The First Case Report of a Primary Mast Cell Tumor Originating from the Inguinal Lymph Node in a Nine-Year-Old Female Maltese Dog and a Comparative Literature Review in Humans
Abstract
1. Introduction
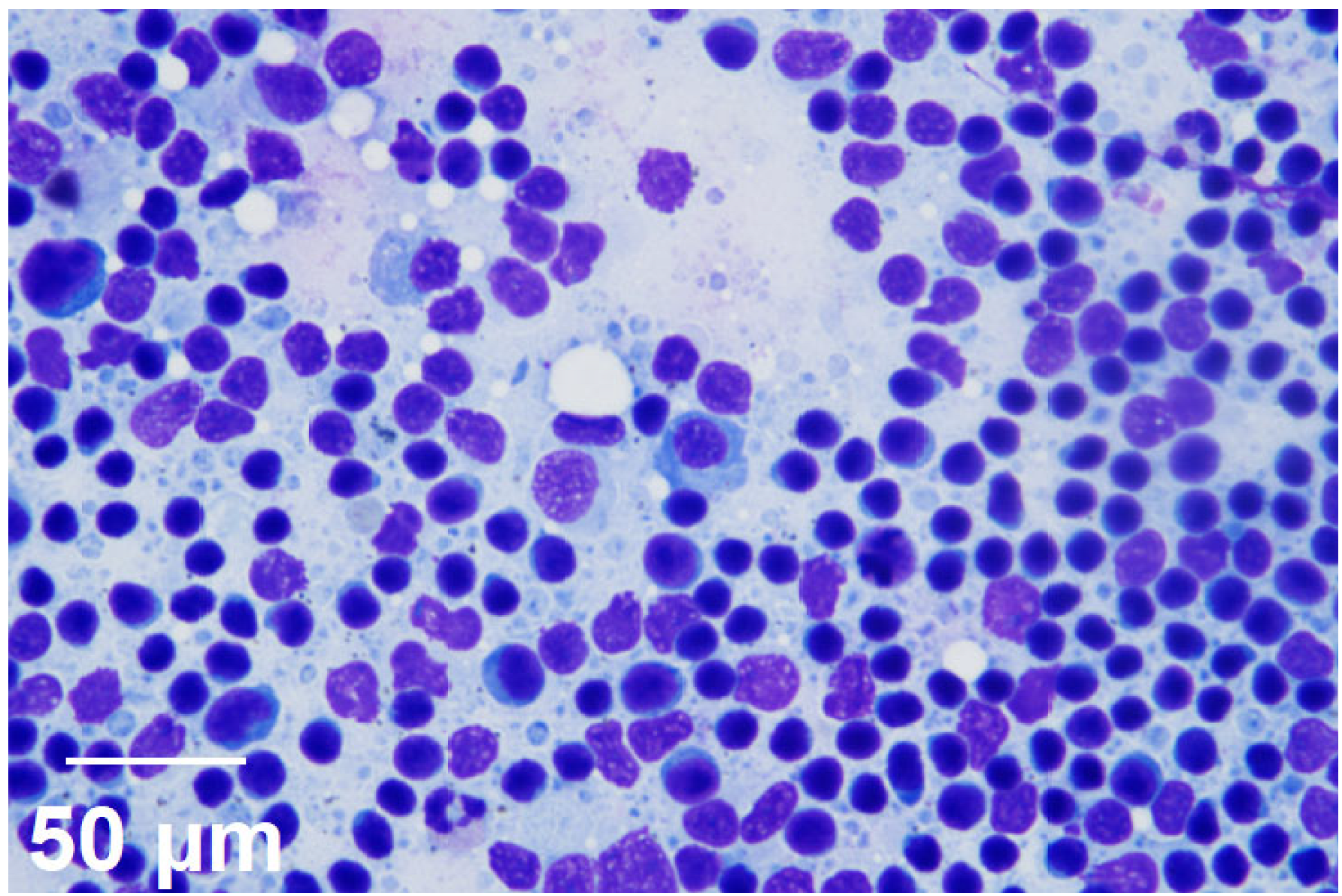
2. Case Presentation
3. Discussion
3.1. Correlation Between MCT and MGT Growth
3.2. Hypothesis of a Rare Primary Lymph Node MCT
3.3. Spatial Transcriptomics and Single-Cell Sequencing of the Lymph Node MCT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A


References
- Amin, K. The role of mast cells in allergic inflammation. Respir. Med. 2012, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M. Mast cell tumors. In Tumors in Domestic Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 176–202. [Google Scholar]
- Shoop, S.J.; Marlow, S.; Church, D.B.; English, K.; McGreevy, P.D.; Stell, A.J.; Thomson, P.C.; O’Neill, D.G.; Brodbelt, D.C. Prevalence and risk factors for mast cell tumours in dogs in England. Canine Genet. Epidemiol. 2015, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Pearl, D.; Yager, J.; Best, S.; Coomber, B.; Foster, R. Canine subcutaneous mast cell tumor: Characterization and prognostic indices. Vet. Pathol. 2011, 48, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Welle, M.M.; Bley, C.R.; Howard, J.; Rüfenacht, S. Canine mast cell tumours: A review of the pathogenesis, clinical features, pathology and treatment. Vet. Dermatol. 2008, 19, 321–339. [Google Scholar] [CrossRef]
- Ozaki, K.; Yamagami, T.; Nomura, K.; Narama, I. Mast cell tumors of the gastrointestinal tract in 39 dogs. Vet. Pathol. 2002, 39, 557–564. [Google Scholar] [CrossRef]
- Elliott, J.; Cripps, P.; Blackwood, L.; Berlato, D.; Murphy, S.; Grant, I. Canine oral mucosal mast cell tumours. Vet. Comp. Oncol. 2016, 14, 101–111. [Google Scholar] [CrossRef]
- Campbell, O.; de Lorimier, L.-P.; Beauregard, G.; Overvelde, S.; Johnson, S. Presumptive primary pulmonary mast cell tumor in 2 dogs. Can. Vet. J. 2017, 58, 591–596. [Google Scholar]
- Patnaik, A.; MacEwen, E.; Black, A.; Luckow, S. Extracutaneous mast-cell tumor in the dog. Vet. Pathol. 1982, 19, 608–615. [Google Scholar] [CrossRef]
- Clark, D.; Harvey, H.; Roth, L.; Callihan, D. Clostridial peritonitis associated with a mast cell tumor in a dog. J. Am. Vet. Med. Assoc. 1986, 188, 188–190. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakaguchi, K.; Kobayashi, S.; Tominaga, M.; Hirayama, K.; Kadosawa, T.; Taniyama, H. Systemic candidiasis and mesenteric mast cell tumor with multiple metastases in a dog. J. Vet. Med. Sci. 2009, 71, 229–232. [Google Scholar] [CrossRef]
- Conti, P.; Castellani, M.L.; Kempuraj, D.; Salini, V.; Vecchiet, J.; Tetè, S.; Mastrangelo, F.; Perrella, A.; De Lutiis, M.A.; Tagen, M. Role of mast cells in tumor growth. Ann. Clin. Lab. Sci. 2007, 37, 315–322. [Google Scholar] [PubMed]
- Lavalle, G.; Bertagnolli, A.; Tavares, W.; Ferreira, M.; Cassali, G. Mast cells and angiogenesis in canine mammary tumor. Arq. Bras. Med. Vet. Zootec. 2010, 62, 1348–1351. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. Controversial role of mast cells in breast cancer tumor progression and angiogenesis. Clin. Breast Cancer 2021, 21, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Bertola, L.; Pellizzoni, B.; Giudice, C.; Grieco, V.; Ferrari, R.; Chiti, L.E.; Stefanello, D.; Manfredi, M.; De Zani, D.; Recordati, C. Tumor-associated macrophages and tumor-infiltrating lymphocytes in canine cutaneous and subcutaneous mast cell tumors. Vet. Pathol. 2024, 61, 882–4895. [Google Scholar] [CrossRef]
- Yale, A.D.; Szladovits, B.; Stell, A.J.; Fitzgerald, S.D.; Priestnall, S.L.; Suarez-Bonnet, A. High-grade cutaneous mast cell tumour with widespread intrathoracic metastasis and neoplastic pericardial effusion in a dog. J. Comp. Pathol. 2020, 180, 29–34. [Google Scholar] [CrossRef]
- Marconato, L.; Bettini, G.; Giacoboni, C.; Romanelli, G.; Cesari, A.; Zatelli, A.; Zini, E. Clinicopathological features and outcome for dogs with mast cell tumors and bone marrow involvement. J. Vet. Intern. Med. 2008, 22, 1001–1007. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Bianchi, M.V.; Bandinelli, M.B.; Rosa, R.B.; Echenique, J.V.Z.; Serpa Stolf, A.; Driemeier, D.; Sonne, L.; Pavarini, S.P. Pathological aspects of cutaneous mast cell tumors with metastases in 49 dogs. Vet. Pathol. 2022, 59, 922–930. [Google Scholar] [CrossRef]
- Hikasa, Y.; Morita, T.; Futaoka, Y.; Sato, K.; Shimada, A.; Kagota, K.; Matsuda, H. Connective tissue-type mast cell leukemia in a dog. J. Vet. Med. Sci. 2000, 62, 187–190. [Google Scholar] [CrossRef]
- Coelho, Y.N.B.; Soldi, L.R.; Silva, P.H.R.d.; Mesquita, C.M.; Paranhos, L.R.; Santos, T.R.d.; Silva, M.J.B. Tyrosine kinase inhibitors as an alternative treatment in canine mast cell tumor. Front. Vet. Sci. 2023, 10, 1188795. [Google Scholar] [CrossRef]
- Davies, D.R.; Wyatt, K.M.; Jardine, J.E.; Robertson, I.D.; Irwin, P.J. Vinblastine and prednisolone as adjunctive therapy for canine cutaneous mast cell tumors. J. Am. Anim. Hosp. Assoc. 2004, 40, 124–130. [Google Scholar] [CrossRef]
- Rifici, C.; Sfacteria, A.; Di Giorgio, S.; Giambrone, G.; Marino, G.; Mazzullo, G. Mast Cell Tumour and Mammary Gland Carcinoma Collision Tumour. Case report and literature review. J. Hell. Vet. Med. Soc. 2022, 73, 4675–4680. [Google Scholar] [CrossRef]
- Kawai, H.; Li, H.; Chun, P.; Avraham, S.; Avraham, H.K. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene 2002, 21, 7730–7739. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. Mast cells and angiogenesis. APMIS 2002, 110, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, L.; Willmann, M.; Sonneck, K.; Gleixner, K.V.; Florian, S.; Kondo, R.; Mayerhofer, M.; Vales, A.; Gruze, A.; Pickl, W.F. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet. Immunol. Immunopathol. 2007, 115, 320–333. [Google Scholar] [CrossRef]
- Sakalauskaitė, S.; Šaltenienė, V.; Nikitina, D.; Ugenskienė, R.; Riškevičienė, V.; Karvelienė, B.; Juodžiukynienė, N. VEGF-B, VEGF-A, FLT-1, KDR, ERBB2, EGFR, GRB2, RAC1, CDH1 and HYAL-1 genes expression analysis in canine mammary gland tumors and the association with tumor clinicopathological parameters and dog breed assessment. Vet. Sci. 2021, 8, 212. [Google Scholar] [CrossRef]
- Marone, G.; Varricchi, G.; Loffredo, S.; Granata, F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2016, 778, 146–151. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Monteiro, L.; Rodrigues, M.; Gomes, D.; Salgado, B.; Cassali, G. Tumour-associated macrophages: Relation with progression and invasiveness, and assessment of M1/M2 macrophages in canine mammary tumours. Vet. J. 2018, 234, 119–125. [Google Scholar] [CrossRef]
- Ribatti, D. Mast cells and macrophages exert beneficial and detrimental effects on tumor progression and angiogenesis. Immunol. Lett. 2013, 152, 83–88. [Google Scholar] [CrossRef]
- Sfacteria, A.; Napoli, E.; Rifici, C.; Commisso, D.; Giambrone, G.; Mazzullo, G.; Marino, G. Immune cells and immunoglobulin expression in the mammary gland tumors of dog. Animals 2021, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Grutzkau, A.; Kruger-Krasagakes, S.; Baumeister, H.; Schwarz, C.; Kogel, H.; Welker, P.; Lippert, U.; Henz, B.M.; Moller, A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol. Biol. Cell 1998, 9, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Larcher, F.; Murillas, R.; Bolontrade, M.; Conti, C.J.; Jorcano, J.L. VEGF/VPF overexpression in skin of transgenic mice induces angiogenesis, vascular hyperpermeability and accelerated tumor development. Oncogene 1998, 17, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kunder, C.A.; St John, A.L.; Abraham, S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood J. Am. Soc. Hematol. 2011, 118, 5383–5393. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Thompson, H.L.; Burbelo, P.D.; Yamada, Y.; Kleinman, H.K.; Metcalfe, D. Mast cells chemotax to laminin with enhancement after IgE-mediated activation. J. Immunol. 1989, 143, 4188–4192. [Google Scholar] [CrossRef]
- Evans, R.; Flores-Borja, F.; Nassiri, S.; Miranda, E.; Lawler, K.; Grigoriadis, A.; Monypenny, J.; Gillet, C.; Owen, J.; Gordon, P. Integrin-mediated macrophage adhesion promotes lymphovascular dissemination in breast cancer. Cell Rep. 2019, 27, 1967–1978-E4. [Google Scholar] [CrossRef]
- Shapiro, E.; Biezuner, T.; Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 2013, 14, 618–630. [Google Scholar] [CrossRef]
- Moses, L.; Pachter, L. Museum of spatial transcriptomics. Nat. Methods 2022, 19, 534–546. [Google Scholar] [CrossRef]



| Organ | Mammary Gland | Spleen | Left Inguinal Lymph Node | Mast Cell Leukemia | Others (Including Inguinal and Hind Body Regions) | |
|---|---|---|---|---|---|---|
| Method | ||||||
| Full CT scan | Not applicable | Not applicable | Not applicable | Not applicable | Three inguinal nodules and three masses were found | |
| Biopsy (or histopathology) results | Mammary complex carcinoma, grade 1 | Nodular lymphoid hyperplasia | Mast cell tumor | Not applicable | Mild lymphoid follicular hyperplasia, nodular sebaceous hyperplasia, lipoma, and normal-haired skin | |
| Blood smear | Not applicable | Not applicable | Not applicable | Ruled out | Not applicable | |
| Procedure | Result |
|---|---|
| C-kit PCR specimen | Formalin-Fixed Paraffin-Embedded Tissue, positive |
| C-kit PCR 8 K9 Only | Negative |
| C-kit PCR 11 K9 Only | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Kwon, G.; Choi, K. The First Case Report of a Primary Mast Cell Tumor Originating from the Inguinal Lymph Node in a Nine-Year-Old Female Maltese Dog and a Comparative Literature Review in Humans. Life 2025, 15, 1029. https://doi.org/10.3390/life15071029
Lee N, Kwon G, Choi K. The First Case Report of a Primary Mast Cell Tumor Originating from the Inguinal Lymph Node in a Nine-Year-Old Female Maltese Dog and a Comparative Literature Review in Humans. Life. 2025; 15(7):1029. https://doi.org/10.3390/life15071029
Chicago/Turabian StyleLee, Nuri, Gibum Kwon, and Kyuhyung Choi. 2025. "The First Case Report of a Primary Mast Cell Tumor Originating from the Inguinal Lymph Node in a Nine-Year-Old Female Maltese Dog and a Comparative Literature Review in Humans" Life 15, no. 7: 1029. https://doi.org/10.3390/life15071029
APA StyleLee, N., Kwon, G., & Choi, K. (2025). The First Case Report of a Primary Mast Cell Tumor Originating from the Inguinal Lymph Node in a Nine-Year-Old Female Maltese Dog and a Comparative Literature Review in Humans. Life, 15(7), 1029. https://doi.org/10.3390/life15071029






