Prognostic Impact of Right Ventricular Damage Markers on CT in Patients Undergoing TAVI
Abstract
1. Introduction
2. Material and Method
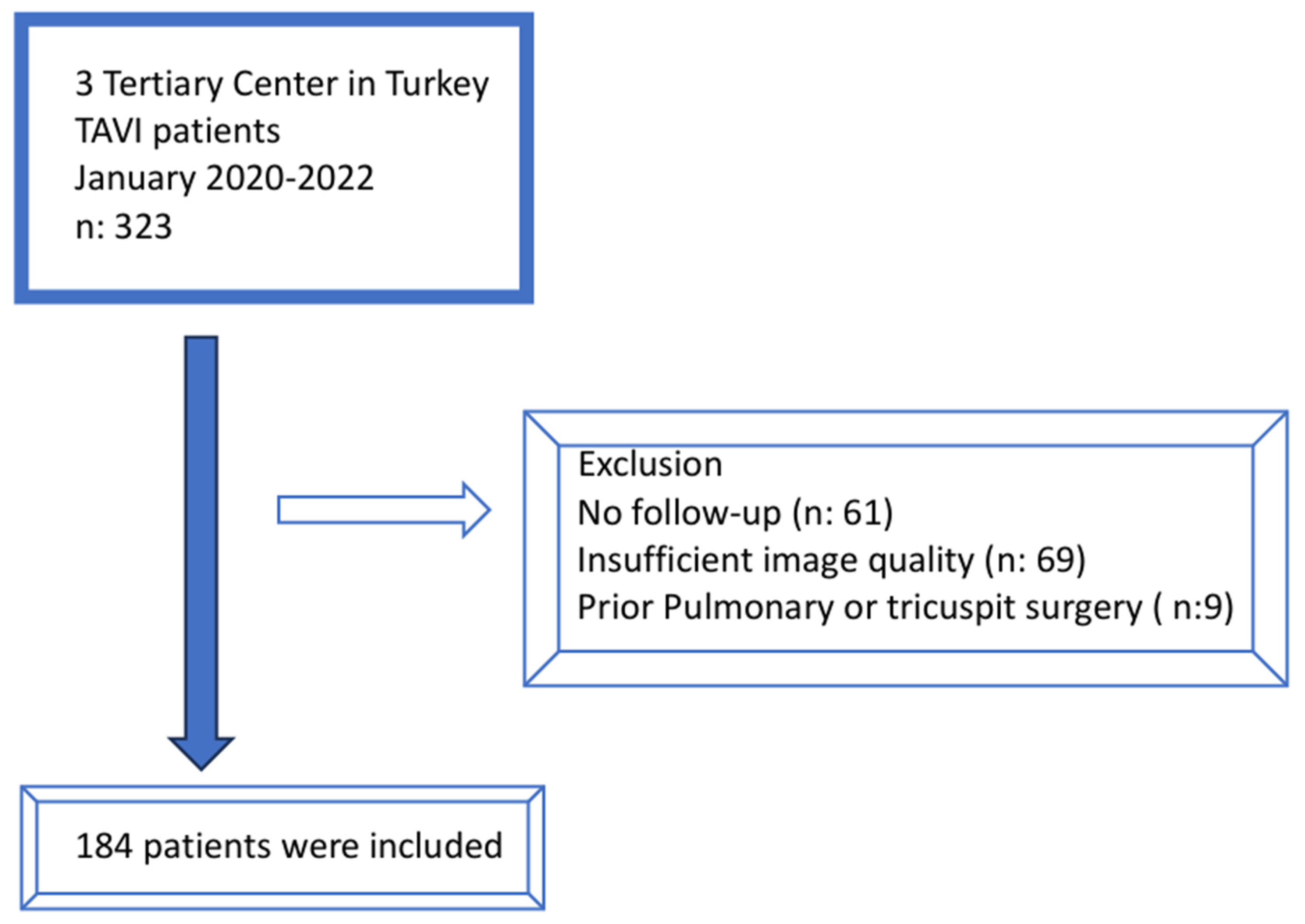
2.1. Study Population
2.2. Echocardiography
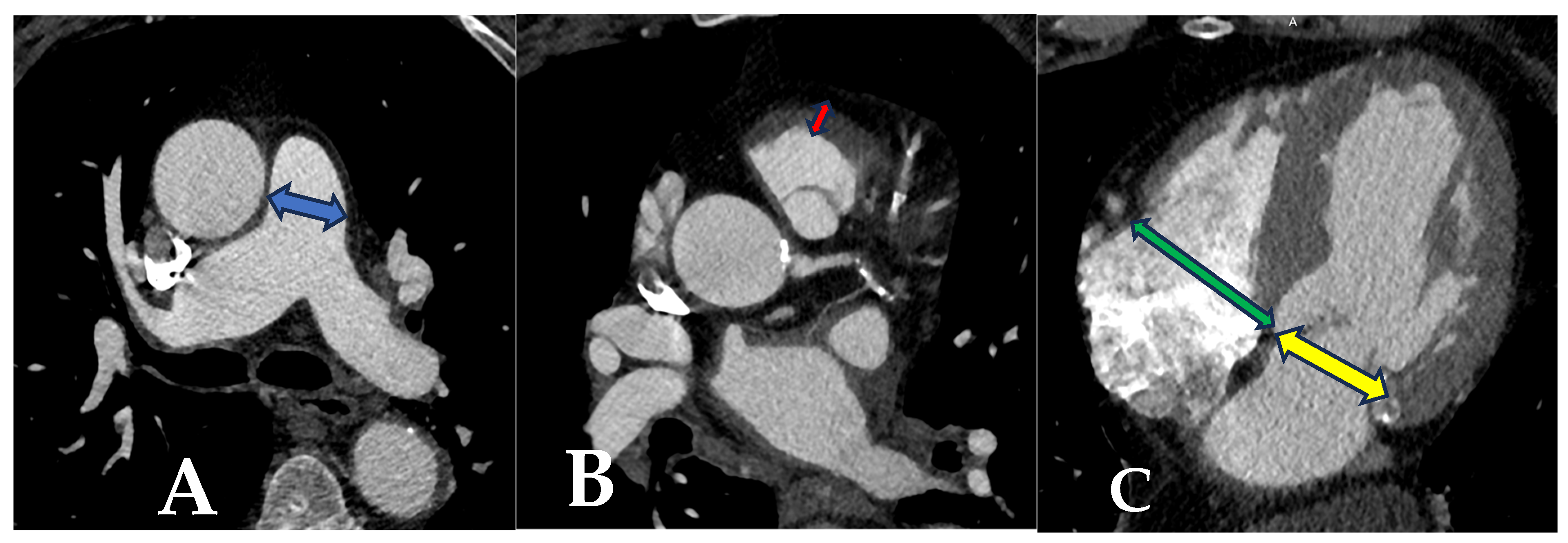
2.3. Tomographic Measurements
2.4. Operative Details
2.5. Endpoint
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genereux, P.; Sharma, R.P.; Cubeddu, R.J.; Aaron, L.; Abdelfattah, O.M.; Koulogiannis, K.P.; Marcoff, L.; Naguib, M.; Kapadia, S.R.; Makkar, R.R.; et al. The Mortality Burden of Untreated Aortic Stenosis. J. Am. Coll. Cardiol. 2023, 82, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Genereux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Marano, R.; Pontone, G.; Agricola, E.; Alushi, B.; Bartorelli, A.; Cameli, M.; Carrabba, N.; Esposito, A.; Faletti, R.; Francone, M.; et al. Recommendations in pre-procedural imaging assessment for TAVI intervention: SIC-SIRM position paper part 2 (CT and MR angiography, standard medical reporting, future perspectives). Radiol. Med. 2022, 127, 277–293. [Google Scholar] [CrossRef]
- Swift, A.J.; Dwivedi, K.; Johns, C.; Garg, P.; Chin, M.; Currie, B.J.; Rothman, A.M.; Capener, D.; Shahin, Y.; Elliot, C.A.; et al. Diagnostic accuracy of CT pulmonary angiography in suspected pulmonary hypertension. Eur. Radiol. 2020, 30, 4918–4929. [Google Scholar] [CrossRef]
- Kang, D.K.; Thilo, C.; Schoepf, U.J.; Barraza, J.M., Jr.; Nance, J.W., Jr.; Bastarrika, G.; Abro, J.A.; Ravenel, J.G.; Costello, P.; Goldhaber, S.Z. CT signs of right ventricular dysfunction: Prognostic role in acute pulmonary embolism. JACC Cardiovasc. Imaging 2011, 4, 841–849. [Google Scholar] [CrossRef]
- Kang, D.K.; Ramos-Duran, L.; Schoepf, U.J.; Armstrong, A.M.; Abro, J.A.; Ravenel, J.G.; Thilo, C. Reproducibility of CT signs of right ventricular dysfunction in acute pulmonary embolism. AJR Am. J. Roentgenol. 2010, 194, 1500–1506. [Google Scholar] [CrossRef]
- Writing Committee, M.; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Clavel, M.A.; Malouf, J.; Michelena, H.I.; Suri, R.M.; Jaffe, A.S.; Mahoney, D.W.; Enriquez-Sarano, M. B-type natriuretic peptide clinical activation in aortic stenosis: Impact on long-term survival. J. Am. Coll. Cardiol. 2014, 63, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Rafique, A.M.; Biner, S.; Ray, I.; Forrester, J.S.; Tolstrup, K.; Siegel, R.J. Meta-analysis of prognostic value of stress testing in patients with asymptomatic severe aortic stenosis. Am. J. Cardiol. 2009, 104, 972–977. [Google Scholar] [CrossRef]
- Vollema, E.M.; Sugimoto, T.; Shen, M.; Tastet, L.; Ng, A.C.T.; Abou, R.; Marsan, N.A.; Mertens, B.; Dulgheru, R.; Lancellotti, P.; et al. Association of Left Ventricular Global Longitudinal Strain with Asymptomatic Severe Aortic Stenosis: Natural Course and Prognostic Value. JAMA Cardiol. 2018, 3, 839–847. [Google Scholar] [CrossRef]
- Prihadi, E.A.; Vollema, E.M.; Ng, A.C.T.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Determinants and prognostic implications of left ventricular mechanical dispersion in aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 740–748. [Google Scholar] [CrossRef]
- Marechaux, S.; Hachicha, Z.; Bellouin, A.; Dumesnil, J.G.; Meimoun, P.; Pasquet, A.; Bergeron, S.; Arsenault, M.; Le Tourneau, T.; Ennezat, P.V.; et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur. Heart J. 2010, 31, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, M.C.; Tsuruta, H.; Hayashida, K.; Hase, H.; Yoshijima, N.; Saito, T.; Myojin, S.; Kobari, Y.; Ryuzaki, T.; Imaeda, S.; et al. Right Ventricular Dysfunction in Patients with Concomitant Tricuspid Regurgitation Undergoing Transcatheter Aortic Valve Implantation. Circ. J. 2024, 88, 451–459. [Google Scholar] [CrossRef]
- Miyamoto, J.; Ohno, Y.; Kamioka, N.; Ikari, Y.; Otsuka, T.; Tada, N.; Naganuma, T.; Yamawaki, M.; Yamanaka, F.; Shirai, S.; et al. Impact of Periprocedural Pulmonary Hypertension on Outcomes After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2022, 80, 1601–1613. [Google Scholar] [CrossRef]
- Calabro, P.; Niccoli, G.; Gragnano, F.; Grove, E.L.; Vergallo, R.; Mikhailidis, D.P.; Patti, G.; Spaccarotella, C.; Katsiki, N.; Masiero, G.; et al. Are we ready for a gender-specific approach in interventional cardiology? Int. J. Cardiol. 2019, 286, 226–233. [Google Scholar] [CrossRef]
- Lin, F.Y.; Devereux, R.B.; Roman, M.J.; Meng, J.; Jow, V.M.; Jacobs, A.; Weinsaft, J.W.; Shaw, L.J.; Berman, D.S.; Callister, T.Q.; et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: Mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc. Imaging 2008, 1, 782–786. [Google Scholar] [CrossRef]
- Kuriyama, K.; Gamsu, G.; Stern, R.G.; Cann, C.E.; Herfkens, R.J.; Brundage, B.H. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Investig. Radiol. 1984, 19, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef] [PubMed]
| Survival Group (n: 142) | Mortality Group (n: 42) | p | |
|---|---|---|---|
| Age (year) Median (IQR) | 76 (68–85) | 81 (72–90) | 0.002 |
| Gender Female sex, n (%) | 76 (53.5%) | 23 (54.7%) | 0.94 |
| Coronary artery disease, n (%) | 102 (71.8%) | 36 (85.7%) | 0.04 |
| Diabetes, n (%) | 78 (54.9%) | 24 (57.1%) | 0.835 |
| Hypertension, n (%) | 28 (19.9%) | 9 (21.4%) | 0.177 |
| COPD, n (%) | 37 (26%) | 12 (28.6%) | 0.172 |
| Creatinine level Median (IQR) | 0.95 (0.5–1.37) | 1.10 (0.6–1.6) | 0.019 |
| GFR Median (IQR) | 71 (32) | 52 (33) | <0.001 |
| Albumin Median (IQR) | 4.1 (3.5–4.7) | 4.1 (3.7–4.5) | 0.9 |
| Haemoglobin Median (IQR) | 12.1 (8.7–15.5) | 11.5 (9.5–14.5) | 0.18 |
| WBC Median (IQR) | 7.32 (4.5–10.3) | 7.0 (4.2–10.2) | 0.163 |
| LV EF Median (IQR) | 50.0 (35.0–65.0) | 50.0 (30.0–65.0) | 0.375 |
| Systolic pulmonary artery pressure Median (IQR) | 40 (25–65) | 45 (30.0–70.0) | 0.014 |
| Echocardiographic RV dilatation, n (%) | 22 (22.9%) | 18 (48.6%) | 0.03 |
| Severe Tricuspid Regurgitation, n (%) | 17 (11.9%) | 9 (21%) | 0.008 |
| Euroscore 2 Median (IQR) | 12 (6.5–16.5) | 12.1 (9.5–16.5) | 0.155 |
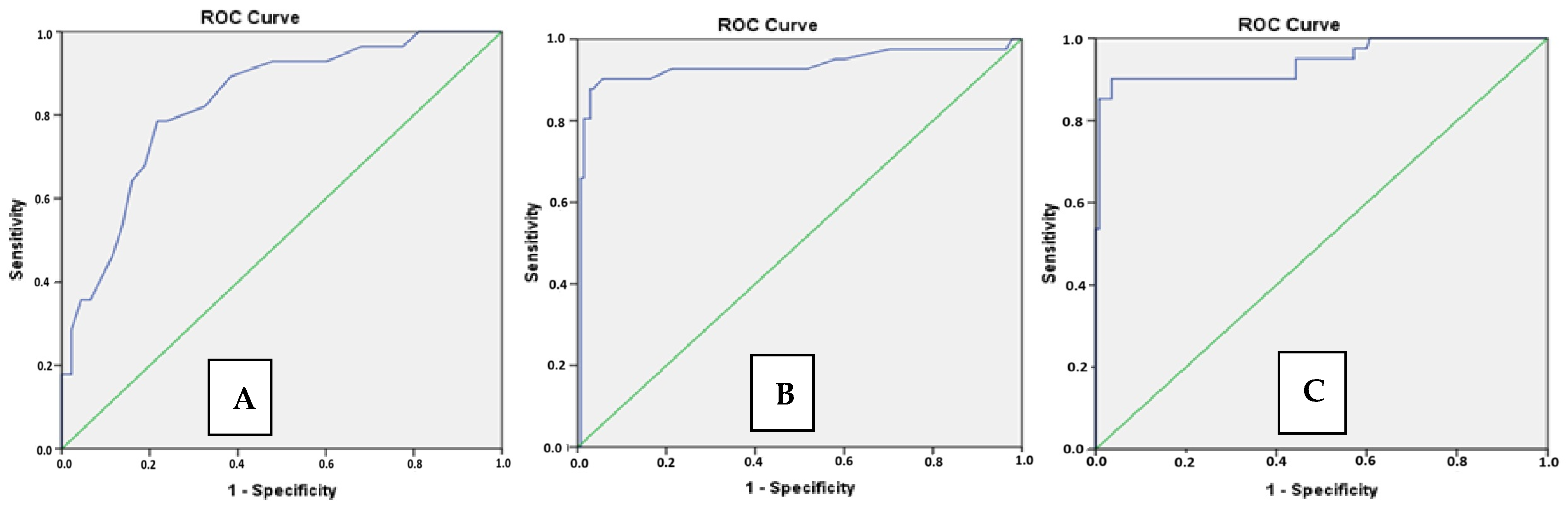
| PAD (mm) Median (IQR) | 25 (15–36) | 34.5 (28–43) | <0.001 |
| RVOTMT (mm) Median (IQR) | 3.5 (2.5–4.5) | 6,4 (4.9–7.2) | <0.001 |
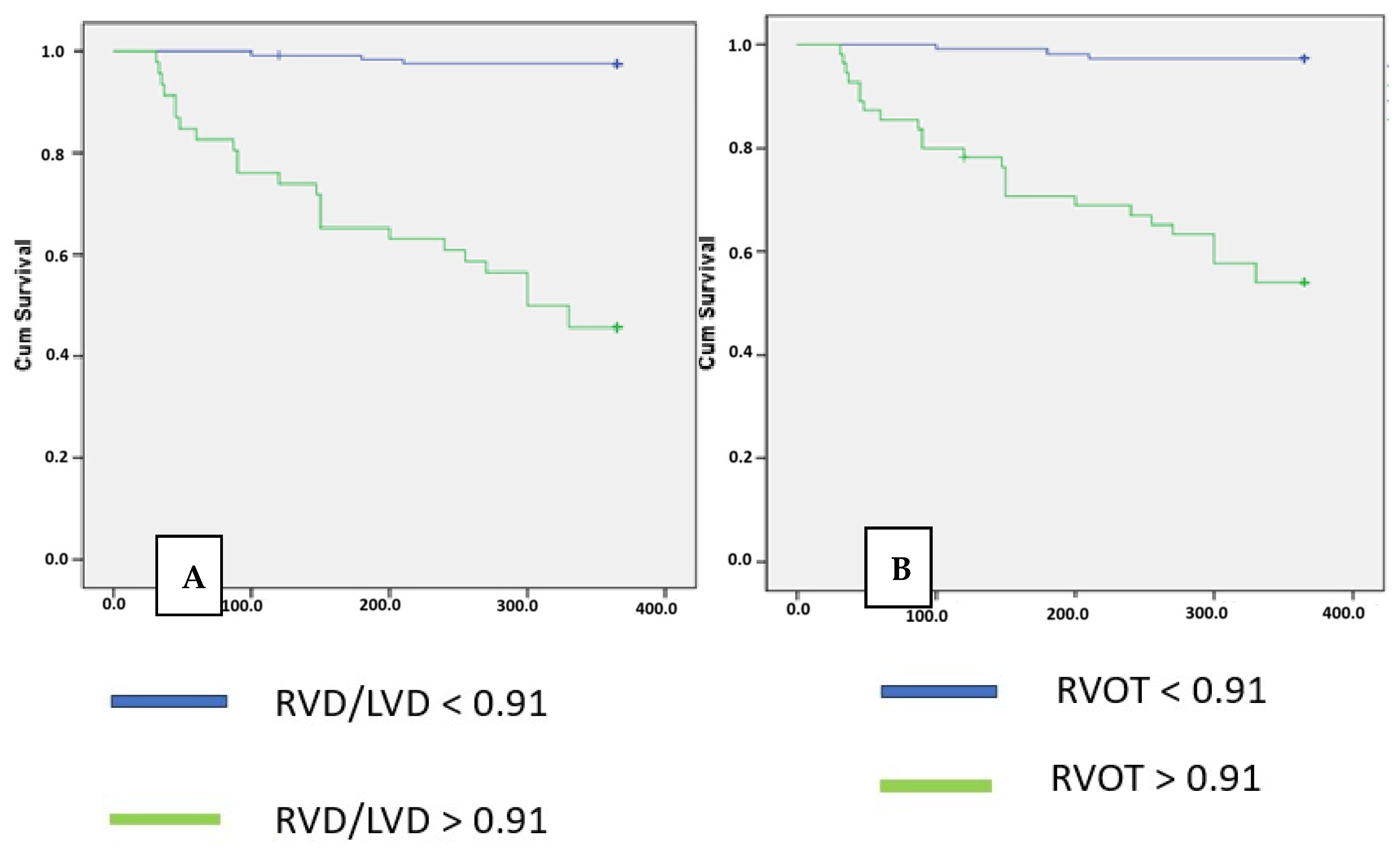
| Maximal RVD/LVD Mean ± SD | 0.77 ± 0.12 | 1.15 ± 0.19 | <0.001 |
| Variables in the Equation | |||
|---|---|---|---|
| B | Wald | p | |
| Age | −0.018 | 0.302 | 0.583 |
| Coronary artery disease | −0.929 | 1.299 | 0.254 |
| GFR | 0.005 | 0.184 | 0.668 |
| Systolic pulmonary artery pressure | −0.007 | 0.206 | 0.650 |
| Severe tricuspid regurgitation | 0.344 | 0.085 | 0.771 |
| RV dilatation | −0.362 | 0.000 | 0.998 |
| PAD > 30.5 mm | −0.0227 | 0.146 | 0.703 |
| RVOTMT > 4 mm | −1.729 | 4.666 | 0.031 |
| RVD/LVD ratio > 0.91 | −2.326 | 8.685 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barış, V.Ö.; Karaca, Ö.; Harman, M.; Poyraz, F.; Köksal, F.; Öztürk, A.; Kaya, E.E.; Kaya, M.; Ballı, M.; Kobat, M.A. Prognostic Impact of Right Ventricular Damage Markers on CT in Patients Undergoing TAVI. Life 2025, 15, 1020. https://doi.org/10.3390/life15071020
Barış VÖ, Karaca Ö, Harman M, Poyraz F, Köksal F, Öztürk A, Kaya EE, Kaya M, Ballı M, Kobat MA. Prognostic Impact of Right Ventricular Damage Markers on CT in Patients Undergoing TAVI. Life. 2025; 15(7):1020. https://doi.org/10.3390/life15071020
Chicago/Turabian StyleBarış, Veysel Özgür, Özkan Karaca, Murat Harman, Fatih Poyraz, Fatma Köksal, Alperen Öztürk, Emin Erdem Kaya, Murat Kaya, Mehmet Ballı, and Mehmet Ali Kobat. 2025. "Prognostic Impact of Right Ventricular Damage Markers on CT in Patients Undergoing TAVI" Life 15, no. 7: 1020. https://doi.org/10.3390/life15071020
APA StyleBarış, V. Ö., Karaca, Ö., Harman, M., Poyraz, F., Köksal, F., Öztürk, A., Kaya, E. E., Kaya, M., Ballı, M., & Kobat, M. A. (2025). Prognostic Impact of Right Ventricular Damage Markers on CT in Patients Undergoing TAVI. Life, 15(7), 1020. https://doi.org/10.3390/life15071020









