Physical Therapy Interventions for Gait and Balance in Charcot-Marie-Tooth Disease: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Review Question
2.2. Eligibility Criteria
2.3. Exclusion Criteria
- Studies involving patients with comorbid neurological disorders not related to Charcot-Marie-Tooth disease.
- Studies that did not assess both gait and balance outcomes.
- Studies investigating orthotic or surgical interventions without an associated physiotherapeutic programme.
- Protocols, ongoing trials, abstracts without full text, and studies published in languages other than English (if not translatable).
- Reviews or opinion papers that did not include original clinical data or outcome measures.
2.4. Search Strategy
- MEDLINE (PubMed):(“charcot marie tooth disease” [MeSH Terms] OR “charcot marie tooth” [All Fields] OR “hereditary motor and sensory neuropathy” [All Fields] OR HMSN) AND (“rehabilitation” [MeSH Terms] OR “physiotherapy” [All Fields] OR “physical therapy modalities” [MeSH Terms] OR “exercise therapy”) AND (“gait” [MeSH Terms] OR “walking” [MeSH Terms] OR “balance” [MeSH Terms] OR “postural balance”)
- Cochrane Central:(charcot marie tooth OR hereditary motor and sensory neuropathy) AND (rehabilitation OR physiotherapy OR physical therapy) AND (gait OR walking OR balance)
- Scopus:TITLE-ABS-KEY (“charcot marie tooth” OR “hereditary motor and sensory neuropathy”) AND TITLE-ABS-KEY (“rehabilitation” OR “physiotherapy” OR “physical therapy”) AND TITLE-ABS-KEY (“gait” OR “balance” OR “walking”)
- PEDro:The search within the PEDro (Physiotherapy Evidence Database) platform was conducted using the advanced search function. The term “charcot marie tooth” was entered in the “Title & Abstract” field, and the subdiscipline was restricted to “Neurology” to enhance the specificity of the results. To ensure a comprehensive retrieval of relevant studies, an alternative search was also performed using the term “hereditary motor and sensory neuropathy” in the same field, again under the neurology subdiscipline. The search was not limited by publication type, language, or date, and all study designs available in the PEDro database were considered eligible. Due to the constraints of the platform, Boolean operators such as AND/OR could not be applied across multiple fields simultaneously. Therefore, broader terms were used, and the relevance of each record was verified manually through title and abstract screening. All retrieved records were exported and catalogued using Zotero for subsequent de-duplication and eligibility assessment.
- Web of Science:TS = (“charcot marie tooth” OR “hereditary motor and sensory neuropathy”) AND TS = (“rehabilitation” OR “physiotherapy” OR “physical therapy”) AND TS = (“gait” OR “walking” OR “balance”)
2.5. Study Selection
2.6. Data Extraction and Data Synthesis
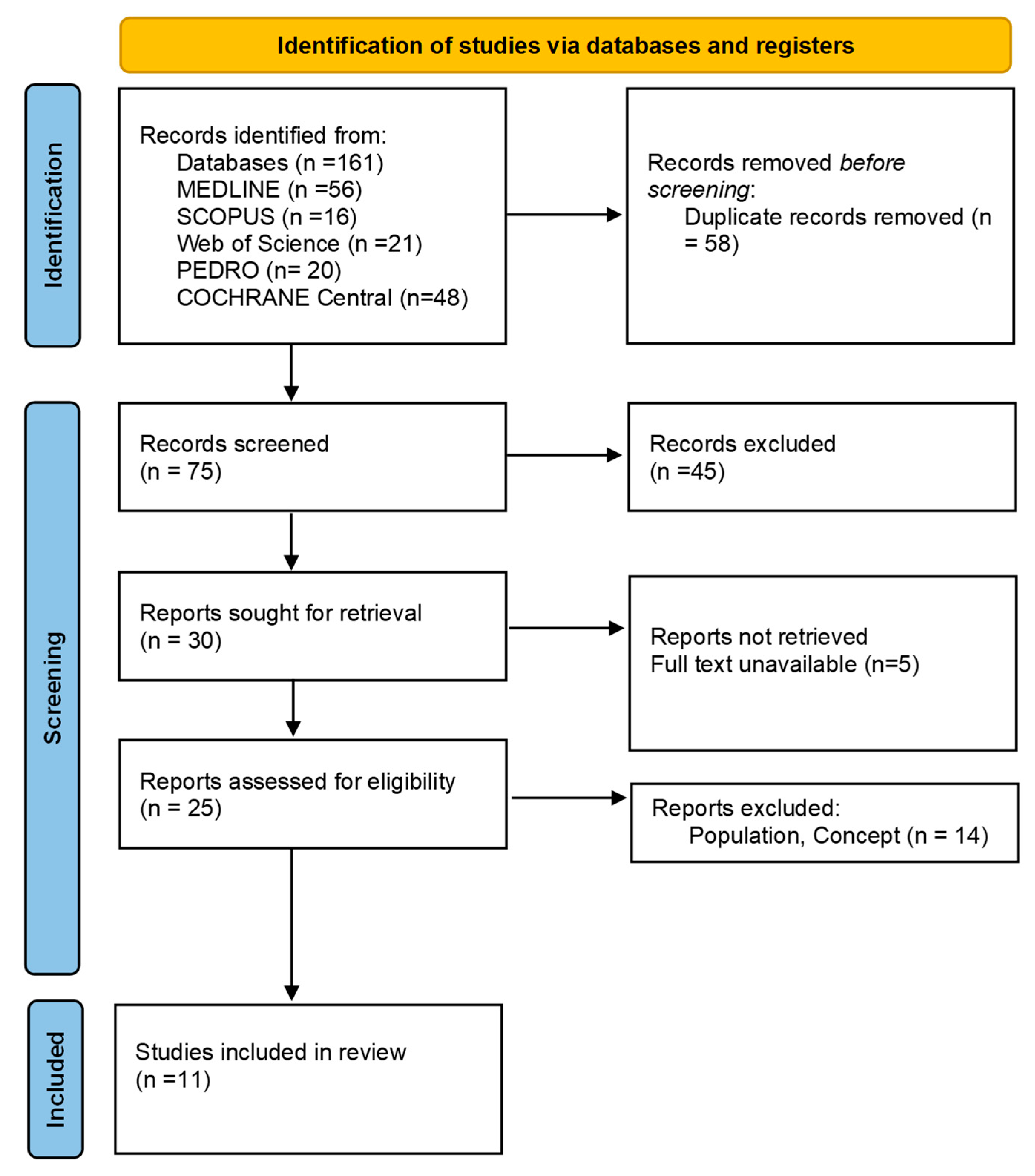
3. Results
3.1. Walking Performance
3.2. Balance
3.3. Muscle Strength
3.4. Fatigue
3.5. Pain and Cramping
3.6. Quality of Life and Patient-Reported Outcomes
4. Discussion
5. Limitations
6. Clinical Practice Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nagappa, M.; Sharma, S.; Taly, A.B. Charcot-Marie-Tooth Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Van den Bergh, P.Y.K.; van Doorn, P.A.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Allen, J.A.; Attarian, S.; Blomkwist-Markens, P.H.; Cornblath, D.R.; Eftimov, F.; et al. European Academy of Neurology/Peripheral Nerve Society Guideline on Diagnosis and Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Report of a Joint Task Force-Second Revision. Eur. J. Neurol. 2021, 28, 3556–3583. [Google Scholar] [CrossRef] [PubMed]
- Pipis, M.; Rossor, A.M.; Laura, M.; Reilly, M.M. Next-Generation Sequencing in Charcot-Marie-Tooth Disease: Opportunities and Challenges. Nat. Rev. Neurol. 2019, 15, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Aprile, I.; Cavallaro, T.; Commodari, I.; La Torre, G.; Pareyson, D.; Quattrone, A.; Rizzuto, N.; Vita, G.; Tonali, P.; et al. Variables Influencing Quality of Life and Disability in Charcot Marie Tooth (CMT) Patients: Italian Multicentre Study. Neurol. Sci. 2006, 27, 417–423. [Google Scholar] [CrossRef]
- Vinci, P.; Serrao, M.; Millul, A.; Deidda, A.; De Santis, F.; Capici, S.; Martini, D.; Pierelli, F.; Santilli, V. Quality of Life in Patients with Charcot-Marie-Tooth Disease. Neurology 2005, 65, 922–924. [Google Scholar] [CrossRef]
- Johnson, N.E.; Heatwole, C.R.; Dilek, N.; Sowden, J.; Kirk, C.A.; Shereff, D.; Shy, M.E.; Herrmann, D.N.; Inherited Neuropathies Consortium. Quality-of-Life in Charcot-Marie-Tooth Disease: The Patient’s Perspective. Neuromuscul. Disord. 2014, 24, 1018–1023. [Google Scholar] [CrossRef]
- Fridman, V.; Reilly, M.M. Inherited Neuropathies. Semin. Neurol. 2015, 35, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Corrado, B.; Ciardi, G.; Bargigli, C. Rehabilitation Management of the Charcot-Marie-Tooth Syndrome: A Systematic Review of the Literature. Medicine 2016, 95, e3278. [Google Scholar] [CrossRef]
- Ferraro, F.; Calafiore, D.; Curci, C.; Fortunato, F.; Carantini, I.; Genovese, F.; Lucchini, G.; Merlo, A.; Ammendolia, A.; de Sire, A. Effects of Intensive Rehabilitation on Functioning in Patients with Mild and Moderate Charcot-Marie-Tooth Disease: A Real-Practice Retrospective Study. Neurol. Sci. 2024, 45, 289–297. [Google Scholar] [CrossRef]
- Matjacić, Z.; Zupan, A. Effects of Dynamic Balance Training during Standing and Stepping in Patients with Hereditary Sensory Motor Neuropathy. Disabil. Rehabil. 2006, 28, 1455–1459. [Google Scholar] [CrossRef]
- Knak, K.L.; Andersen, L.K.; Vissing, J. Aerobic Anti-gravity Exercise in Patients with Charcot–Marie–Tooth Disease Types 1A and X: A Pilot Study. Brain Behav. 2017, 7, e00794. [Google Scholar] [CrossRef]
- Kobesova, A.; Kolar, P.; Mlckova, J.; Svehlik, M.; Morris, C.E.; Frank, C.; Lepsikova, M.; Kozak, J. Effect of Functional Stabilization Training on Balance and Motor Patterns in a Patient with Charcot-Marie-Tooth Disease. Neuro Endocrinol. Lett. 2012, 33, 3–10. [Google Scholar]
- Pazzaglia, C.; Camerota, F.; Germanotta, M.; Di Sipio, E.; Celletti, C.; Padua, L. Efficacy of Focal Mechanic Vibration Treatment on Balance in Charcot-Marie-Tooth 1A Disease: A Pilot Study. J. Neurol. 2016, 263, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R. Podological Analysis in Children with Neuromotor Disabilities. Reabil. Moksl. Slauga Kineziter. Ergoter. 2024, 2, 63–71. [Google Scholar] [CrossRef]
- Dudziec, M.M.; Lee, L.E.; Massey, C.; Tropman, D.; Skorupinska, M.; Laurá, M.; Reilly, M.M.; Ramdharry, G.M. Home-Based Multi-Sensory and Proximal Strengthening Program to Improve Balance in Charcot-Marie-Tooth Disease Type 1A: A Proof of Concept Study. Muscle Nerve 2024, 69, 354–361. [Google Scholar] [CrossRef]
- Bottoni, G.; Crisafulli, O.; Pisegna, C.; Serra, M.; Brambilla, S.; Feletti, F.; Cremonte, G.; D’Antona, G. An 8-Month Adapted Motor Activity Program in a Young CMT1A Male Patient. Front. Physiol. 2024, 15, 1347319. [Google Scholar] [CrossRef] [PubMed]
- Mori, L.; Signori, A.; Prada, V.; Pareyson, D.; Piscosquito, G.; Padua, L.; Pazzaglia, C.; Fabrizi, G.M.; Picelli, A.; Schenone, A.; et al. Treadmill Training in Patients Affected by Charcot-Marie-Tooth Neuropathy: Results of a Multicenter, Prospective, Randomized, Single-Blind, Controlled Study. Eur. J. Neurol. 2020, 27, 280–287. [Google Scholar] [CrossRef]
- Tedeschi, R.; Labanca, L.; Platano, D.; Benedetti, M.G. Assessment of Balance During a Single-Limb Stance Task in Healthy Adults: A Cross-Sectional Study. Percept. Mot. Skills 2024, 131, 1504–1516. [Google Scholar] [CrossRef]
- Jennings, M.J.; Lochmüller, A.; Atalaia, A.; Horvath, R. Targeted Therapies for Hereditary Peripheral Neuropathies: Systematic Review and Steps Towards a ‘Treatabolome’. J. Neuromuscul. Dis. 2021, 8, 383–400. [Google Scholar] [CrossRef]
- Luglio, A.; Maggi, E.; Riviello, F.N.; Conforti, A.; Sorrentino, U.; Zuccarello, D. Hereditary Neuromuscular Disorders in Reproductive Medicine. Genes 2024, 15, 1409. [Google Scholar] [CrossRef]
- Pagliano, E.; Foscan, M.; Marchi, A.; Corlatti, A.; Aprile, G.; Riva, D. Intensive Strength and Balance Training with the Kinect Console (Xbox 360) in a Patient with CMT1A. Dev. Neurorehabil. 2018, 21, 542–545. [Google Scholar] [CrossRef]
- Peters: Joanna Briggs Institute Reviewer’s Manual, JBI—Google Scholar. Available online: https://scholar-google-com.ezproxy.unibo.it/scholar_lookup?hl=en&publication_year=2020&author=MDJ+Peters&author=C+Godfrey&author=P+McInerney&author=Z+Munn&author=AC+Tricco&author=H+Khalil&title=Joanna+Briggs+Institute+Reviewer%27s+Manual%2C+JBI (accessed on 9 June 2022).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Raymond, J.; Ouvrier, R. Feasibility of Foot and Ankle Strength Training in Childhood Charcot-Marie-Tooth Disease. Neuromuscul. Disord. 2009, 19, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.; Dusina, B.; Carantini, I.; Strambi, R.; Galante, E.; Gaiani, L. The Efficacy of Functional Surgery Associated with Early Intensive Rehabilitation Therapy in Charcot-Marie-Tooth Type 1A Disease. Eur. J. Phys. Rehabil. Med. 2017, 53, 788–793. [Google Scholar] [CrossRef]
- Mori, L.; Schenone, C.; Cotellessa, F.; Ponzano, M.; Aiello, A.; Lagostina, M.; Massucco, S.; Marinelli, L.; Grandis, M.; Trompetto, C.; et al. Quality of Life and Upper Limb Disability in Charcot-Marie-Tooth Disease: A Pilot Study. Front. Neurol. 2022, 13, 964254. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R. Kinematic and Plantar Pressure Analysis in Strumpell-Lorrain Disease: A Case Report. Brain Disorders 2023, 11, 100097. [Google Scholar] [CrossRef]
- Tedeschi, R. Reevaluating the Drucebo Effect: Implications for Physiotherapy Practice. J. Psychosoc. Rehabil. Ment. Health 2024, 11, 391–393. [Google Scholar] [CrossRef]
- Tedeschi, R. The Forgotten DOMS: Recognising Delayed Muscle Soreness in Hand Rehabilitation. Br. J. Sports Med. 2025. [Google Scholar] [CrossRef]
| Authors, Year | Enrolled Patients | Experimental Protocol | Evaluation Metrics | Quantitative Results |
|---|---|---|---|---|
| Knak et al., 2017 [11] | 5 patients (CMT1A = 4, CMTX = 1) | 10-week control phase followed by 10-week treadmill aerobic training (30 min, 3x/week) | 6MWT, BBS, stabilometric tests, FSS, SF-36 | Improved BBS; walking non-significant; 2 cases of temporary pain |
| Bottoni et al., 2024 [16] | 1 male, 16 y.o. (CMT1A) | Adapted motor activity (1 h twice/week, 8 months) | SF-36, CIS-20R, 6MWT, 10MWT, SPPB, BBS, YBT, MVC, TOE, EMG | Improved fatigue, balance, and walking; mixed muscle strength |
| Kobesova et al., 2012 [12] | 1 male, 55 y.o. (CMTX) | 3-week intensive rehab (2.5 h/day) | mCTSIB, LOS, FL (dynamic posturography) | Improved balance and walking stability; reduced plantar and back pain |
| Matjacić & Zupan, 2006 [10] | 16 ambulant patients (CMT1) | 2-week rehab: Balance Trainer vs. conventional physio | BBS, TUG, 10MWT | Significant improvements in both groups; greater with Balance Trainer |
| Ferraro et al., 2024 (1) [9] | 37 patients (CMT1, CMT2, mixed) | 3-week intensive rehab (2–4 h/day, 5x/week) | MRC, VRS, BBS, Walk12, 10MWT | Significant gains post-treatment; partial regression at 12-month follow-up |
| Pazzaglia et al., 2016 [13] | 14 patients (CMT1A) | 3-day focused mechanical vibration on lower limb muscles | BBS, DGI, 6MWT, MRC, stabilometry, SF-36 | Improved BBS and DGI; mild changes in 6MWT, MRC, SF-36 |
| Burns et al., 2009 [24] | 1 female, 15 y.o. (AR-CMT2) | 12-week dorsiflexor strengthening home programme (3x/week) | Dynamometry, BOT2, jump test, 6MWT, gait analysis | Strength gains in dorsiflexors; unchanged balance/endurance |
| Dudziec et al., 2024 [15] | 14 patients (CMT1A, fall history) | 12-week home-based exercise + fall education | BBS, BESTest, posturography, 10MWT, FGA, dynamometry, Walk12, SF-36, FES-I, IPAQ, HADS | Improved balance and walking in the intervention group; better subjective outcomes |
| Pagliano et al., 2018 [21] | 1 male, 9 y.o. (CMT1A) | 5-week home programe: ankle strengthening + Kinect balance games (3x/week) | BOT2, jump test, 6MWT, dynamometry, Walk-12 | Improved balance and endurance; mixed strength outcomes |
| Ferraro et al., 2017 [25] | 5 patients (CMT1A) | Foot surgery, 3 weeks in a cast, then 3-week of intensive rehab (2x/day, 5x/week) | BBS, WHS, 10MWT, Gait Analysis, Walk-12, VAS, MRC, OHS, CMTNS | Improved proximal strength, balance, and pain; limited gait gains |
| Mori et al., 2022 [26] | 53 ambulant patients (CMT1A) | 3-month TreSPE vs. SPE (treadmill, proprioception, etc.) | 6MWT, 10MWT, BBS, SPPB, dynamometry, CMTNS, Walk-12, SF-36 | Improved gait and plantarflexor strength; better balance in the TreSPE group |
| Author | Study Type | Walking | Balance | Strength | Fatigue | QoL |
|---|---|---|---|---|---|---|
| Knak et al. [11] | Case series | ↑ | ↑ | → | → | → |
| Bottoni et al. [16] | Case report | ↑ | ↑ | ↑ | ↑ | ↑ |
| Kobesova et al. [12] | Case report | ↑ | ↑ | → | → | ↑ |
| Matjacić and Zupan [10] | RCT | ↑ | ↑ | ↑ | → | → |
| Ferraro et al. (1) [9] | Cohort study | ↑ | ↑ | ↑ | ↑ | ↑ |
| Pazzaglia et al. [13] | Case series | ↑ | ↑ | → | → | ↑ |
| Burns et al. [24] | Case report | → | → | ↑ | → | → |
| Dudziec et al. [15] | RCT | ↑ | ↑ | → | → | ↑ |
| Pagliano et al. [21] | Case report | ↑ | ↑ | → | → | → |
| Ferraro et al. (2) [25] | Case series | ↑ | ↑ | ↑ | → | → |
| Mori et al. [26] | Cohort study | ↑ | ↑ | ↑ | → | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, R.; Donati, D.; Giorgi, F. Physical Therapy Interventions for Gait and Balance in Charcot-Marie-Tooth Disease: A Scoping Review. Life 2025, 15, 1036. https://doi.org/10.3390/life15071036
Tedeschi R, Donati D, Giorgi F. Physical Therapy Interventions for Gait and Balance in Charcot-Marie-Tooth Disease: A Scoping Review. Life. 2025; 15(7):1036. https://doi.org/10.3390/life15071036
Chicago/Turabian StyleTedeschi, Roberto, Danilo Donati, and Federica Giorgi. 2025. "Physical Therapy Interventions for Gait and Balance in Charcot-Marie-Tooth Disease: A Scoping Review" Life 15, no. 7: 1036. https://doi.org/10.3390/life15071036
APA StyleTedeschi, R., Donati, D., & Giorgi, F. (2025). Physical Therapy Interventions for Gait and Balance in Charcot-Marie-Tooth Disease: A Scoping Review. Life, 15(7), 1036. https://doi.org/10.3390/life15071036





