, 9, 69. [Google Scholar] [CrossRef]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross Talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23, 8. [Google Scholar] [CrossRef]
- Venema, K.; Van Den Abbeele, P. Experimental Models of the Gut Microbiome. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole Grain Benefit: Synergistic Effect of Oat Phenolic Compounds and β-Glucan on Hyperlipidemia via Gut Microbiota in High-Fat-Diet Mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Goris, T.; Cuadrat, R.R.C.; Braune, A. Flavonoid-Modifying Capabilities of the Human Gut Microbiome—An In Silico Study. Nutrients 2021, 13, 2688. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of Dietary Flavonoid Glycosides by Strains of Intestinal Bacteroides from Humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K.; Osawa, T. Metabolism of Antioxidant in Lemon Fruit (Citrus limon BURM. f.) by Human Intestinal Bacteria. J. Agric. Food Chem. 1997, 45, 3738–3742. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Morishima, K.; Ishiguro-Watanabe, M. KEGG Pathway Database: Degradation of Flavonoids—Eubacterium Siraeum V10Sc8a. Available online: https://www.kegg.jp/pathway/map00946 (accessed on 29 January 2025).
- Schneider, H.; Blaut, M. Anaerobic Degradation of Flavonoids by Eubacterium Ramulus. Arch. Microbiol. 2000, 173, 71–75. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A Candidate for the next-Generation Probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, L.R.; Bryant, M.P. Eubacterium Oxidoreducens Sp. Nov. Requiring H2 or Formate to Degrade Gallate, Pyrogallol, Phloroglucinol and Quercetin. Arch. Microbiol. 1986, 144, 8–14. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the Rutinose-Conjugates Flavonoids Rutin and Hesperidin by the Gut Microbiota and Bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef]
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; Pascual-Teresa, S.D. Bioconversion of Anthocyanin Glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Bang, S.-H.; Hyun, Y.-J.; Shim, J.; Hong, S.-W.; Kim, D.-H. Metabolism of Rutin and Poncirin by Human Intestinal Microbiota and Cloning of Their Metabolizing α-L-Rhamnosidase from Bifidobacterium Dentium. J. Microbiol. Biotechnol. 2015, 25, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Garro, M.S.; Aguirre, L.; Savoy De Giori, G. Biological Activity of Bifidobacterium Longum in Response to Environmental pH. Appl. Microbiol. Biotechnol. 2006, 70, 612–617. [Google Scholar] [CrossRef]
- Marotti, I.; Bonetti, A.; Biavati, B.; Catizone, P.; Dinelli, G. Biotransformation of Common Bean ( Phaseolus vulgaris L.) Flavonoid Glycosides by Bifidobacterium Species from Human Intestinal Origin. J. Agric. Food Chem. 2007, 55, 3913–3919. [Google Scholar] [CrossRef]
- Breinig, F.; Diehl, B.; Rau, S.; Zimmer, C.; Schwab, H.; Schmitt, M.J. Cell Surface Expression of Bacterial Esterase A by Saccharomyces cerevisiae and Its Enhancement by Constitutive Activation of the Cellular Unfolded Protein Response. Appl. Environ. Microbiol. 2006, 72, 7140–7147. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.L.; Lindstad, L.J.; Westereng, B. Carbohydrate Esterases Involved in Deacetylation of Food Components by the Human Gut Microbiota. Essays Biochem. 2023, 67, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Calvete-Torre, I.; Sabater, C.; Muñoz-Almagro, N.; Campelo, A.B.; Moreno, F.J.; Margolles, A.; Ruiz, L. A Methyl Esterase from Bifidobacterium longum Subsp. Longum Reshapes the Prebiotic Properties of Apple Pectin by Triggering Differential Modulatory Capacity in Faecal Cultures. Microb. Biotechnol. 2024, 17, e14443. [Google Scholar] [CrossRef]
- Panda, T.; Gowrishankar, B.S. Production and Applications of Esterases. Appl. Microbiol. Biotechnol. 2005, 67, 160–169. [Google Scholar] [CrossRef]
- Rini, J.M.; Moremen, K.W.; Davis, B.G. Glycosyltransferases and Glycan-Processing Enzymes. In Essentials of Glycobiology; Cold Spring Harbor: New York, NY, USA, 2022. [Google Scholar]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. IJMS 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic Metabolites of Berry Polyphenols: The Missing Link to Biological Activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.S.; Niamah, A.K.; Ali, H.I. Survival and Viability of Limosilactobacillus reuteri Bacteria: A Comparative Study between Free and Microencapsulated Forms under Gastrointestinal and Thermal Stress Conditions. IOP Conf. Ser. Earth Environ. Sci. 2025, 1449, 012148. [Google Scholar] [CrossRef]
- Osborne, M.G.; Geiger, C.J.; Corzett, C.H.; Kram, K.E.; Finkel, S.E. Removal of Toxic Volatile Compounds in Batch Culture Prolongs Stationary Phase and Delays Death of Escherichia Coli. Appl. Environ. Microbiol. 2021, 87, e01860-21. [Google Scholar] [CrossRef]
- Pirkola, L.; Dicksved, J.; Loponen, J.; Marklinder, I.; Andersson, R. Fecal Microbiota Composition Affects in Vitro Fermentation of Rye, Oat, and Wheat Bread. Sci. Rep. 2023, 13, 99. [Google Scholar] [CrossRef] [PubMed]
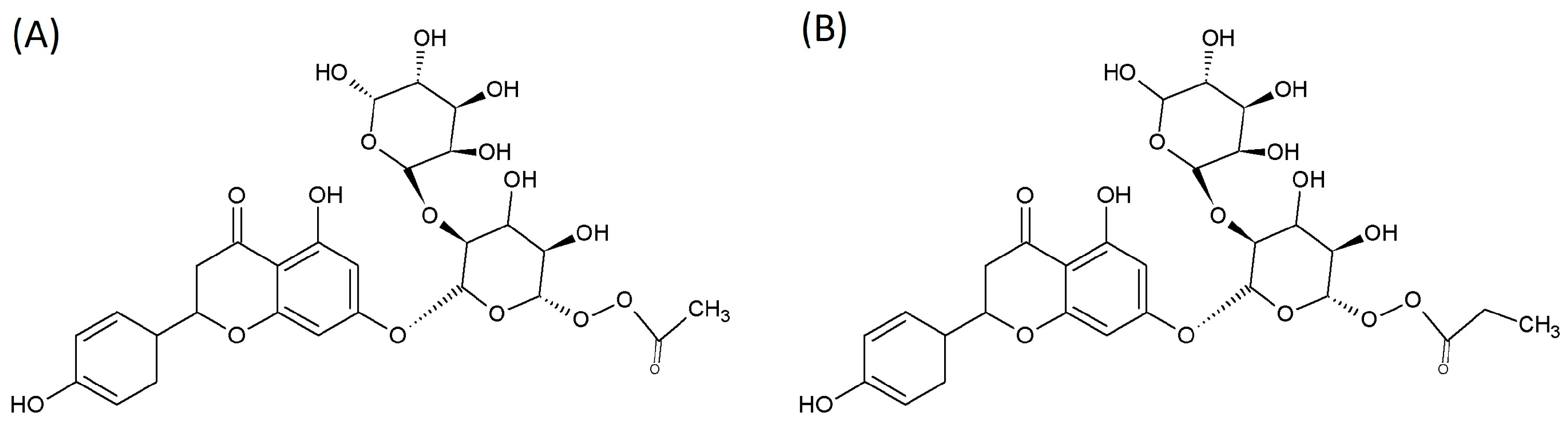

| Compound | Molecular Weight | %SCFAs | %Naringin | Naringin (g) | SCFAs (g/Mole) |
|---|---|---|---|---|---|
| Naringin acetate | 621.0 | 9.1 | 90.9 | 564 | 56.51 |
| Naringin propionate | 635 | 11.5 | 88.5 | 562 | 73.03 |
| Naringin | 580.5 | - | 100.0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Álvarez, B.E.; Padilla-de la Rosa, J.D.; González Avila, M.; Sandoval, G.; Desjardins, Y. Novel Acylated Naringin Enhances Propionate Release and Stimulates the Growth of Flavanone-Metabolizing Bacteria in an In Vitro Batch Fermentation Model. Life 2025, 15, 967. https://doi.org/10.3390/life15060967
Ruiz-Álvarez BE, Padilla-de la Rosa JD, González Avila M, Sandoval G, Desjardins Y. Novel Acylated Naringin Enhances Propionate Release and Stimulates the Growth of Flavanone-Metabolizing Bacteria in an In Vitro Batch Fermentation Model. Life. 2025; 15(6):967. https://doi.org/10.3390/life15060967
Chicago/Turabian StyleRuiz-Álvarez, Blanca Elizabeth, José Daniel Padilla-de la Rosa, Marisela González Avila, Georgina Sandoval, and Yves Desjardins. 2025. "Novel Acylated Naringin Enhances Propionate Release and Stimulates the Growth of Flavanone-Metabolizing Bacteria in an In Vitro Batch Fermentation Model" Life 15, no. 6: 967. https://doi.org/10.3390/life15060967
APA StyleRuiz-Álvarez, B. E., Padilla-de la Rosa, J. D., González Avila, M., Sandoval, G., & Desjardins, Y. (2025). Novel Acylated Naringin Enhances Propionate Release and Stimulates the Growth of Flavanone-Metabolizing Bacteria in an In Vitro Batch Fermentation Model. Life, 15(6), 967. https://doi.org/10.3390/life15060967






