Review on the Clinical, Imaging, and Therapeutic Aspects of Cardiac Masses in Dog
Abstract
1. Introduction
2. Relevant Sections
3. Discussion
3.1. Clinical Examination
Diagnostic Imaging Techniques
3.2. Echocardiographic Diagnosis of Atrial Hemangiosarcoma
3.3. Echocardiographic Diagnosis of Chemodectoma
3.4. Echocardiographic Diagnosis in the Case of Other Cardiac and Pericardial Masses
3.5. Therapeutic Protocols
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ware, W.A. Cardiovascular Disease in Small Animal Medicine; Manson Publishing: London, UK, 2011. [Google Scholar]
- Meurs, K.M. Clinical features of cardiac tumors in the dog. J. Vet. Cardiol. 2005, 7, 15–20. [Google Scholar]
- Atkins, C.E.; Meurs, K.M. Cardiac tumors in dogs and cats. Clin. Tech. Small Anim. Pract. 2004, 19, 267–276. [Google Scholar]
- Schultheiss, P.C. A retrospective study of primary cardiac neoplasia in dogs (1992–1999). J. Vet. Diagn. Invest. 2004, 16, 547–552. [Google Scholar] [CrossRef]
- MacDonald, K.A. Cardiac neoplasia in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1085–1102. [Google Scholar]
- Yamamoto, S.; Hoshi, K.; Hirakawa, A.; Chimura, S.; Kobayashi, M.; Machida, N. Epidemiological, Clinical and Pathological Features of Primary Cardiac Hemangiosarcoma in Dogs: A Review of 51 Cases. J. Vet. Med. Sci. 2013, 75, 1433–1441. [Google Scholar] [CrossRef]
- Tidholm, A.; Menciotti, G.; Borgarelli, M. Current Use of Real-Time Three-Dimensional Transthoracic Echocardiography in Animals. J. Vet. Cardiol. 2024, 51, 97–104. [Google Scholar] [CrossRef]
- Detweiler, D.K.; Patterson, D.F. The prevalence and types of cardiovascular disease in dogs. Ann. N. Y. Acad. Sci. 1965, 127, 481–516. [Google Scholar] [CrossRef]
- Ware, W.A. Tumors of the cardiovascular system. In Textbook of Veterinary Internal Medicine; Ettinger, S.J., Feldman, E.C., Eds.; Elsevier: St. Louis, MO, USA, 1995. [Google Scholar]
- Ware, W.A.; Hopper, D.L. Cardiac tumors in dogs: 1982–1995. J. Vet. Intern. Med. 1999, 13, 95–103. [Google Scholar] [CrossRef]
- Alvarez, F.J.; Hosoya, K.; Lara-Garcia, A.; Kisseberth, W.; Couto, G. VAC protocol for treatment of dogs with stage III hemangiosarcoma. J. Am. Anim. Hosp. Assoc. 2013, 49, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bussadori, C.; Santilli, R.A.; Borgarelli, M.; Moise, N.S. Echocardiography in Veterinary Cardiology, 2nd ed.; Edra: Milan, Italy, 2020. [Google Scholar]
- Baisan, R.; Borda, C.; Bălăceanu, A. Cardiac tumors in dogs: Retrospective review. Rev. Rom. Med. Vet. 2018, 28, 35–42. [Google Scholar]
- Tilley, L.P.; Smith, F.W.K. Blackwell’s Five-Minute Veterinary Consult: Canine and Feline, 6th ed.; Wiley-Blackwell: Ames, IA, USA, 2015. [Google Scholar]
- Schultheiss, P.C. A retrospective study of visceral and nonvisceral hemangiosarcoma and hemangiomas in domestic animals. J. Vet. Diagn. Invest. 2004, 16, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Côté, E.; MacDonald, K.A.; Meurs, K.M.; Sleeper, M.M. Feline Cardiology; Wiley-Blackwell: Ames, IA, USA, 2011. [Google Scholar]
- Johnson, R.L. Cardiovascular diseases in dogs. In Textbook of Veterinary Internal Medicine, 8th ed.; Sisson, D., Thomas, W.P., Oyama, M.A., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 123–145. [Google Scholar]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J. Vet. Intern. Med. 1993, 7, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Brăslaşu, C.M.; Lungu, A.; Brăslaşu, E.D.; Verman, G.I.; Săvulescu, I. Cardiologie Veterinară: Diagnosticul Bolilor Cardiovasculare la Animale; Editura Artprint: București, Romania, 2007. [Google Scholar]
- Boon, J.A. Veterinary Echocardiography, 2nd ed.; Wiley-Blackwell: Ames, IA, USA, 2011. [Google Scholar]
- Ciobotaru, E. Pericardial Pathology in Dogs—Clinical Cases. Ph.D. Thesis, USAMV, Bucharest, Romania, 2013. [Google Scholar]
- Silverstein, D.C.; Hopper, K. (Eds.) Small Animal Critical Care Medicine, 3rd ed.; Elsevier: St. Louis, MO, USA, 2023. [Google Scholar]
- Warman, S. Pericardial effusion and cardiac tamponade. In Textbook of Veterinary Internal Medicine, 7th ed.; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Elsevier: St. Louis, MO, USA, 2006. [Google Scholar]
- Fuentes, V.L. Cardiomegaly in dogs: Diagnostic implications. Vet. J. 2010, 185, 68–75. [Google Scholar]
- Neagu, C. Right heart failure and digestive signs. Rev. Rom. Med. Vet. 2014, 24, 50–55. [Google Scholar]
- May, W. Diagnostic Imaging of Dogs and Cats: Advanced Imaging Techniques; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Newell, S.M.; Mahaffey, M.B.; Roberts, G.D. Use of iodinated contrast media in veterinary patients. Vet. Radiol. Ultrasound 2017, 58, 465–475. [Google Scholar]
- Leca, F. Echocardiography in Veterinary Practice. Veterinary Medical Ed.: Bucharest, Romania, 2016. [Google Scholar]
- Treggiari, E.; Pedro, B.; Dukes-McEwan, J.; Gelzer, A.R.; Blackwood, L. A descriptive review of cardiac tumours in dogs and cats. Vet. Comp. Oncol. 2017, 15, 273–288. [Google Scholar] [CrossRef]
- Mihai, A.S.; Georgescu, B.; Dănilă, A.M.; Cotuțiu, C. Cardiac tumors in dogs—Imaging diagnosis and classification. Rev. Rom. Med. Vet. 2019, 29, 22–27. [Google Scholar]
- Brownlee, L.; Barber, L.G.; Baumgartner, W.; Norris, A.M. Association between pericardial effusion and right atrial hemangiosarcoma in dogs. J. Am. Vet. Med. Assoc. 1999, 215, 861–867. [Google Scholar]
- Marcu, C.B.; Becher, H. Cardiac masses: The role of cardiovascular imaging in the differential diagnosis. Diagnostics 2020, 10, 1088. [Google Scholar] [CrossRef]
- Cave, T.A.; Johnson, V.S.; Rottman, J.B. Primary cardiac tumors in dogs and cats. Clin. Tech. Small Anim. Pract. 2002, 17, 151–156. [Google Scholar]
- MacLachlan, N.J.; Meuten, D.J. Tumors of the Cardiovascular System. In Tumors in Domestic Animals, 5th ed.; Wiley-Blackwell: Ames, IA, USA, 2017; pp. 433–456. [Google Scholar]
- Abbott, J.A. Feline and Canine Cardiac Tumors. In Kirk’s Current Veterinary Therapy XV; Bonagura, J.D., Twedt, D.C., Eds.; Elsevier: St. Louis, MO, USA, 2010. [Google Scholar]
- Clifford, C.A.; Mackin, A.J.; Henry, C.J. Treatment of canine hemangiosarcoma: 2000 and beyond. J. Vet. Intern. Med. 2000, 14, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.S.; Couto, C.G.; Filppi, J.; Getzy, D.M.; Shank, K. Epidemiology and prognosis for canine hemangiosarcoma of the right atrium. J. Vet. Intern. Med. 1991, 5, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, E. Hemangiosarcoma in dogs: Clinical update. Vet. Focus 2015, 25, 14–20. [Google Scholar]
- Noszczyk-Nowak, A.; Nowak, M.; Paslawska, U.; Cepiel, A.; Janiszewski, A.; Staszczyk, M.; Nicpon, J. A retrospective study of cardiac hemangiosarcoma in dogs. Turk. J. Vet. Anim. Sci. 2014, 38, 77–81. [Google Scholar] [CrossRef]
- Berry, C.R.; Lombard, C.W.; Hager, D.A.; Ackerman, N.; King, R.R. Echocardiographic evaluation of cardiac tamponade in dogs before and after pericardiocentesis: Four cases. J. Am. Vet. Med. Assoc. 1988, 192, 1597–1603. [Google Scholar] [CrossRef]
- Magne, M.; MacDonald, K.A.; Cagney, O. Echocardiographic and clinicopathologic characterization of pericardial effusion in dogs. Vet. Cardiol. 2009, 235, 1456–1461. [Google Scholar]
- Köster, L.S.; Lawson, P.B.; Aisa, J. Cardiac tumors in dogs and cats. Clinician’s Brief, 12 February 2021. [Google Scholar]
- Vanderweerd, V.; Lambrechts, N. Metastatic cardiac hemangiosarcoma diagnostic evaluation including 2D/M mode and Doppler echocardiography. Vet. Sci. 2019, 6, 65. [Google Scholar] [CrossRef]
- Lorbach, S.K.; Romsland, T.D.; Carroll, V.W.; Block, C.L. Pathology in Practice. J. Am. Vet. Med. Assoc. 2022, 259, 1–4. [Google Scholar] [CrossRef]
- Boz, E.; Papa, M.; Claretti, M.; Bussadori, R.; Lopez, B.S.; Rossi, C.; Mazzoni, L.; Pradelli, D.; Bussadori, C.M. Real-time three-dimensional echocardiographic study of a cardiac mass in a dog. J. Vet. Cardiol. 2020, 28, 31–36. [Google Scholar] [CrossRef]
- Popescu, D.; Ionescu, V.; Marin, A. Cardiac tumor, dog, pericardial effusion. Sci. Work. Ser. C Vet. Med. 2013, 19, 45–52. [Google Scholar]
- Stepien, R.L. Cardiac tumors: Clinical findings and diagnostic approach. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 889–906. [Google Scholar]
- Șerdean, C.; Codreanu, M.D. Tumorile cardiace la câine—Aspecte clinice și imagistice. Pract. Vet. 2017, 10, 45–50. [Google Scholar]
- Ferasin, L. Cardiac tumors and pericardial disease in small animals. Vet. Focus 2019, 29, 10–17. [Google Scholar]
- Ferasin, L. Cardiac masses in dogs: Clinical presentation, diagnosis and management. Vet. Focus 2017, 27, 2–9. [Google Scholar]
- Bussadori, C.; Amberger, C.; Le Bobinnec, G. Diagnostic imaging of canine chemodectoma: Echocardiographic patterns and differential diagnosis. Vet. Radiol. Ultrasound 2010, 51, 183–189. [Google Scholar]
- Young, L.N.; Gordon, S.G.; Roland, R.M. Advanced echocardiographic assessment of canine heart base tumors using 3D and CEUS modalities. J. Vet. Cardiol. 2021, 35, 73–79. [Google Scholar] [CrossRef]
- Lamb, C.R. Imaging the heart and pericardium. In Textbook of Veterinary Diagnostic Radiology, 4th ed.; Thrall, D.E., Ed.; Saunders: St. Louis, MO, USA, 2002; pp. 673–693. [Google Scholar]
- Ware, W.A.; Bonagura, J.D. Pericardial Diseases and Cardiac Tumors. In Cardiovascular Disease in Small Animal Medicine; CRC Press: Boca Raton, FL, USA, 2019; pp. 1234–1250. [Google Scholar]
- Meurs, K.M. Cardiac tumors in dogs: Clinical presentation, diagnosis, and management. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 1001–1010. [Google Scholar] [CrossRef]
- Ioniță, L.; Mincă, N.A.; Țogoe, D.; Brăslașu, D.E.; Ioniță, C. Use of pericardial fluid pH in the differentiation of neoplastic and nonneoplastic pericardial effusion. Rev. Rom. Med. Vet. 2024, 34, 45–50. [Google Scholar]
- Fine, D.M.; Tobias, A.H.; Bonagura, J.D.; Fox, P.R.; Jacob, K.A.; Moïse, N.S. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions in dogs. J. Vet. Intern. Med. 2003, 17, 525–529. [Google Scholar] [CrossRef]
- Jones, M.D. Cardiac neoplasia in dogs. In Textbook of Veterinary Internal Medicine, 8th ed.; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 1350–1370. [Google Scholar]
- Gordon, S.G. Surgical intervention in cardiac neoplasia. J. Vet. Cardiol. 2010, 12, 127–135. [Google Scholar]
- Brownlie, S.; Cobb, M. Pericardiectomy in dogs with neoplasia. J. Small Anim. Pract. 2005, 46, 179–184. [Google Scholar]
- Vail, D.M.; Thamm, D.H.; Liptak, J.M. Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: St. Louis, MO, USA, 2019. [Google Scholar]
- Bender, C.E. Toceranib phosphate therapy in dogs with cardiac tumors. Vet. Comp. Oncol. 2020, 18, 349–357. [Google Scholar]
- Ogilvie, G.K. Evaluation of doxorubicin chemotherapy in dogs with hemangiosarcoma. J. Am. Vet. Med. Assoc. 2016, 249, 391–396. [Google Scholar]
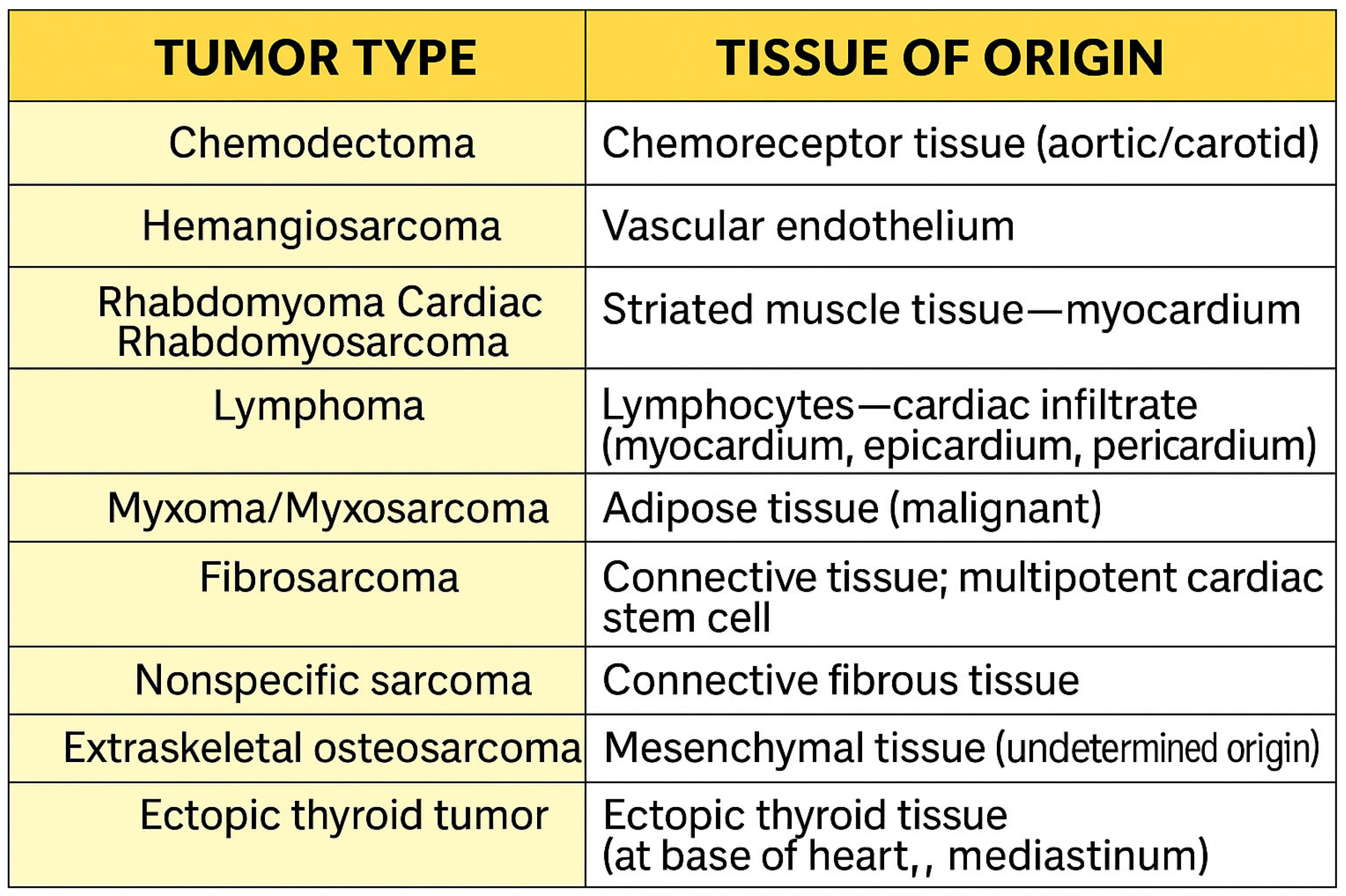
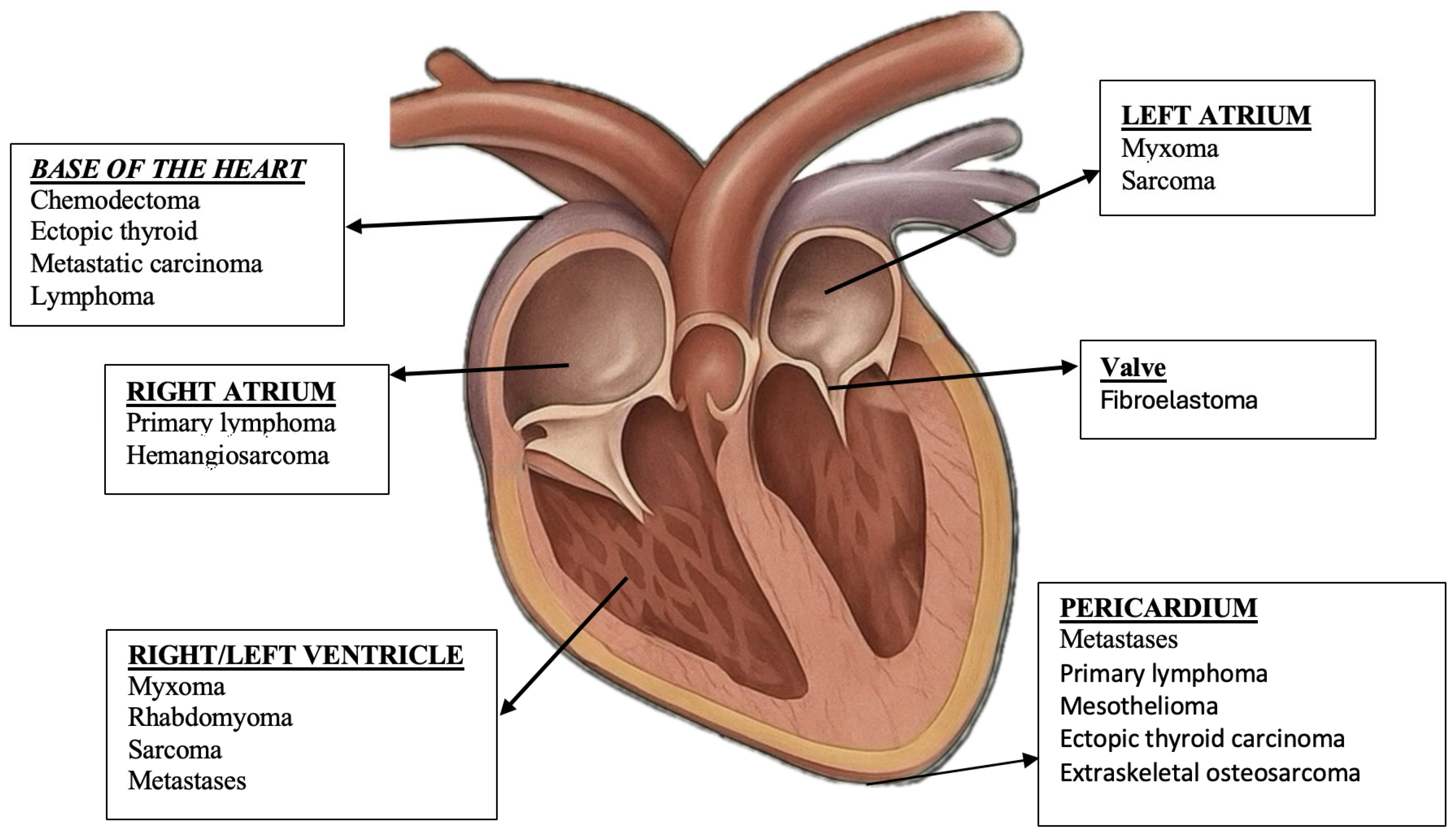
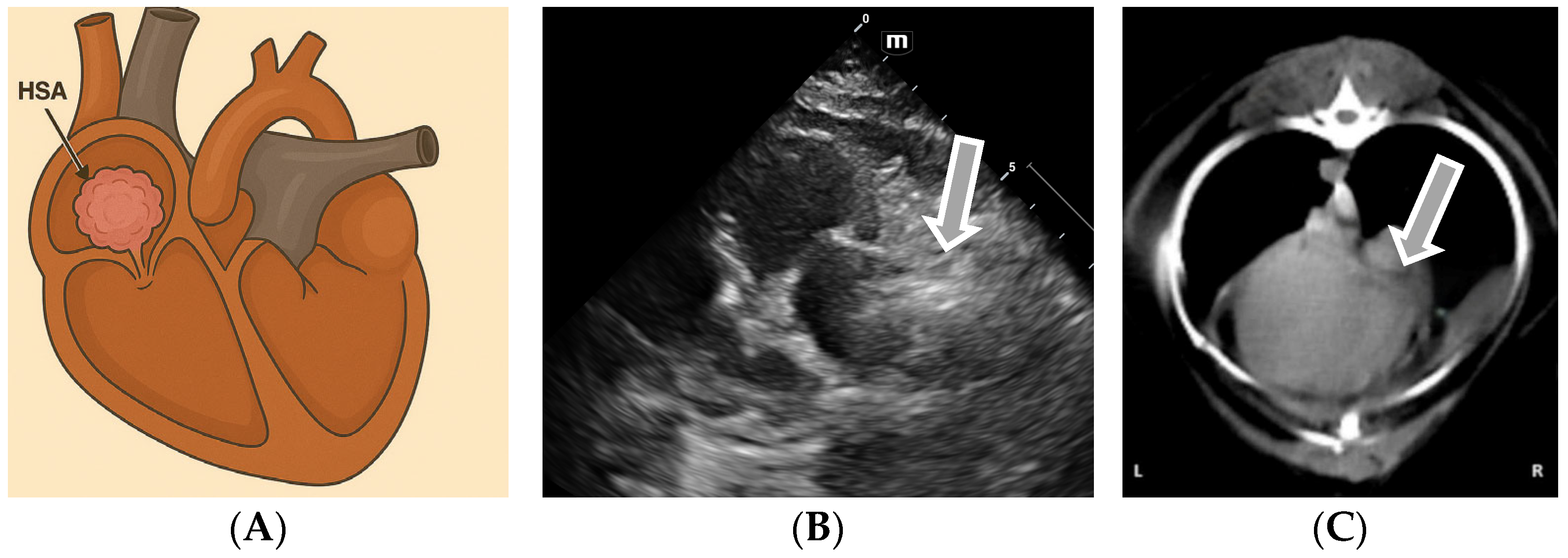
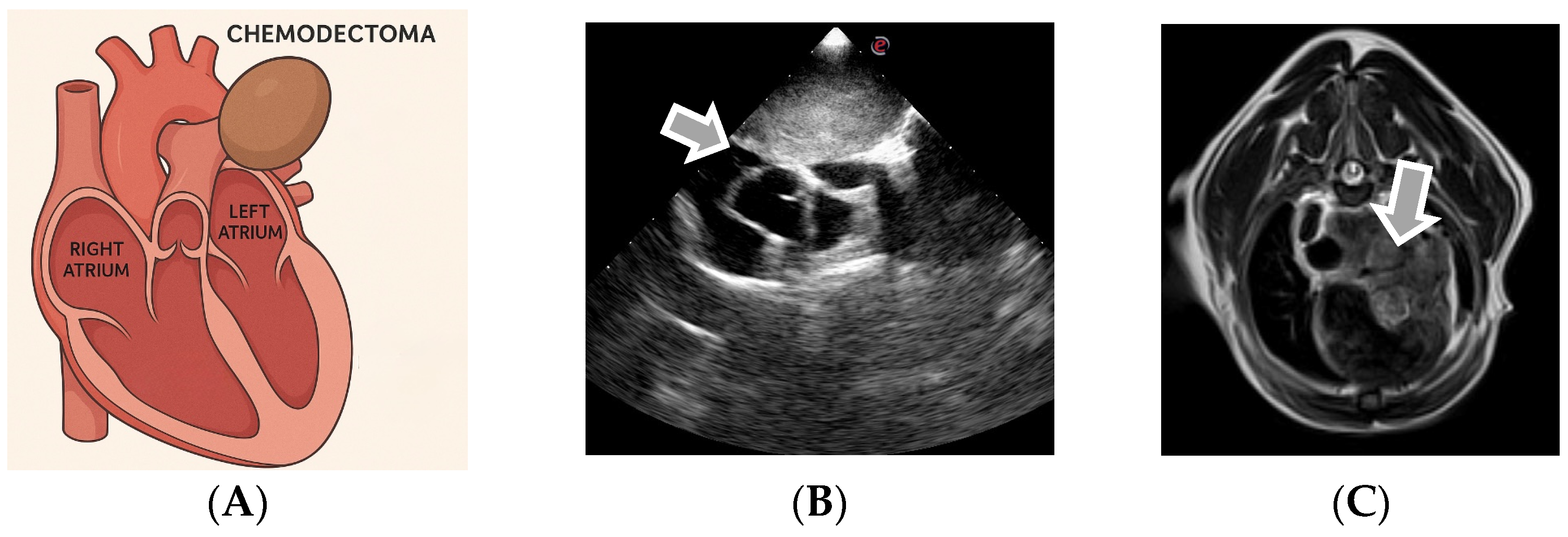
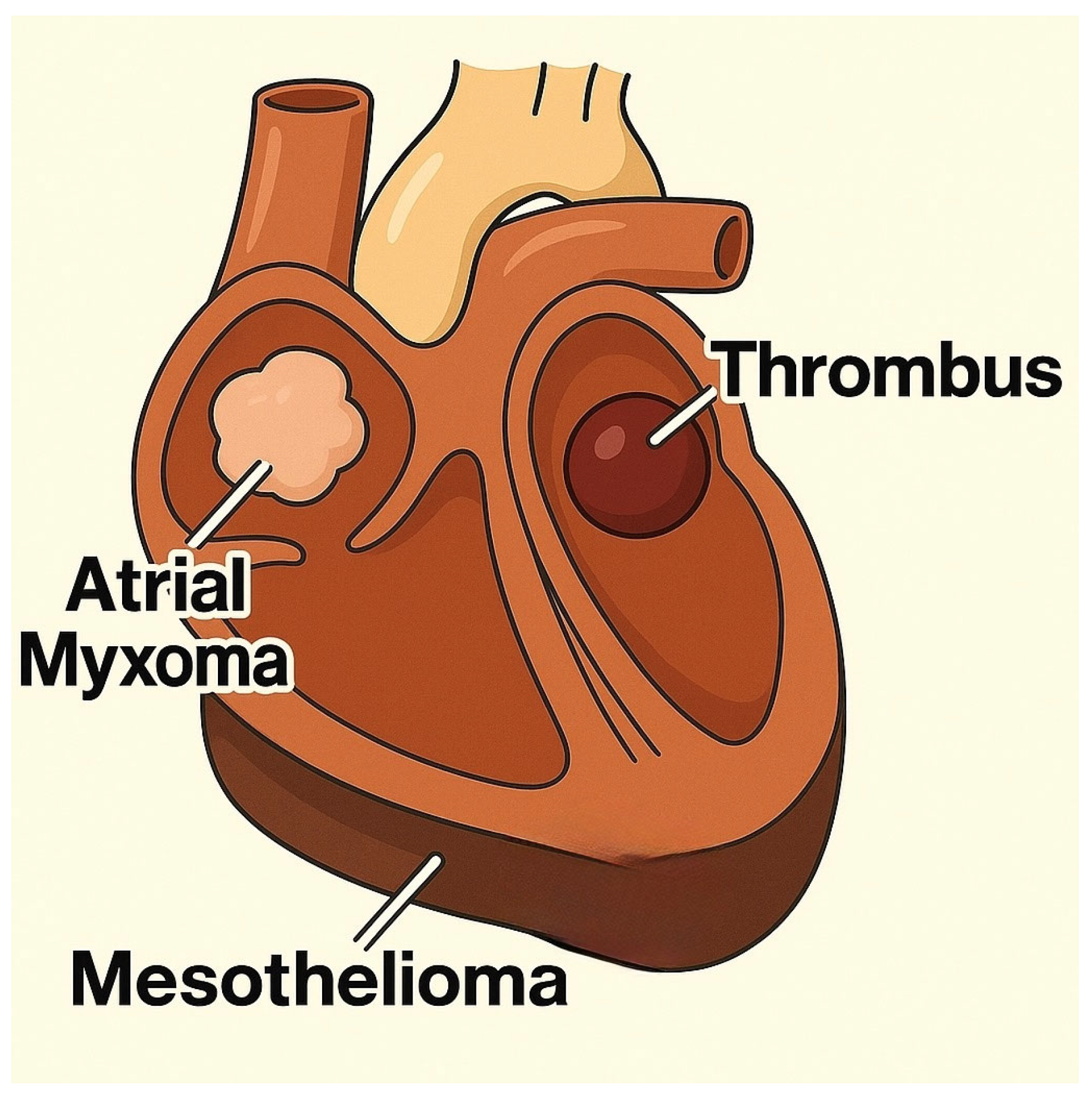
| Tumor Type | Auscultation Findings |
|---|---|
| Hemangiosarcoma |
|
| Chemodectoma/Aortic-body Tumor |
|
| Lymphoma (cardiac infiltration) |
|
| Fibrosarcoma/Undifferentiated Sarcoma |
|
| Extraskeletal Osteosarcoma |
|
| Mesothelioma |
|
| Ectopic Thyroid Tumor |
|
| Myxoma/Myxosarcoma |
|
| Tumor Type | Common Location | Main Clinical Signs |
|---|---|---|
| Hemangiosarcoma | Right atrium, pericardium | Lethargy, exertional syncope, jugular distension, ascites, collapse, dyspnea |
| Chemodectoma (Paraganglioma) | Heart base (aortic bifurcation) | Chronic cough, dyspnea, bradycardia, exercise intolerance, tracheal compression |
| Lymphoma | Myocardium, pericardium, atria | Arrhythmias, tachycardia, lethargy, congestive heart failure, generalized lymphadenopathy |
| Mesothelioma | Pericardium | Dyspnea, gallop rhythm, pericardial effusion, cough, signs of right-sided heart failure |
| Myxomas/Myxosarcomas | Cardiac valves, chambers | Heart murmur, signs of obstruction, mild dyspnea, reduced exercise tolerance |
| Ectopic thyroid tumors | Heart base/anterior mediastinum | Heart murmur, mediastinal compression, dyspnea, cough |
| Feature | Chemodectoma | Ectopic Thyroid Tumor |
|---|---|---|
| Common Location | The base of the heart, near aortic root and pulmonary artery | Cranial mediastinum, near the base of the heart |
| Vascular Involvement | Encircles pulmonary arteries and may compress atria | Surrounds great vessels (cranial vena cava, aorta) |
| Echogenicity | Homogeneous, hyperechoic | Homogeneous, hyperechoic |
| Mobility on Echo | Non-mobile | Non-mobile |
| Contour | Smooth margins | Smooth margins |
| Growth Speed | Very slow | Slow |
| Infiltration | Extremely rare | None |
| Number of Masses | Typically single | Single |
| Other Clues | Often incidental and predisposed to brachycephalic breeds | May be functional (thyroid hormones) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mincă, N.A.; Dumbravă, I.C.; Tudor, N.; Ștefănescu, A.; Vițălaru, A.B.; Ioniță, L.; Țogoe, D. Review on the Clinical, Imaging, and Therapeutic Aspects of Cardiac Masses in Dog. Life 2025, 15, 1092. https://doi.org/10.3390/life15071092
Mincă NA, Dumbravă IC, Tudor N, Ștefănescu A, Vițălaru AB, Ioniță L, Țogoe D. Review on the Clinical, Imaging, and Therapeutic Aspects of Cardiac Masses in Dog. Life. 2025; 15(7):1092. https://doi.org/10.3390/life15071092
Chicago/Turabian StyleMincă, Nicoleta Andreea, Ionuț Cătălin Dumbravă, Niculae Tudor, Alina Ștefănescu, Alexandru Bogdan Vițălaru, Lucian Ioniță, and Dorin Țogoe. 2025. "Review on the Clinical, Imaging, and Therapeutic Aspects of Cardiac Masses in Dog" Life 15, no. 7: 1092. https://doi.org/10.3390/life15071092
APA StyleMincă, N. A., Dumbravă, I. C., Tudor, N., Ștefănescu, A., Vițălaru, A. B., Ioniță, L., & Țogoe, D. (2025). Review on the Clinical, Imaging, and Therapeutic Aspects of Cardiac Masses in Dog. Life, 15(7), 1092. https://doi.org/10.3390/life15071092





