Mycobacterium Ulcerans Ulcer: Current Trends in Antimicrobial Management and Reconstructive Surgical Strategies
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
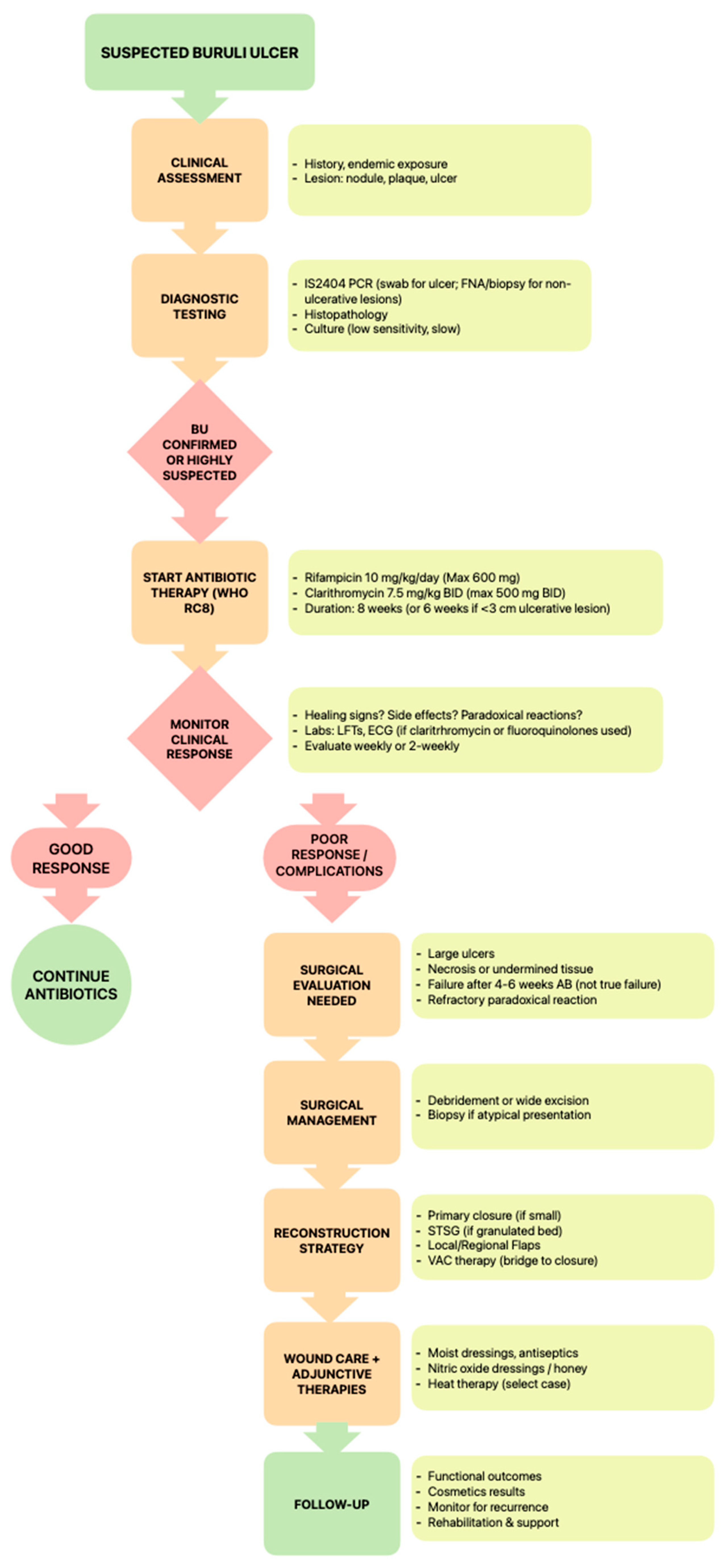
3.1. Antimicrobial Management
3.1.1. Current WHO-Recommended Regimens
3.1.2. Drug Resistance Concerns, Duration, and Compliance Challenges
3.1.3. Role of Topical Versus Systemic Agents
3.1.4. Emerging Therapies
4. Diagnostic Challenges
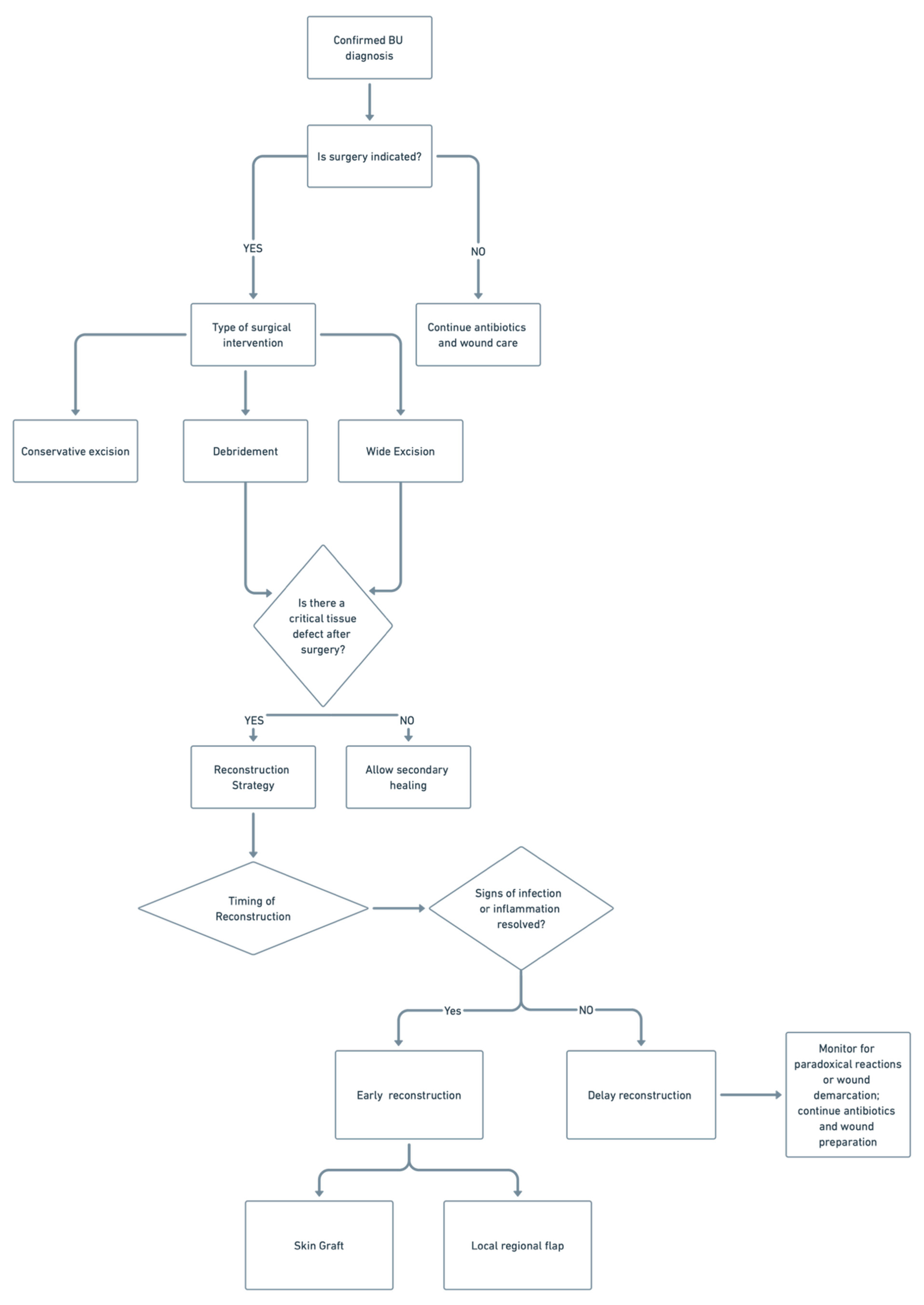
5. Surgical Management
5.1. Debridement and Excision Margins
5.2. Flap and Graft Option
5.3. Indications for Early Versus Delayed Reconstruction
6. Reconstructive Outcomes
6.1. Functional and Cosmetic Outcomes
6.2. Limb Preservation Versus Amputation in Advanced Cases
6.3. Role of Multidisciplinary Care
7. Pediatric Considerations
Special Approaches in Children (Common in Endemic Areas)
8. Research Gaps and Future Directions
8.1. Need for Randomized Controlled Trials on Antibiotic Duration
8.2. Better Guidelines for Surgery Timing
8.3. Vaccine Potential
8.4. Public Health Interventions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BU | Buruli Ulcer |
| NPWT | Negative Pressure Wound Therapy |
| FNA | Fine Needle Aspirate |
| PCR | Polymerase Chain Reaction |
| DNA | Deoxyribonucleic Acid |
| HBOT | Hyperbaric Oxygen Therapy |
| VAC | Vacuum Assisted Closure |
| RIF + STR | Rifampicin + Streptomycin |
| RIF + CLR | Rifampicin + Clarithromycin |
| RIF + CFZ | Rifampicin + Clofazimine |
References
- Röltgen, K.; Pluschke, G. Buruli Ulcer: History and Disease Burden. In Buruli Ulcer: Mycobacterium ulcerans Disease; Pluschke, G., Röltgen, K., Eds.; Springer: Cham, Switzerland, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553836/ (accessed on 12 June 2025). [CrossRef]
- Yotsu, R.R.; Suzuki, K.; Simmonds, R.E.; Bedimo, R.; Ablordey, A.; Yeboah-Manu, D.; Phillips, R.; Asiedu, K. Buruli Ulcer: A Review of the Current Knowledge. Curr. Trop. Med. Rep. 2018, 5, 247–256. [Google Scholar] [CrossRef]
- Muhi, S.; Cox, V.R.; O’BRien, M.; Priestley, J.T.; Hill, J.; Murrie, A.; McDonald, A.; Callan, P.; Jenkin, G.A.; Friedman, N.D.; et al. Management of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: Consensus statement. Med. J. Aust. 2025, 222, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.C.; Athan, E.; Friedman, N.D.; Hughes, A.; Walton, A.; Callan, P.; McDonald, A.; O’Brien, D.P. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med. J. Aust. 2012, 196, 341–344. [Google Scholar] [CrossRef]
- Wong, C.C.W.; Muhi, S.; O’Brien, D. An overview of Buruli ulcer in Australia. Aust. J. Gen. Pract. 2024, 53, 671–674. [Google Scholar] [CrossRef]
- Fenner, F. The significance of the incubation period in infectious diseases. Med. J. Aust. 1950, 2, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, P.; Tolhurst, J.C. A new mycobacterial infection in man. J. Pathol. Bacteriol. 1948, 60, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Global Buruli Ulcer Initiative. Buruli Ulcer (Mycobacterium ulcerans Infection). World Health Organization 2018. Available online: http://www.who.int/buruli/en/ (accessed on 30 April 2025).
- Tabah, E.N.; Johnson, C.R.; Degnonvi, H.; Pluschke, G.; Röltgen, K. Buruli Ulcer in Africa. 2019 Apr 30. In Buruli Ulcer: Mycobacterium Ulcerans Disease [Internet]; Pluschke, G., Röltgen, K., Eds.; Springer: Cham, Switzerland, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553829/ (accessed on 12 June 2025). [CrossRef]
- O’Brien, D.P.; Jeanne, I.; Blasdell, K.; Avumegah, M.; Athan, E. The changing epidemiology worldwide of Mycobacterium ulcerans. Epidemiol. Infect. 2018, 147, e19. [Google Scholar] [CrossRef]
- World Health Organization. Buruli Ulcer (Mycobacterium ulcerans Infection). WHO Fact Sheet. Updated 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection) (accessed on 29 April 2025).
- Coudereau, C.; Besnard, A.; Robbe-Saule, M.; Bris, C.; Kempf, M.; Johnson, R.C.; Brou, T.Y.; Gnimavo, R.; Eyangoh, S.; Khater, F.; et al. Stable and Local Reservoirs of Mycobacterium ulcerans Inferred from the Nonrandom Distribution of Bacterial Genotypes, Benin. Emerg. Infect. Dis. 2020, 26, 491–503. [Google Scholar] [CrossRef]
- Muleta, A.J.; Lappan, R.; Stinear, T.P.; Greening, C. Understanding the transmission of Mycobacterium ulcerans: A step towards controlling Buruli ulcer. PLoS Negl. Trop. Dis. 2021, 15, e0009678. [Google Scholar] [CrossRef]
- Victoria State Government Department of Health. Mycobacterium ulcerans Infection (Buruli Ulcer). Updated 18 October 2024. Available online: https://www.health.vic.gov.au/infectious-diseases/mycobacterium-ulcerans-infection (accessed on 29 April 2025).
- Vandelannoote, K.; Buultjens, A.H.; Porter, J.L.; Velink, A.; Wallace, J.R.; Blasdell, K.R.; Dunn, M.; Boyd, V.; Fyfe, J.A.; Tay, E.L.; et al. Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. eLife 2023, 12, e84983. [Google Scholar] [CrossRef]
- Hall, B.S.; Hill, K.; McKenna, M.; Ogbechi, J.; High, S.; Willis, A.E.; Simmonds, R.E.; Deretic, V. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014, 10, e1004061. [Google Scholar] [CrossRef] [PubMed]
- Demangel, C.; Stinear, T.; Cole, S. Buruli ulcer: Reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat. Rev. Microbiol. 2009, 7, 50–60. [Google Scholar] [CrossRef]
- Guenin-Macé, L.; Ruf, M.T.; Pluschke, G.; Demangel, C. Mycolactone: More than Just a Cytotoxin. In Buruli Ulcer: Mycobacterium ulcerans Disease; Pluschke, G., Röltgen, K., Eds.; Springer: Cham, Switzerland, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553838/ (accessed on 12 June 2025). [CrossRef]
- Foulon, M.; Robbe-Saule, M.; Manry, J.; Esnault, L.; Boucaud, Y.; Alcaïs, A.; Malloci, M.; D’aNdon, M.F.; Beauvais, T.; Labarriere, N.; et al. Mycolactone toxin induces an inflammatory response by targeting the IL-1β pathway: Mechanistic insight into Buruli ulcer pathophysiology. PLoS Pathog. 2020, 16, e1009107. [Google Scholar] [CrossRef]
- Strong, E.; Hart, B.; Wang, J.; Orozco, M.G.; Lee, S. Induced Synthesis of Mycolactone Restores the Pathogenesis of Mycobacterium ulcerans In Vitro and In Vivo. Front. Immunol. 2022, 13, 750643. [Google Scholar] [CrossRef] [PubMed]
- Akolgo, G.A.; Asiedu, K.B.; Amewu, R.K. Exploring Mycolactone—The Unique Causative Toxin of Buruli Ulcer: Biosynthetic, Synthetic Pathways, Biomarker for Diagnosis, and Therapeutic Potential. Toxins 2024, 16, 528. [Google Scholar] [CrossRef]
- Tello Rubio, B.; Bugault, F.; Baudon, B.; Raynal, B.; Brûlé, S.; Morel, J.-D.; Saint-Auret, S.; Blanchard, N.; Demangel, C.; Guenin-Macé, L. Molecular Mechanisms Underpinning the Circulation and Cellular Uptake of Mycobacterium ulcerans Toxin Mycolactone. Front. Pharmacol. 2021, 12, 733496. [Google Scholar] [CrossRef] [PubMed]
- Omansen, T.F.; van der Werf, T.S.; Phillips, R.O. Antimicrobial Treatment of Mycobacterium ulcerans Infection. In Buruli Ulcer: Mycobacterium ulcerans Disease; Pluschke, G., Röltgen, K., Eds.; Springer: Cham, Switzerland, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553822/ (accessed on 12 June 2025). [CrossRef]
- Van Der Werf, T.S.; Barogui, Y.T.; Converse, P.J.; Phillips, R.O.; Stienstra, Y. Pharmacologic management of Mycobacterium ulcerans infection. Expert Rev. Clin. Pharmacol. 2020, 13, 391–401. [Google Scholar] [CrossRef]
- Klis, S.; Stienstra, Y.; Phillips, R.O.; Abass, K.M.; Tuah, W.; van der Werf, T.S. Long term streptomycin toxicity in the treatment of Buruli Ulcer: Follow-up of participants in the BURULICO drug trial. PLoS Negl. Trop. Dis. 2014, 8, e2739. [Google Scholar] [CrossRef]
- O’bRien, D.P.; Hughes, A.J.; Cheng, A.C.; Henry, M.J.; Callan, P.; McDonald, A.; Holten, I.; Birrell, M.; Sowerby, J.M.; Johnson, P.D.R.; et al. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: Findings from a south-eastern Australian case series. Med. J. Aust. 2007, 186, 58–61. [Google Scholar] [CrossRef]
- Phillips, R.O.; Robert, J.; Abass, K.M.; Thompson, W.; Sarfo, F.S.; Wilson, T.; Sarpong, G.; Gateau, T.; Chauty, A.; Omollo, R.; et al. Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: A randomised, open-label, non-inferiority phase 3 trial. Lancet 2020, 395, 1259–1267. [Google Scholar] [CrossRef]
- Dhople, A.M.; Namba, K. In vitro activity of sitafloxacin (DU-6859a) alone, or in combination with rifampicin, against Mycobacterium ulcerans. J. Antimicrob. Chemother. 2002, 50, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, H.S.; Adjei, O.; Allen, B.W.; Portaels, F.; Evans, M.R.W.; Banerjee, D.K.; Wansbrough-Jones, M.H. In vitro activity of ciprofloxacin, sparfloxacin, ofloxacin, amikacin and rifampicin against Ghanaian isolates of Mycobacterium ulcerans. J. Antimicrob. Chemother. 2000, 45, 231–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bentoucha, A.; Robert, J.; Dega, H.; Lounis, N.; Jarlier, V.; Grosset, J. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 2001, 45, 3109–3112. [Google Scholar] [CrossRef]
- Ji, B.; Lefrançois, S.; Robert, J.; Chauffour, A.; Truffot, C.; Jarlier, V. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob. Agents Chemother. 2006, 50, 1921–1926. [Google Scholar] [CrossRef]
- Yan, A.; Bryant, E.E. Quinolones. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557777/ (accessed on 1 January 2025).
- Sansone, J.M.; Wilsman, N.J.; Leiferman, E.M.; Conway, J.; Hutson, P.; Noonan, K.J. The effect of fluoroquinolone antibiotics on growing cartilage in the lamb model. J. Pediatr. Orthop. 2009, 29, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Grady, R. Safety profile of quinolone antibiotics in the pediatric population. Pediatr. Infect. Dis. J. 2003, 22, 1128–1132. [Google Scholar] [CrossRef]
- O’Brien, D.P.; McDonald, A.; Callan, P.; Robson, M.; Friedman, N.D.; Hughes, A.; Holten, I.; Walton, A.; Athan, E.; Johnson, C. Successful outcomes with oral fluoroquinolones combined with rifampicin in the treatment of Mycobacterium ulcerans: An observational cohort study. PLoS Negl. Trop. Dis. 2012, 6, e1473. [Google Scholar] [CrossRef]
- Friedman, N.D.; Athan, E.; Walton, A.L.; O’brien, D.P. Increasing Experience with Primary Oral Medical Therapy for Mycobacterium ulcerans Disease in an Australian Cohort. Antimicrob. Agents Chemother. 2016, 60, 2692–2695. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Friedman, D.; Hughes, A.; Walton, A.; Athan, E. Antibiotic complications during the treatment of Mycobacterium ulcerans disease in Australian patients. Intern. Med. J. 2017, 47, 1011–1019. [Google Scholar] [CrossRef]
- Patel, P.H.; Hashmi, M.F. Macrolides. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551495/ (accessed on 1 January 2025).
- Vázquez-Laslop, N.; Mankin, A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018, 43, 668–684. [Google Scholar] [CrossRef]
- Portaels, F.; Traore, H.; De Ridder, K.; Meyers, W.M. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob. Agents Chemother. 1998, 42, 2070–2073. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dega, H.; Robert, J.; Bonnafous, P.; Jarlier, V.; Grosset, J. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 2000, 44, 2367–2372. [Google Scholar] [CrossRef]
- Dubuisson, T.; Bogatcheva, E.; Krishnan, M.Y.; Collins, M.T.; Einck, L.; Nacy, C.A.; Reddy, V.M. In vitro antimicrobial activities of capuramycin analogues against non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2010, 65, 2590–2597. [Google Scholar] [CrossRef]
- Rastogi, N.; Goh, K.S.; Berchel, M.; Bryskier, A. In vitro activities of the ketolides telithromycin (HMR 3647) and HMR 3004 compared to those of clarithromycin against slowly growing mycobacteria at pHs 6.8 and 7.4. Antimicrob. Agents Chemother. 2000, 44, 2848–2852. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Yotsu, R.; Nuremberger, E.; Srivastava, S. Repurposing drugs to advance the treatment of Buruli ulcer. Antimicrob. Agents Chemother. 2025, 69, e00029-25. [Google Scholar] [CrossRef]
- Ji, B.; Chauffour, A.; Robert, J.; Lefrançois, S.; Jarlier, V. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 2007, 51, 3737–3739. [Google Scholar] [CrossRef]
- Ji, B.; Chauffour, A.; Robert, J.; Jarlier, V. Bactericidal and sterilizing activities of several orally administered combined regimens against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 2008, 52, 1912–1916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chauffour, A.; Robert, J.; Veziris, N.; Aubry, A.; Jarlier, V. Sterilizing Activity of Fully Oral Intermittent Regimens against Mycobacterium Ulcerans Infection in Mice. PLoS Negl. Trop. Dis. 2016, 10, e0005066. [Google Scholar] [CrossRef]
- O’BRien, D.P.; Jenkin, G.; Buntine, J.; Steffen, C.M.; McDonald, A.; Horne, S.; Friedman, N.D.; Athan, E.; Hughes, A.; Callan, P.P.; et al. Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: Guideline update. Med. J. Aust. 2014, 200, 267–270. [Google Scholar] [CrossRef]
- Alffenaar, J.W.C.; Nienhuis, W.A.; de Velde, F.; Zuur, A.T.; Wessels, A.M.A.; Almeida, D.; Grosset, J.; Adjei, O.; Uges, D.R.A.; van der Werf, T.S. Pharmacokinetics of rifampin and clarithromycin in patients treated for Mycobacterium ulcerans infection. Antimicrob. Agents Chemother. 2010, 54, 3878–3883. [Google Scholar] [CrossRef]
- Christopher, K.; Hyatt, P.A.; Horkan, C.; Yodice, P.C. Clarithromycin use preceding fulminant hepatic failure. Am. J. Gastroenterol. 2002, 97, 489–490. [Google Scholar] [CrossRef]
- Shaheen, N.; Grimm, I.S. Fulminant hepatic failure associated with clarithromycin. Am. J. Gastroenterol. 1996, 91, 394–395. [Google Scholar]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352.e7. [Google Scholar] [CrossRef]
- Björnsson, E.S.; Bergmann, O.M.; Björnsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008, 135, 1924–1934.e19344. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.P.; Friedman, N.D.; Walton, A.; Hughes, A.; Athan, E. Risk Factors Associated with Antibiotic Treatment Failure of Buruli Ulcer. Antimicrob. Agents Chemother. 2020, 64, e00722-20. [Google Scholar] [CrossRef]
- Phillips, R.; Adjei, O.; Lucas, S.; Benjamin, N.; Wansbrough-Jones, M. Pilot randomized double-blind trial of treatment of Mycobacterium ulcerans disease (Buruli ulcer) with topical nitrogen oxides. Antimicrob. Agents Chemother. 2004, 48, 2866–2870. [Google Scholar] [CrossRef] [PubMed]
- Adjei, O.; Evans, M.R.; Asiedu, A. Phenytoin in the treatment of Buruli ulcer. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 108–109. [Google Scholar] [CrossRef]
- Reid, I.S. Mycobacterium ulcerans infection: A report of 13 cases at the Port Moresby General Hospital, Papua. Med. J. Aust. 1967, 1, 427–431. [Google Scholar] [CrossRef]
- Meyers, W.M.; Shelly, W.M.; Connor, D.H. Heat treatment of Mycobacterium ulcerans infections without surgical excision. Am. J. Trop. Med. Hyg. 1974, 23, 924–929. [Google Scholar] [CrossRef]
- Vogel, M.; Bayi, P.F.; Ruf, M.T.; Bratschi, M.W.; Bolz, M.; Um Boock, A.; Zwahlen, M.; Pluschke, G.; Junghanss, T. Local Heat Application for the Treatment of Buruli Ulcer: Results of a Phase II Open Label Single Center Non Comparative Clinical Trial. Clin. Infect. Dis. 2016, 62, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Yotsu, R.R.; Richardson, M.; Ishii, N. Drugs for treating Buruli ulcer (Mycobacterium ulcerans disease). Cochrane Database Syst. Rev. 2018, 8, CD012118. [Google Scholar] [CrossRef] [PubMed]
- Fehr, H.; Egger, M.; Senn, I. Cotrimoxazol in the treatment of Mycobacterium ulcerans infection (Buruli ulcer) in west Africa. Trop. Doct. 1994, 24, 61–63. [Google Scholar] [CrossRef]
- Dhople, A.M. Antimicrobial activities of dihydrofolate reductase inhibitors, used singly or in combination with dapsone, against Mycobacterium ulcerans. J. Antimicrob. Chemother. 2001, 47, 93–96. [Google Scholar] [CrossRef][Green Version]
- Espey, D.K.; Djomand, G.; Diomande, I.; Dosso, M.; Saki, M.Z.; Kanga, J.-M.; Spiegel, R.A.; Marston, B.J.; Gorelkin, L.; Meyers, W.M.; et al. A pilot study of treatment of Buruli ulcer with rifampin and dapsone. Int. J. Infect. Dis. 2002, 6, 60–65. [Google Scholar] [CrossRef]
- Converse, P.J.; Tyagi, S.; Xing, Y.; Li, S.-Y.; Kishi, Y.; Adamson, J.; Nuermberger, E.L.; Grosset, J.H.; Phillips, R.O. Efficacy of Rifampin Plus Clofazimine in a Murine Model of Mycobacterium ulcerans Disease. PLoS Negl. Trop. Dis. 2015, 9, e0003823. [Google Scholar] [CrossRef][Green Version]
- Revill, W.D.L.; Morrow, R.H.; Pike, M.C.; Ateng, J. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet 1973, 302, 873–877. [Google Scholar] [CrossRef]
- Almeida, D.V.; Converse, P.J.; Omansen, T.F.; Tyagi, S.; Tasneen, R.; Kim, J.; Nuermberger, E.L. Telacebec for Ultrashort Treatment of Buruli Ulcer in a Mouse Model. Antimicrob. Agents Chemother. 2020, 64, e00259-20. [Google Scholar] [CrossRef] [PubMed]
- Converse, P.J.; Almeida, D.V.; Tyagi, S.; Xu, J.; Nuermberger, E.L. Shortening Buruli ulcer treatment with combination therapy targeting the respiratory chain and exploiting Mycobacterium ulcerans gene decay. Antimicrob. Agents Chemother. 2019, 63, e00426-19. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Liu, J.; Tan, Y.; Liu, Z.; Chhotaray, C.; Jiang, H.; Lu, Z.; Chiwala, G.; Wang, S.; et al. The compound TB47 is highly bactericidal against Mycobacterium ulcerans in a Buruli ulcer mouse model. Nat. Commun. 2019, 10, 524. [Google Scholar] [CrossRef]
- Bolhuis, M.S.; Tiberi, S.; Sotgiu, G.; De Lorenzo, S.; Kosterink, J.G.; van der Werf, T.S.; Migliori, G.B.; Alffenaar, J.-W.C. Linezolid tolerability in multidrug-resistant tuberculosis: A retrospective study. Eur. Respir. J. 2015, 46, 1205–1207. [Google Scholar] [CrossRef] [PubMed]
- Thirot, H.; Briquet, C.; Frippiat, F.; Jacobs, F.; Holemans, X.; Henrard, S.; Tulkens, P.M.; Spinewine, A.; Van Bambeke, F. Clinical Use and Adverse Drug Reactions of Linezolid: A Retrospective Study in Four Belgian Hospital Centers. Antibiotics 2021, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Arenaz-Callao, M.P.; González Del Río, R.; Lucía Quintana, A.; Thompson, C.J.; Mendoza-Losana, A.; Ramón-García, S. Triple oral beta-lactam containing therapy for Buruli ulcer treatment shortening. PLoS Negl. Trop. Dis. 2019, 13, e0007126. [Google Scholar] [CrossRef]
- D’Agate, S.; Velickovic, P.; García-Barrios, N.; Ramón-García, S.; Della Pasqua, O. Optimizing β-lactam-containing antibiotic combination therapy for the treatment of Buruli ulcer. Br. J. Clin. Pharmacol. 2025, 91, 179–189. [Google Scholar] [CrossRef]
- Palomino, J.C.; Obiang, A.M.; Realini, L.; Meyers, W.M.; Portaels, F. Effect of oxygen on growth of Mycobacterium ulcerans in the BACTEC system. J. Clin. Microbiol. 1998, 36, 3420–3422. [Google Scholar] [CrossRef]
- Pszolla, N.; Sarkar, M.R.; Strecker, W.; Kern, P.; Kinzl, L.; Meyers, W.M.; Portaels, F. Buruli ulcer: A systemic disease. Clin. Infect. Dis. 2003, 37, e78–e82. [Google Scholar] [CrossRef]
- Krieg, R.E.; Wolcott, J.H.; Confer, A. Treatment of Mycobacterium ulcerans infection by hyperbaric oxygenation. Aviat. Space Environ. Med. 1975, 46, 1241–1245. [Google Scholar]
- Suzuki, K.; Luo, Y.; Miyamoto, Y.; Murase, C.; Mikami-Sugawara, M.; Yotsu, R.R.; Ishii, N. (Eds.) Buruli ulcer in Japan. In Buruli Ulcer; Springer: Cham, Switzerland, 2019; pp. 87–105. [Google Scholar] [CrossRef]
- Röltgen, K.; Cruz, I.; Ndung’u, J.M.; Pluschke, G. Laboratory diagnosis of Buruli ulcer: Challenges and future perspectives. In Buruli Ulcer; Pluschke, G., Röltgen, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 183–202. [Google Scholar] [CrossRef]
- Guarner, J.; Bartlett, J.; Whitney, E.A.S.; Raghunathan, P.L.; Stienstra, Y.; Asamoa, K.; Etuaful, S.; Klutse, E.; Quarshie, E.; van der Werf, T.S.; et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg. Infect. Dis. 2003, 9, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Osei-Owusu, J.; Aidoo, O.F.; Eshun, F.; Gaikpa, D.S.; Dofuor, A.K.; Vigbedor, B.Y.; Turkson, B.K.; Ochar, K.; Opata, J.; Opoku, M.J.; et al. Buruli ulcer in Africa: Geographical distribution, ecology, risk factors, diagnosis, and indigenous plant treatment options—A comprehensive review. Heliyon 2023, 9, e22018. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Globan, M.; Fyfe, J.M.; Lavender, C.J.; Murrie, A.; Flanagan, D.; Meggyesy, P.; Priestley, J.T.; Leslie, D. Diagnosis of Mycobacterium ulcerans disease: Be alert to the possibility of negative initial PCR results. Med. J. Aust. 2019, 210, 416. [Google Scholar] [CrossRef]
- Quek, T.Y.; Henry, M.J.; Pasco, J.A.; O’Brien, D.P.; Johnson, P.D.; Hughes, A.; Cheng, A.C.; Redden-Hoare, J.; Athan, E. Mycobacterium ulcerans infection: Factors influencing diagnostic delay. Med. J. Aust. 2007, 187, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Winburn, B.; Sharman, T. Atypical Mycobacterial Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556117/ (accessed on 1 January 2025).
- Phillips, R.O.; Sarfo, F.S.; Osei-Sarpong, F.; Boateng, A.; Tetteh, I.; Lartey, A.; Adentwe, E.; Opare, W.; Asiedu, K.B.; Wansbrough-Jones, M. Sensitivity of PCR targeting Mycobacterium ulcerans by use of fine-needle aspirates for diagnosis of Buruli ulcer. J. Clin. Microbiol. 2009, 47, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Herbinger, K.; Adjei, O.; Awua-Boateng, N.; Nienhuis, W.A.; Kunaa, L.; Siegmund, V.; Nitschke, J.; Thompson, W.; Klutse, E.; Agbenorku, P.; et al. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin. Infect. Dis. 2009, 48, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Li, H.; Hu, Q.; Zhang, Y.; Chen, S.-B.; Wang, C.; Zhou, X.-N.; Chen, J.-H.; Yin, K. A versatile microfluidic platform for malaria infection screening and Plasmodium species genotyping. eBioMedicine 2023, 98, 104898. [Google Scholar] [CrossRef]
- Obeagu, E.I.; Okoroiwu, G.I.; Ubosi, N.I.; Obeagu, G.U.B.N.; Onohuean, H.; Muhammad, T.; Adias, T.C. Revolution in malaria detection: Unveiling current breakthroughs and tomorrow’s possibilities in biomarker innovation. Ann. Med. Surg. 2024, 86, 5859–5876. [Google Scholar] [CrossRef]
- Aborode, A.T.; Adesola, R.O.; Scott, G.Y.; Arthur-Hayford, E.; Otorkpa, O.J.; Kwaku, S.D.; Elebesunu, E.E.; Nibokun, E.O.; Aruorivwooghene, I.J.; Bakre, A.A.; et al. Bringing lab to the field: Exploring innovations in point-of-care diagnostics for the rapid detection and management of tropical diseases in resource-limited settings. Adv. Biomark. Sci. Technol. 2025, 7, 28–43. [Google Scholar] [CrossRef]
- Heidt, B.; Siqueira, W.F.; Eersels, K.; Diliën, H.; van Grinsven, B.; Fujiwara, R.T.; Cleij, T.J. Point of Care Diagnostics in Resource-Limited Settings: A Review of the Present and Future of PoC in Its Most Needed Environment. Biosensors 2020, 10, 133. [Google Scholar] [CrossRef]
- Hengel, B.; Guy, R.J.; Casey, D.; Anderson, L.; Smith, K.; Andrewartha, K.; Applegate, T.D.; Saha, A.; Cunningham, P.; DeToca, L.; et al. Decentralised COVID-19 molecular point-of-care testing: Lessons from implementing a primary care-based network in remote Australian communities. Med. J. Aust. 2025, 222, 172–178. [Google Scholar] [CrossRef]
- O’BRien, D.P.; Callan, P.; Friedman, N.D.; Athan, E.; Hughes, A.; McDonald, A. Mycobacterium ulcerans disease management in Australian patients: The re-emergence of surgery as an important treatment modality. ANZ J. Surg. 2019, 89, 653–658. [Google Scholar] [CrossRef]
- Wadagni, A.C.; Barogui, Y.T.; Johnson, R.C.; Sopoh, G.E.; Affolabi, D.; van der Werf, T.S.; de Zeeuw, J.; Kleinnijenhuis, J.; Stienstra, Y. Delayed versus standard assessment for excision surgery in patients with Buruli ulcer in Benin: A randomised controlled trial. Lancet Infect. Dis. 2018, 18, 650–656. [Google Scholar] [CrossRef]
- WHO. Treatment of Mycobacterium ulcerans Disease (Buruli Ulcer): Guidance for Health Workers; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Johnson, P.D.R.; Stinear, T.; Small, P.L.C.; Pluschke, G.; Merritt, R.W.; Portaels, F.; Huygen, K.; Hayman, J.A.; Asiedu, K. Buruli ulcer (M. ulcerans infection): New insights, new hope for disease control. PLoS Med. 2005, 2, e108. [Google Scholar] [CrossRef] [PubMed]
- Adu, E.; Ampadu, E.; Acheampong, D. Surgical management of buruli ulcer disease: A four-year experience from four endemic districts in ghana. Ghana. Med. J. 2011, 45, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Elmasry, M.; El-Serafi, A.; Sjöberg, F.; Vuola, J.; Kankuri, E.; Grigoriadi, M.P.; Valtonen, J.; Abdelrahman, I.; Steinvall, I.; et al. A prospective dual-centre intra-individual controlled study for the treatment of burns comparing dermis graft with split-thickness skin autograft. Sci. Rep. 2022, 12, 21666. [Google Scholar] [CrossRef] [PubMed]
- Calonge, W.M.; Meredith, P.; Kouakou-Adonis, K.A.; Yao, C.K.; N’da Assie, K.M.; Asse, H. Reconstructive surgery for sequellae of Mycobacterium ulcerans infection (Buruli ulcer) of the upper limb. J. Plast. Surg. Hand Surg. 2021, 55, 339–344. [Google Scholar] [CrossRef]
- Ilori, O.S.; Ilori, O.R.; Alabi, A.O.; Adedokun, S.I. Challenges in the management of Buruli ulcer in Nigeria: A case report and literature review. Arch. Med. Health Sci. 2023, 11, 127–130. [Google Scholar] [CrossRef]
- Murase, C.; Kono, M.; Nakanaga, K.; Ishii, N.; Akiyama, M. Buruli ulcer successfully treated with negative-pressure wound therapy. JAMA Dermatol. 2015, 151, 1137–1139. [Google Scholar] [CrossRef]
- Loftus, M.J.; Kettleton-Butler, N.; Wade, D.; Whitby, R.M.; Johnson, P.D. A severe case of Mycobacterium ulcerans (Buruli ulcer) osteomyelitis requiring a below-knee amputation. Med. J. Aust. 2018, 208, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, F.; Rozen, W.; Seth, I.; Cuomo, R. Exploring the efficacy of negative pressure wound therapy in the management of Mycobacterium ulcerans wounds: A comprehensive literature review. Plast. Aesthet. Res. 2024, 11, 43. [Google Scholar] [CrossRef]
- Popa, G.L.; Muntean, A.A.; Popa, M.I. Recent Advances in the Management Strategies for Buruli Ulcers. Pathogens 2023, 12, 1088. [Google Scholar] [CrossRef]
- Beissner, M.; Arens, N.; Wiedemann, F.; Piten, E.; Kobara, B.; Bauer, M.; Herbinger, K.-H.; Badziklou, K.; Kere, A.B.; Löscher, T.; et al. Treatment Outcome of Patients with Buruli Ulcer Disease in Togo. PLoS Negl. Trop. Dis. 2015, 9, e0004170. [Google Scholar] [CrossRef]
- Uygur, F.; Duman, H.; Ulkür, E.; Celiköz, B. Are reverse flow fasciocutaneous flaps an appropriate option for the reconstruction of severe postburn lower extremity contractures? Ann. Plast. Surg. 2008, 61, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.N.; Weidemann, F.; O’Loughlin, P.F.; Krettek, C.; Gaulke, R. Mid- to Long-term Outcomes After Split-thickness Skin Graft vs. Skin Extension by Multiple Incisions. In Vivo 2019, 33, 453–464. [Google Scholar] [CrossRef]
- Sharma, M.; Wakure, A. Scar revision. Indian J. Plast. Surg. 2013, 46, 408–418. [Google Scholar] [CrossRef]
- Velink, A.; Woolley, R.J.; Phillips, R.O.; Abass, K.M.; van der Werf, T.S.; Agumah, E.; de Zeeuw, J.; Klis, S.; Stienstra, Y.; Vinetz, J.M. Former Buruli Ulcer Patients’ Experiences and Wishes May Serve as a Guide to Further Improve Buruli Ulcer Management. PLoS Negl. Trop. Dis. 2016, 10, e0005261. [Google Scholar] [CrossRef]
- Pommelet, V.; Vincent, Q.B.; Ardant, M.-F.; Adeye, A.; Tanase, A.; Tondeur, L.; Rega, A.; Landier, J.; Marion, E.; Alcaïs, A.; et al. Findings in patients from Benin with osteomyelitis and polymerase chain reaction–confirmed Mycobacterium ulcerans infection. Clin. Infect. Dis. 2014, 59, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Agbenorku, P.; Akpaloo, J. Mycobacterium ulcerans skin ulcers: Review of surgical management. Eur. J. Plast. Surg. 2001, 24, 188–191. [Google Scholar] [CrossRef]
- Atinga, A.G.; Anthony, A.; Janet, P.; Joseph, T.; Konama, K.N.; Albert, P.; Gladys, A.; Agyapomaa, C.A.; Kwarteng, D.S.; Richard, A. Integrated management strategies (diagnosis, treatment, and wound care management) for improved clinical outcomes of Buruli ulcer in Ghana: A retrospective case report in the Ga West Municipal Hospital, Amasaman. Clin. Med. Rev. Case Rep. 2022, 9, 379. [Google Scholar] [CrossRef]
- Tuwor, R.D.; Mtuy, T.B.; Amoako, Y.A.; Owusu, L.; Oppong, M.N.; Agbanyo, A.; Agbavor, B.; Marks, M.; Walker, S.L.; Yeboah-Manu, D.; et al. Stigma experiences, effects and coping among individuals affected by Buruli ulcer and yaws in Ghana. PLoS Negl. Trop. Dis. 2024, 18, e0012093. [Google Scholar] [CrossRef]
- Amoako, Y.A.; Ackam, N.; Omuojine, J.-P.; Oppong, M.N.; Owusu-Ansah, A.G.; Boateng, H.; Abass, M.K.; Amofa, G.; Ofori, E.; Okyere, P.B.; et al. Mental health and quality of life burden in Buruli ulcer disease patients in Ghana. Infect. Dis. Poverty 2021, 10, 109. [Google Scholar] [CrossRef]
- Johnson, R.C.; Phanzu, D.M.; Guédénon, A.; Portaels, F. Clinical features of Buruli ulcer. In Leprosy and Buruli Ulcer; Nunzi, E., Massone, C., Portaels, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Nienhuis, W.A.; Stienstra, Y.; Thompson, W.A.; Awuah, P.C.; Abass, K.M.; Tuah, W.; Awua-Boateng, N.Y.; Ampadu, E.O.; Siegmund, V.; Schouten, J.P.; et al. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: A randomised controlled trial. Lancet 2010, 375, 664–672. [Google Scholar] [CrossRef]
- Tanywe, A.; Fernandez, R.S. Effectiveness of rifampicin-streptomycin for treatment of Buruli ulcer: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Converse, P.J.; Almeida, D.V.; Tasneen, R.; Saini, V.; Tyagi, S.; Ammerman, N.C.; Li, S.-Y.; Anders, N.M.; Rudek, M.A.; Grosset, J.H.; et al. Shorter-course treatment for Mycobacterium ulcerans disease with high-dose rifamycins and clofazimine in a mouse model of Buruli ulcer. PLoS Negl. Trop. Dis. 2018, 12, e0006728. [Google Scholar] [CrossRef]
- Cowan, R.; Athan, E.; Friedman, N.D.; Hughes, A.J.; McDonald, A.; Callan, P.; Fyfe, J.; O’bRien, D.P.; Johnson, C. Mycobacterium ulcerans treatment—Can antibiotic duration be reduced in selected patients? PLoS Negl. Trop. Dis. 2015, 9, e0003503. [Google Scholar] [CrossRef] [PubMed]
- Tweedale, B.; Collier, F.; Waidyatillake, N.T.; Athan, E.; O’Brien, D.P. Mycobacterium ulcerans culture results according to duration of prior antibiotic treatment: A cohort study. PLoS ONE 2023, 18, e0284201. [Google Scholar] [CrossRef] [PubMed]
- Boakye-Appiah, J.; Hall, B.; Reljic, R.; Simmonds, R.E. Current progress and prospects for a Buruli ulcer vaccine. In Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges; Christodoulides, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Boakye-Appiah, J.K.; Tran, A.C.; Paul, M.J.; Hart, P.; Phillips, R.O.; Harrison, T.S.; Wansbrough-Jones, M.; Reljic, R. A composite subunit vaccine confers full protection against Buruli ulcer disease in the mouse footpad model of Mycobacterium ulcerans infection. PLoS Negl. Trop. Dis. 2025, 19, e0012710. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Blasdell, K.; Muhi, S.; Marais, B.J.; Buddle, B.; McNamara, B.; Athan, E. Is BCG vaccination of possums the solution to the Buruli ulcer epidemic in south-eastern Australia? Med. J. Aust. 2023, 219, 520–522. [Google Scholar] [CrossRef]
- Abass, K.M.; van der Werf, T.S.; Phillips, R.O.; Sarfo, F.S.; Abotsi, J.; Mireku, S.O.; Thompson, W.N.; Asiedu, K.; Stienstra, Y.; Klis, S.-A. Buruli ulcer control in a highly endemic district in Ghana: Role of community-based surveillance volunteers. Am. J. Trop. Med. Hyg. 2015, 92, 115–117. [Google Scholar] [CrossRef]
- Stienstra, Y.; van der Graaf, W.T.; Asamoa, K.; van der Werf, T.S. Beliefs and attitudes toward Buruli ulcer in Ghana. Am. J. Trop. Med. Hyg. 2002, 67, 207–213. [Google Scholar] [CrossRef]
- World Health Organization. Buruli Ulcer: Objective and Strategy for Control and Research. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/buruli-ulcer/objective-and-strategy-for-control-and-research (accessed on 2 May 2025).
- Ahorlu, C.K.; Koka, E.; Yeboah-Manu, D.; Lamptey, I.; Ampadu, E. Enhancing Buruli ulcer control in Ghana through social interventions: A case study from the Obom sub-district. BMC Public Health 2013, 13, 59. [Google Scholar] [CrossRef]
- McNamara, B.J.; Cornish, J.; Blasdell, K.R.; Athan, E.; Clarke, N.E.; Pe, T.; Hussain, M.A.; Muleme, M.; Tay, E.L.; Dunn, M.; et al. Mycobacterium ulcerans in Possum Feces before Emergence in Humans, Australia. Emerg. Infect. Dis. 2025, 31, 569–573. [Google Scholar] [CrossRef]
- Collinson, S.; Frimpong, V.N.B.; Agbavor, B.; Montgomery, B.; Oppong, M.; Frimpong, M.; Amoako, Y.A.; Marks, M.; Phillips, R.O.; O’BRien, D.P. Barriers to Buruli ulcer treatment completion in the Ashanti and Central Regions, Ghana. PLoS Negl. Trop. Dis. 2020, 14, e0008369. [Google Scholar] [CrossRef] [PubMed]
| Antimicrobial Agent/Regimen | Indications | Efficacy | Side Effects | Evidence Level |
|---|---|---|---|---|
| Rifampicin + Clarithromycin (RC8) | First-line; WHO-recommended for all age groups | Cure rates > 90%; proven in RCTs | Gastrointestinal, hepatotoxicity, QTc prolongation, dysgeusia, and drug interactions | High (WHO and RCTs) |
| Rifampicin + Moxifloxacin | Alternative first-line in Australia (esp. adults) | Comparable to clarithromycin in practice | QTc prolongation, tendon issues (esp. elderly), photosensitivity | Moderate (lab + observational studies) |
| Rifampicin + Ciprofloxacin | Alternative option for children | Effective, supported by observational data | Similar to moxifloxacin, cartilage toxicity in children | Moderate (lab + observational studies) |
| Rifampicin + Streptomycin (Discontinued) | Previously first-line; now discontinued | Comparable efficacy, more side effects | High ototoxicity, nephrotoxicity | High (RCTs) but now outdated |
| Rifampicin + Clofazimine | Emerging; suitable in combination therapy | Effective in the murine model, relapse prevention | Skin discoloration, GI upset (rare) | Low (preclinical + murine models) |
| Telacebec (Q203) | Investigational; ultrashort regimen candidate | Sterile cures in mice; ongoing trials in humans | Minimal in small clinical studies | High (preclinical + Phase 2 ongoing) |
| TB47 | Experimental; high activity in murine models | Cures BU in mice in 2–3 weeks | No severe reports in animals | Low (murine models) |
| Cotrimoxazole (TMP-SMX) | Investigational; weak evidence, small RCTs | No significant clinical benefit | Well tolerated, minor skin discoloration | Low (small RCT, observational) |
| Dapsone + Rifampicin | Pilot study, limited efficacy | Non-significant improvement over placebo | Can cause SJS, limited sample size | Low (pilot, not statistically significant) |
| Beta-lactam (Amox/Clavulanic Acid) + Rifampicin + Clarithromycin | Investigational; RCT underway | Potential four-week alternative: synergistic action | Minimal known so far | Moderate (in vitro + modeling, RCT ongoing) |
| Linezolid | Resistant TB; not favored for BU due to toxicity | Effective but high toxicity risk | Hematologic, neurologic, and lactic acidosis | Low (case reports, side-effect profile) |
| Bedaquiline | Investigational; high cost limits use | Promising in vitro/in vivo | Cost, QTc prolongation, liver impact | Low (preclinical, expensive) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.; Shadid, O.; Novo, J.; Mon, Y.; Seth, I.; Marcaccini, G.; Cuomo, R.; O’Brien, D.P.; Rozen, W.M. Mycobacterium Ulcerans Ulcer: Current Trends in Antimicrobial Management and Reconstructive Surgical Strategies. Life 2025, 15, 1096. https://doi.org/10.3390/life15071096
Lim B, Shadid O, Novo J, Mon Y, Seth I, Marcaccini G, Cuomo R, O’Brien DP, Rozen WM. Mycobacterium Ulcerans Ulcer: Current Trends in Antimicrobial Management and Reconstructive Surgical Strategies. Life. 2025; 15(7):1096. https://doi.org/10.3390/life15071096
Chicago/Turabian StyleLim, Bryan, Omar Shadid, Jennifer Novo, Yi Mon, Ishith Seth, Gianluca Marcaccini, Roberto Cuomo, Daniel P. O’Brien, and Warren M. Rozen. 2025. "Mycobacterium Ulcerans Ulcer: Current Trends in Antimicrobial Management and Reconstructive Surgical Strategies" Life 15, no. 7: 1096. https://doi.org/10.3390/life15071096
APA StyleLim, B., Shadid, O., Novo, J., Mon, Y., Seth, I., Marcaccini, G., Cuomo, R., O’Brien, D. P., & Rozen, W. M. (2025). Mycobacterium Ulcerans Ulcer: Current Trends in Antimicrobial Management and Reconstructive Surgical Strategies. Life, 15(7), 1096. https://doi.org/10.3390/life15071096









