Cardiovascular Remodeling and Potential Controversies in Master Endurance Athletes—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Structural, Functional, and Electrical Cardiac Remodeling—Specific Athlete Risk
3.1. Association Between Endurance Training and Atrial Fibrillation
3.2. Accelerated Coronary Artery Atherosclerosis in Endurance MAs
3.3. Exercise-Induced Cardiac Remodeling, Cardiac Fibrosis, and Arrhythmogenesis in Endurance MAs
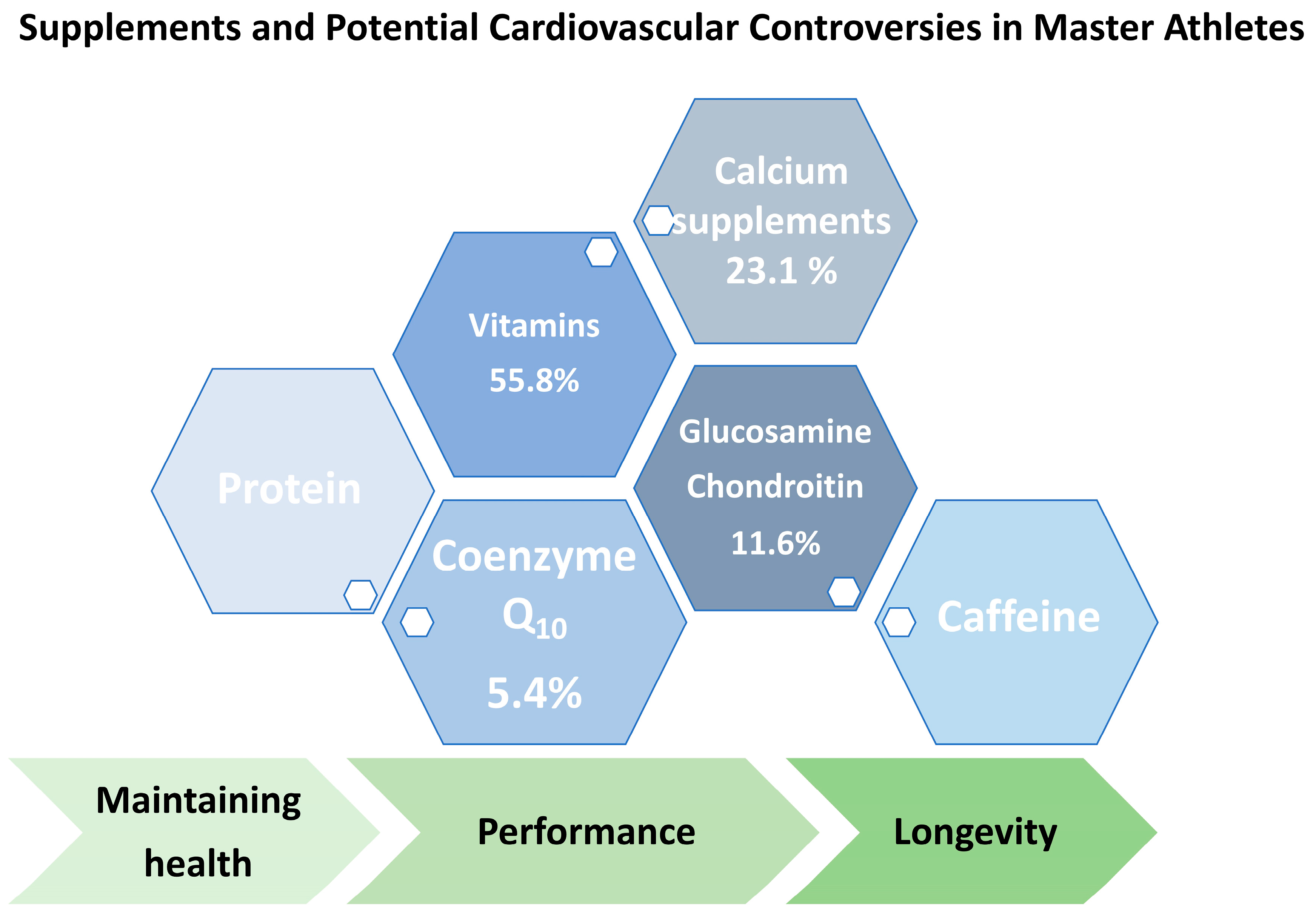
4. Use of Medication and Dietary Supplements in MAs—Potential Interactions
5. Clinical Implications and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tso, J.; Kim, J.H. Master Endurance Athletes and Cardiovascular Controversies. Curr. Sports Med. Rep. 2020, 19, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V.; Tiller, N.B.; Doutreleau, S.; Khodaee, M.; Knechtle, B.; Pasternak, A.; Rojas-Valverde, D. Potential Long-Term Health Problems Associated with Ultra-Endurance Running: A Narrative Review. Sports Med. 2022, 52, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Merghani, A.; Maestrini, V.; Rosmini, S.; Cox, A.T.; Dhutia, H.; Bastiaenan, R.; David, S.; Yeo, T.J.; Narain, R.; Malhotra, A.; et al. Prevalence of Subclinical Coronary Artery Disease in Masters Endurance Athletes With a Low Atherosclerotic Risk Profile. Circulation 2017, 136, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Fruytier, L.A.; van de Sande, D.A.; Kemps, H.M. Exercise-Related Major Adverse Cardiovascular Events in Asymptomatic Recreational Master Athletes: A Case Series. Eur. Heart J. Case Rep. 2022, 6, ytac309. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Zimmermann, P.; Lutter, C. Establishing Stable Sinus Rhythm in an Endurance Athlete with Paroxysmal Supraventricular Tachycardia Improves Haemodynamical Performance during Exercise Testing. BMJ Case Rep. 2020, 13, e238674. [Google Scholar] [CrossRef]
- Henning, R.J. The Differentiation of the Competitive Athlete with Physiologic Cardiac Remodeling from the Athlete with Cardiomyopathy. Curr. Probl. Cardiol. 2024, 49, 102473. [Google Scholar] [CrossRef]
- Weiner, R.B.; Baggish, A.L. Cardiovascular Adaptation and Remodeling to Rigorous Athletic Training. Clin. Sports Med. 2015, 34, 405–418. [Google Scholar] [CrossRef]
- Churchill, T.W.; Baggish, A.L. Cardiovascular Care of Masters Athletes. J. Cardiovasc. Transl. Res. 2020, 13, 313–321. [Google Scholar] [CrossRef]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Van De Heyning, C.M.; Van Herck, P.; Paelinck, B.; et al. Lifelong Endurance Exercise and Its Relation with Coronary Atherosclerosis. Eur. Heart J. 2023, 44, 2388–2399. [Google Scholar] [CrossRef]
- D’Ambrosio, P.; De Paepe, J.; Janssens, K.; Mitchell, A.M.; Rowe, S.J.; Spencer, L.W.; Van Puyvelde, T.; Bogaert, J.; Ghekiere, O.; Pauwels, R.; et al. Arrhythmias and Structural Remodeling in Lifelong and Retired Master Endurance Athletes. J. Sport. Health Sci. 2025, 101043. [Google Scholar] [CrossRef]
- Liu, B.; Pagan, E.; Beccarino, N.; Chang, D.; Dulmovits, E.; Beldner, S. Voltage Mapping and Pulmonary Vein Isolation in Master Athletes with Atrial Fibrillation. Pacing Clin. Electrophysiol. 2022, 45, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Fyyaz, S.; Papadakis, M. Arrhythmogenesis of Sports: Myth or Reality? Arrhythm. Electrophysiol. Rev. 2022, 11, e05. [Google Scholar] [CrossRef]
- Opondo, M.A.; Aiad, N.; Cain, M.A.; Sarma, S.; Howden, E.; Stoller, D.A.; Ng, J.; van Rijckevorsel, P.; Hieda, M.; Tarumi, T.; et al. Does High-Intensity Endurance Training Increase the Risk of Atrial Fibrillation? A Longitudinal Study of Left Atrial Structure and Function. Circ. Arrhythm. Electrophysiol. 2018, 11, e005598. [Google Scholar] [CrossRef]
- Iskandar, A.; Mujtaba, M.T.; Thompson, P.D. Left Atrium Size in Elite Athletes. JACC Cardiovasc. Imaging 2015, 8, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Roten, L.; Tanner, H.; Wilhelm, I.; Schmid, J.-P.; Saner, H. Atrial Remodeling, Autonomic Tone, and Lifetime Training Hours in Nonelite Athletes. Am. J. Cardiol. 2011, 108, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Dorian, D.; Gustafson, D.; Quinn, R.; Bentley, R.F.; Dorian, P.; Goodman, J.M.; Fish, J.E.; Connelly, K.A. Exercise-Dependent Modulation of Immunological Response Pathways in Endurance Athletes With and Without Atrial Fibrillation. J. Am. Heart Assoc. 2024, 13, e033640. [Google Scholar] [CrossRef]
- Harnett, J.; Climstein, M.; Walsh, J.; Gifford, J. The Use of Medications and Dietary Supplements by Masters Athletes—A Review. Curr. Nutr. Rep. 2022, 11, 253–262. [Google Scholar] [CrossRef]
- Striegel, H.; Simon, P.; Wurster, C.; Niess, A.; Ulrich, R. The Use of Nutritional Supplements Among Master Athletes. Int. J. Sports Med. 2005, 27, 236–241. [Google Scholar] [CrossRef]
- Sagris, M.; Vardas, E.P.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tousoulis, D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int. J. Mol. Sci. 2021, 23, 6. [Google Scholar] [CrossRef]
- Brundel, B.J.J.M.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial Fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Baman, J.R.; Passman, R.S. Atrial Fibrillation. JAMA 2021, 325, 2218. [Google Scholar] [CrossRef] [PubMed]
- De Bosscher, R.; Dausin, C.; Claus, P.; Bogaert, J.; Dymarkowski, S.; Goetschalckx, K.; Ghekiere, O.; Belmans, A.; Van De Heyning, C.M.; Van Herck, P.; et al. Endurance Exercise and the Risk of Cardiovascular Pathology in Men: A Comparison between Lifelong and Late-Onset Endurance Training and a Non-Athletic Lifestyle—Rationale and Design of the Master@Heart Study, a Prospective Cohort Trial. BMJ Open Sport. Exerc. Med. 2021, 7, e001048. [Google Scholar] [CrossRef]
- Shapero, K.; Deluca, J.; Contursi, M.; Wasfy, M.; Weiner, R.B.; Lewis, G.D.; Hutter, A.; Baggish, A.L. Cardiovascular Risk and Disease Among Masters Endurance Athletes: Insights from the Boston MASTER (Masters Athletes Survey To Evaluate Risk) Initiative. Sports Med. Open 2016, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Peritz, D.C.; Catino, A.B.; Csecs, I.; Kaur, G.; Kheirkhahan, M.; Loveless, B.; Wasmund, S.; Kholmovski, E.; Morris, A.; Marrouche, N.F. High-Intensity Endurance Training Is Associated with Left Atrial Fibrosis. Am. Heart J. 2020, 226, 206–213. [Google Scholar] [CrossRef]
- Johansen, K.R.; Ranhoff, A.H.; Sørensen, E.; Nes, B.M.; Heitmann, K.A.; Apelland, T.; Bucher Sandbakk, S.; Wilsgaard, T.; Løchen, M.-L.; Thelle, D.S.; et al. Risk of Atrial Fibrillation and Stroke among Older Men Exposed to Prolonged Endurance Sport Practice: A 10-Year Follow-up. The Birkebeiner Ageing Study and the Tromsø Study. Open Heart 2022, 9. [Google Scholar] [CrossRef]
- Abdulla, J.; Nielsen, J.R. Is the Risk of Atrial Fibrillation Higher in Athletes than in the General Population? A Systematic Review and Meta-Analysis. Europace 2009, 11, 1156–1159. [Google Scholar] [CrossRef]
- Andersen, K.; Farahmand, B.; Ahlbom, A.; Held, C.; Ljunghall, S.; Michaëlsson, K.; Sundström, J. Risk of Arrhythmias in 52 755 Long-Distance Cross-Country Skiers: A Cohort Study. Eur. Heart J. 2013, 34, 3624–3631. [Google Scholar] [CrossRef]
- Morseth, B.; Graff-Iversen, S.; Jacobsen, B.K.; Jørgensen, L.; Nyrnes, A.; Thelle, D.S.; Vestergaard, P.; Løchen, M.-L. Physical Activity, Resting Heart Rate, and Atrial Fibrillation: The Tromsø Study. Eur. Heart J. 2016, 37, 2307–2313. [Google Scholar] [CrossRef]
- Gay-Jordi, G.; Guash, E.; Benito, B.; Brugada, J.; Nattel, S.; Mont, L.; Serrano-Mollar, A. Losartan Prevents Heart Fibrosis Induced by Long-Term Intensive Exercise in an Animal Model. PLoS ONE 2013, 8, e55427. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, M.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Exercise Type and Intensity in Relation to Coronary Heart Disease in Men. JAMA 2002, 288, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.A.C.; Jensky, N.E.; Criqui, M.H.; Whitt-Glover, M.C.; Lima, J.A.C.; Allison, M.A. The Association between Physical Activity and Both Incident Coronary Artery Calcification and Ankle Brachial Index Progression: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2013, 230, 278–283. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Kraus, S.M.; Schwartz, J.G.; Wickstrom, K.K.; Peichel, G.; Garberich, R.F.; Lesser, J.R.; Oesterle, S.N.; Knickelbine, T.; Harris, K.M.; et al. Increased Coronary Artery Plaque Volume Among Male Marathon Runners. Mo. Med. 2014, 111, 89–94. [Google Scholar]
- Möhlenkamp, S.; Lehmann, N.; Breuckmann, F.; Bröcker-Preuss, M.; Nassenstein, K.; Halle, M.; Budde, T.; Mann, K.; Barkhausen, J.; Heusch, G.; et al. Running: The Risk of Coronary Events: Prevalence and Prognostic Relevance of Coronary Atherosclerosis in Marathon Runners. Eur. Heart J. 2008, 29, 1903–1910. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Mosterd, A.; Braber, T.L.; Prakken, N.H.J.; Doevendans, P.A.; Grobbee, D.E.; Thompson, P.D.; Eijsvogels, T.M.H.; Velthuis, B.K. Relationship Between Lifelong Exercise Volume and Coronary Atherosclerosis in Athletes. Circulation 2017, 136, 138–148. [Google Scholar] [CrossRef] [PubMed]
- DeFina, L.F.; Radford, N.B.; Barlow, C.E.; Willis, B.L.; Leonard, D.; Haskell, W.L.; Farrell, S.W.; Pavlovic, A.; Abel, K.; Berry, J.D.; et al. Association of All-Cause and Cardiovascular Mortality With High Levels of Physical Activity and Concurrent Coronary Artery Calcification. JAMA Cardiol. 2019, 4, 174–181. [Google Scholar] [CrossRef]
- Kim, J.H.; Baggish, A.L. Physical Activity, Endurance Exercise, and Excess-Can One Overdose? Curr. Treat. Options Cardiovasc. Med. 2016, 18, 68. [Google Scholar] [CrossRef]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium Density of Coronary Artery Plaque and Risk of Incident Cardiovascular Events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef]
- Zerath, E.; Holy, X.; Douce, P.; Guezennec, C.Y.; Chatard, J.C. Effect of Endurance Training on Postexercise Parathyroid Hormone Levels in Elderly Men. Med. Sci. Sports Exerc. 1997, 29, 1139–1145. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- van de Schoor, F.R.; Aengevaeren, V.L.; Hopman, M.T.E.; Oxborough, D.L.; George, K.P.; Thompson, P.D.; Eijsvogels, T.M.H. Myocardial Fibrosis in Athletes. Mayo Clin. Proc. 2016, 91, 1617–1631. [Google Scholar] [CrossRef]
- Breuckmann, F.; Möhlenkamp, S.; Nassenstein, K.; Lehmann, N.; Ladd, S.; Schmermund, A.; Sievers, B.; Schlosser, T.; Jöckel, K.-H.; Heusch, G.; et al. Myocardial Late Gadolinium Enhancement: Prevalence, Pattern, and Prognostic Relevance in Marathon Runners. Radiology 2009, 251, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, E.B.; Cao, J.J.; Sigurdsson, S.; Aspelund, T.; Kellman, P.; Aletras, A.H.; Dyke, C.K.; Thorgeirsson, G.; Eiriksdottir, G.; Launer, L.J.; et al. Prevalence and Prognosis of Unrecognized Myocardial Infarction Determined by Cardiac Magnetic Resonance in Older Adults. JAMA 2012, 308, 890–896. [Google Scholar] [CrossRef]
- Barbier, C.E.; Bjerner, T.; Johansson, L.; Lind, L.; Ahlström, H. Myocardial Scars More Frequent than Expected: Magnetic Resonance Imaging Detects Potential Risk Group. J. Am. Coll. Cardiol. 2006, 48, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Maron, M.S.; Autore, C.; Lesser, J.R.; Rega, L.; Casolo, G.; De Santis, M.; Quarta, G.; Nistri, S.; Cecchi, F.; et al. Assessment and Significance of Left Ventricular Mass by Cardiovascular Magnetic Resonance in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 559–566. [Google Scholar] [CrossRef]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: Proposed Modification of the Task Force Criteria. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Sen-Chowdhry, S.; Syrris, P.; Ward, D.; Asimaki, A.; Sevdalis, E.; McKenna, W.J. Clinical and Genetic Characterization of Families with Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Provides Novel Insights into Patterns of Disease Expression. Circulation 2007, 115, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Bhonsale, A.; Tichnell, C.; Murray, B.; Russell, S.D.; Tandri, H.; Tedford, R.J.; Judge, D.P.; Calkins, H. Exercise Increases Age-Related Penetrance and Arrhythmic Risk in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy-Associated Desmosomal Mutation Carriers. J. Am. Coll. Cardiol. 2013, 62, 1290–1297. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; Macisaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-Induced Right Ventricular Dysfunction and Structural Remodelling in Endurance Athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Heidbüchel, H.; Burns, A.T.; Mooney, D.J.; Taylor, A.J.; Pfluger, H.B.; Inder, W.J.; Macisaac, A.I.; Prior, D.L. Disproportionate Exercise Load and Remodeling of the Athlete’s Right Ventricle. Med. Sci. Sports Exerc. 2011, 43, 974–981. [Google Scholar] [CrossRef]
- Sharma, S.; Zaidi, A. Exercise-Induced Arrhythmogenic Right Ventricular Cardiomyopathy: Fact or Fallacy? Eur. Heart J. 2012, 33, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Shave, R.; Baggish, A.; George, K.; Wood, M.; Scharhag, J.; Whyte, G.; Gaze, D.; Thompson, P.D. Exercise-Induced Cardiac Troponin Elevation: Evidence, Mechanisms, and Implications. J. Am. Coll. Cardiol. 2010, 56, 169–176. [Google Scholar] [CrossRef]
- Wilson, M.; O’Hanlon, R.; Prasad, S.; Deighan, A.; Macmillan, P.; Oxborough, D.; Godfrey, R.; Smith, G.; Maceira, A.; Sharma, S.; et al. Diverse Patterns of Myocardial Fibrosis in Lifelong, Veteran Endurance Athletes. J. Appl. Physiol. 2011, 110, 1622–1626. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Januzzi, J.L.; Taylor, B.A.; Isaacs, S.K.; D’Hemecourt, P.; Zaleski, A.; Dyer, S.; Troyanos, C.; Weiner, R.B.; Thompson, P.D.; et al. Impact of Statin Use on Exercise-Induced Cardiac Troponin Elevations. Am. J. Cardiol. 2014, 114, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Eijsvogels, T.M.H.; Hoogerwerf, M.D.; Oudegeest-Sander, M.H.; Hopman, M.T.E.; Thijssen, D.H.J. The Impact of Exercise Intensity on Cardiac Troponin I Release. Int. J. Cardiol. 2014, 171, E3–E4. [Google Scholar] [CrossRef]
- Douglas, P.S.; O’Toole, M.L.; Hiller, W.D.; Hackney, K.; Reichek, N. Cardiac Fatigue after Prolonged Exercise. Circulation 1987, 76, 1206–1213. [Google Scholar] [CrossRef]
- Iacopini, F.; Schurr, M.O.; Costamagna, G.; Scozzarro, A. Over-the-Scope Clip for a Pharyngeal Perforation Caused by a Self-Inflicted Stab Wound to the Neck. Endoscopy 2014, 46 (Suppl. 1), E42–E43. [Google Scholar] [CrossRef]
- Halar, F.; O’Connor, H.; Climstein, M.; Prvan, T.; Black, D.; Reaburn, P.; Stuart-Smith, W.; Wu, X.S.; Gifford, J. Prevalence of Chronic Conditions in Masters Games Athletes: Predictors and Comparison to the General Population. PeerJ 2025, 13, e18912. [Google Scholar] [CrossRef]
- Climstein, M.; Walsh, J.; Heazlewood, T.; Meir, R. An Overview of Risk Factors for Disease in Masters Athletes. In Coaching Masters Athletes; Routledge: New York, NY, USA, 2021; pp. 64–77. [Google Scholar]
- Guthrie, S.K.; Erickson, S.R. Masters Swimmers Use More Dietary Supplements Than a Large National Comparison Population in the United States. Int. J. Sport. Nutr. Exerc. Metab. 2016, 26, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond Muscle: The Effects of Creatine Supplementation on Brain Creatine, Cognitive Processing, and Traumatic Brain Injury. Eur. J. Sport. Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Doering, T.M.; Reaburn, P.R.; Phillips, S.M.; Jenkins, D.G. Postexercise Dietary Protein Strategies to Maximize Skeletal Muscle Repair and Remodeling in Masters Endurance Athletes: A Review. Int. J. Sport. Nutr. Exerc. Metab. 2016, 26, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Brisswalter, J.; Louis, J. Vitamin Supplementation Benefits in Master Athletes. Sports Med. 2014, 44, 311–318. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-N. Drug-Nutrient Interactions. J. Parenter. Enteral Nutr. 2013, 37, 450–459. [Google Scholar] [CrossRef]
- Yang, W.; Sun, C.; He, S.Q.; Chen, J.Y.; Wang, Y.; Zhuo, Q. The Efficacy and Safety of Disease-Modifying Osteoarthritis Drugs for Knee and Hip Osteoarthritis-a Systematic Review and Network Meta-Analysis. J. Gen. Intern. Med. 2021, 36, 2085–2093. [Google Scholar] [CrossRef]
- Rozenfeld, V.; Crain, J.L.; Callahan, A.K. Possible Augmentation of Warfarin Effect by Glucosamine-Chondroitin. Am. J. Health Syst. Pharm. 2004, 61, 306–307. [Google Scholar] [CrossRef]
- Knudsen, J.F.; Sokol, G.H. Potential Glucosamine-Warfarin Interaction Resulting in Increased International Normalized Ratio: Case Report and Review of the Literature and MedWatch Database. Pharmacotherapy 2008, 28, 540–548. [Google Scholar] [CrossRef]
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. Coenzyme Q10 Supplementation: Efficacy, Safety, and Formulation Challenges. Compr. Rev. Food Sci. Food Saf. 2020, 19, 574–594. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.-C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J.; Banach, M. Lipid-Modifying Effects of Nutraceuticals: An Evidence-Based Approach. Nutrition 2016, 32, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, R.A.; Guarneri, E. Coenzyme Q10. Am. Fam. Physician 2005, 72, 1065–1070. [Google Scholar]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Chen, C.-H.; Uang, Y.-S.; Wang, S.-T.; Yang, J.-C.; Lin, C.-J. Interaction between Red Yeast Rice and CYP450 Enzymes/P-Glycoprotein and Its Implication for the Clinical Pharmacokinetics of Lovastatin. Evid. Based Complement. Alternat Med. 2012, 2012, 127043. [Google Scholar] [CrossRef]
- Hong, S.-P.; Yang, J.-S.; Han, J.-Y.; Ha, S.-I.; Chung, J.-W.; Koh, Y.-Y.; Chang, K.-S.; Choi, D.-H. Effects of Lovastatin on the Pharmacokinetics of Diltiazem and Its Main Metabolite, Desacetyldiltiazem, in Rats: Possible Role of Cytochrome P450 3A4 and P-Glycoprotein Inhibition by Lovastatin. J. Pharm. Pharmacol. 2011, 63, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, A.; Szuta, M.; Galanty, A.; Paśko, P. Optimal Dosing Regimen of Osteoporosis Drugs in Relation to Food Intake as the Key for the Enhancement of the Treatment Effectiveness-A Concise Literature Review. Foods 2021, 10, 720. [Google Scholar] [CrossRef]
- Clarke, H.; Kim, D.-H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef]
- Saunders, B.; da Costa, L.R.; de Souza, R.A.S.; Barreto, G.; Marticorena, F.M. Caffeine and Sport. Adv. Food Nutr. Res. 2023, 106, 95–127. [Google Scholar] [CrossRef]
- de Souza, J.G.; Del Coso, J.; Fonseca, F. de S.; Silva, B.V.C.; de Souza, D.B.; da Silva Gianoni, R.L.; Filip-Stachnik, A.; Serrão, J.C.; Claudino, J.G. Risk or Benefit? Side Effects of Caffeine Supplementation in Sport: A Systematic Review. Eur. J. Nutr. 2022, 61, 3823–3834. [Google Scholar] [CrossRef]
- Fletcher, D.K.; Bishop, N.C. Effect of a High and Low Dose of Caffeine on Antigen-Stimulated Activation of Human Natural Killer Cells after Prolonged Cycling. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 155–165. [Google Scholar] [CrossRef]
- Ganio, M.S.; Johnson, E.C.; Lopez, R.M.; Stearns, R.L.; Emmanuel, H.; Anderson, J.M.; Casa, D.J.; Maresh, C.M.; Volek, J.S.; Armstrong, L.E. Caffeine Lowers Muscle Pain during Exercise in Hot but Not Cool Environments. Physiol. Behav. 2011, 102, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Terzi, M.N.; Roberson, D.W.; Burnett, T.R. Effect of Caffeine Intake on Pain Perception during High-Intensity Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Exercise and Sport Performance with Low Doses of Caffeine. Sports Med. 2014, 44 (Suppl. 2), 175–184. [Google Scholar] [CrossRef] [PubMed]
- Gajda, R.; Gajda, J.; Czuba, M.; Knechtle, B.; Drygas, W. Sports Heart Monitors as Reliable Diagnostic Tools for Training Control and Detecting Arrhythmias in Professional and Leisure-Time Endurance Athletes: An Expert Consensus Statement. Sports Med. 2024, 54, 1–21. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Westaby, J.; Sheppard, M.N.; Papadakis, M.; Sharma, S. Sudden Cardiac Death in Young Athletes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 83, 350–370. [Google Scholar] [CrossRef]
- Zaidi, A.; Ghani, S.; Sharma, R.; Oxborough, D.; Panoulas, V.F.; Sheikh, N.; Gati, S.; Papadakis, M.; Sharma, S. Physiological Right Ventricular Adaptation in Elite Athletes of African and Afro-Caribbean Origin. Circulation 2013, 127, 1783–1792. [Google Scholar] [CrossRef]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Maron, B.J. Physiologic Left Ventricular Cavity Dilatation in Elite Athletes. Ann. Intern. Med. 1999, 130, 23–31. [Google Scholar] [CrossRef]
- Drezner, J.A.; Sharma, S.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Criteria for Electrocardiographic Interpretation in Athletes: Consensus Statement. Br. J. Sports Med. 2017, 51, 704–731. [Google Scholar] [CrossRef]
- Panhuyzen-Goedkoop, N.M.; Wellens, H.J.; Verbeek, A.L.; Jørstad, H.T.; Smeets, J.R.; Peters, R.J. ECG Criteria for the Detection of High-Risk Cardiovascular Conditions in Master Athletes. Eur. J. Prev. Cardiol. 2020, 27, 1529–1538. [Google Scholar] [CrossRef]
- Hevia, A.C.; Fernández, M.M.; Palacio, J.M.A.; Martín, E.H.; Castro, M.G.; Reguero, J.J.R. ECG as a Part of the Preparticipation Screening Programme: An Old and Still Present International Dilemma. Br. J. Sports Med. 2011, 45, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Recommendations for Electrocardiographic Interpretation in Athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso, C.; Biffi, A.; Buja, G.; Delise, P.; Gussac, I.; et al. Recommendations for Interpretation of 12-Lead Electrocardiogram in the Athlete. Eur. Heart J. 2010, 31, 243–259. [Google Scholar] [CrossRef]
- Corrado, D.; Pelliccia, A.; Bjørnstad, H.H.; Vanhees, L.; Biffi, A.; Borjesson, M.; Panhuyzen-Goedkoop, N.; Deligiannis, A.; Solberg, E.; Dugmore, D.; et al. Cardiovascular Pre-Participation Screening of Young Competitive Athletes for Prevention of Sudden Death: Proposal for a Common European Protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur. Heart J. 2005, 26, 516–524. [Google Scholar] [CrossRef]
- Gervasi, S.F.; Palumbo, L.; Cammarano, M.; Orvieto, S.; Di Rocco, A.; Vestri, A.; Marano, R.; Savino, G.; Bianco, M.; Zeppilli, P.; et al. Coronary Atherosclerosis in Apparently Healthy Master Athletes Discovered during Pre-PARTECIPATION Screening. Role of Coronary CT Angiography (CCTA). Int. J. Cardiol. 2019, 282, 99–107. [Google Scholar] [CrossRef]
- Baggish, A.L.; Levine, B.D. Coronary Artery Calcification Among Endurance Athletes: “Hearts of Stone”. Circulation 2017, 136, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J.; Edgman-Levitan, S. Shared Decision Making--Pinnacle of Patient-Centered Care. N. Engl. J. Med. 2012, 366, 780–781. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Olaso-Gonzalez, G.; Corella, D.; Gomez-Cabrera, M.C.; Vina, J. Increased Average Longevity among the “Tour de France” Cyclists. Int. J. Sports Med. 2011, 32, 644–647. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; De Luca, R.; Di Paolo, F.M.; Spataro, A.; Culasso, F. Remodeling of Left Ventricular Hypertrophy in Elite Athletes after Long-Term Deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef]
- Pedlar, C.R.; Brown, M.G.; Shave, R.E.; Otto, J.M.; Drane, A.; Michaud-Finch, J.; Contursi, M.; Wasfy, M.M.; Hutter, A.; Picard, M.H.; et al. Cardiovascular Response to Prescribed Detraining among Recreational Athletes. J. Appl. Physiol. 2018, 124, 813–820. [Google Scholar] [CrossRef]
- Ehsani, A.A.; Hagberg, J.M.; Hickson, R.C. Rapid Changes in Left Ventricular Dimensions and Mass in Response to Physical Conditioning and Deconditioning. Am. J. Cardiol. 1978, 42, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Petretta, M.; Cavallaro, V.; Bianchi, V.; La Mura, G.; Conforti, G.; Breglio, R.; Valva, G.; Morgano, G.; Bonaduce, D. Cardiac Changes Induced by Deconditioning in Athletes: An Echocardiographic and Electrocardiographic Study. G. Ital. Cardiol. 1991, 21, 1167–1177. [Google Scholar] [PubMed]
- Luthi, P.; Zuber, M.; Ritter, M.; Oechslin, E.N.; Jenni, R.; Seifert, B.; Baldesberger, S.; Attenhofer Jost, C.H. Echocardiographic Findings in Former Professional Cyclists after Long-Term Deconditioning of More than 30 Years. Eur. J. Echocardiogr. 2008, 9, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.J.; Shah, A.B. Exercise and the Female Heart. Clin. Ther. 2022, 44, 41–49. [Google Scholar] [CrossRef]

| Author | Study Type | Sport | Age | Prevalence |
|---|---|---|---|---|
| D’ Ambrosio et al., 2025 [11] | Cross-sectional analysis of observational studies | Endurance athletes | >40 y | 32% AF 9% ns-VT |
| Liu et al., 2022 [12] | Retrospective single-center study—ablation of AF | Endurance athletes (running, swimming) | n.a. | n.a. |
| Shapero et al., 2016 [24] | Electronic Internet-based survey | Boston master athletes | >35 y | 9% AF and CAD |
| Johansen et al., 2022 [26] | 10-year survey | Long-distance ski racers | >65 y | 28.5% AF |
| Andersen et al., 2013 [28] | Register data | Vasaloppet, 90 km cross-skiing | total | 1.5–2.0% arrhythmias |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moser, O.; Schunk, S.J.; Schöffl, V.; Schierbauer, J.; Zimmermann, P. Cardiovascular Remodeling and Potential Controversies in Master Endurance Athletes—A Narrative Review. Life 2025, 15, 1095. https://doi.org/10.3390/life15071095
Moser O, Schunk SJ, Schöffl V, Schierbauer J, Zimmermann P. Cardiovascular Remodeling and Potential Controversies in Master Endurance Athletes—A Narrative Review. Life. 2025; 15(7):1095. https://doi.org/10.3390/life15071095
Chicago/Turabian StyleMoser, Othmar, Stefan J. Schunk, Volker Schöffl, Janis Schierbauer, and Paul Zimmermann. 2025. "Cardiovascular Remodeling and Potential Controversies in Master Endurance Athletes—A Narrative Review" Life 15, no. 7: 1095. https://doi.org/10.3390/life15071095
APA StyleMoser, O., Schunk, S. J., Schöffl, V., Schierbauer, J., & Zimmermann, P. (2025). Cardiovascular Remodeling and Potential Controversies in Master Endurance Athletes—A Narrative Review. Life, 15(7), 1095. https://doi.org/10.3390/life15071095







