The Impact of Salvage Radiotherapy in Recurrent Endometrial Cancer: A Review Focusing on Early-Stage, Endometrial Cancer Locoregional Relapses
Abstract
1. Introduction
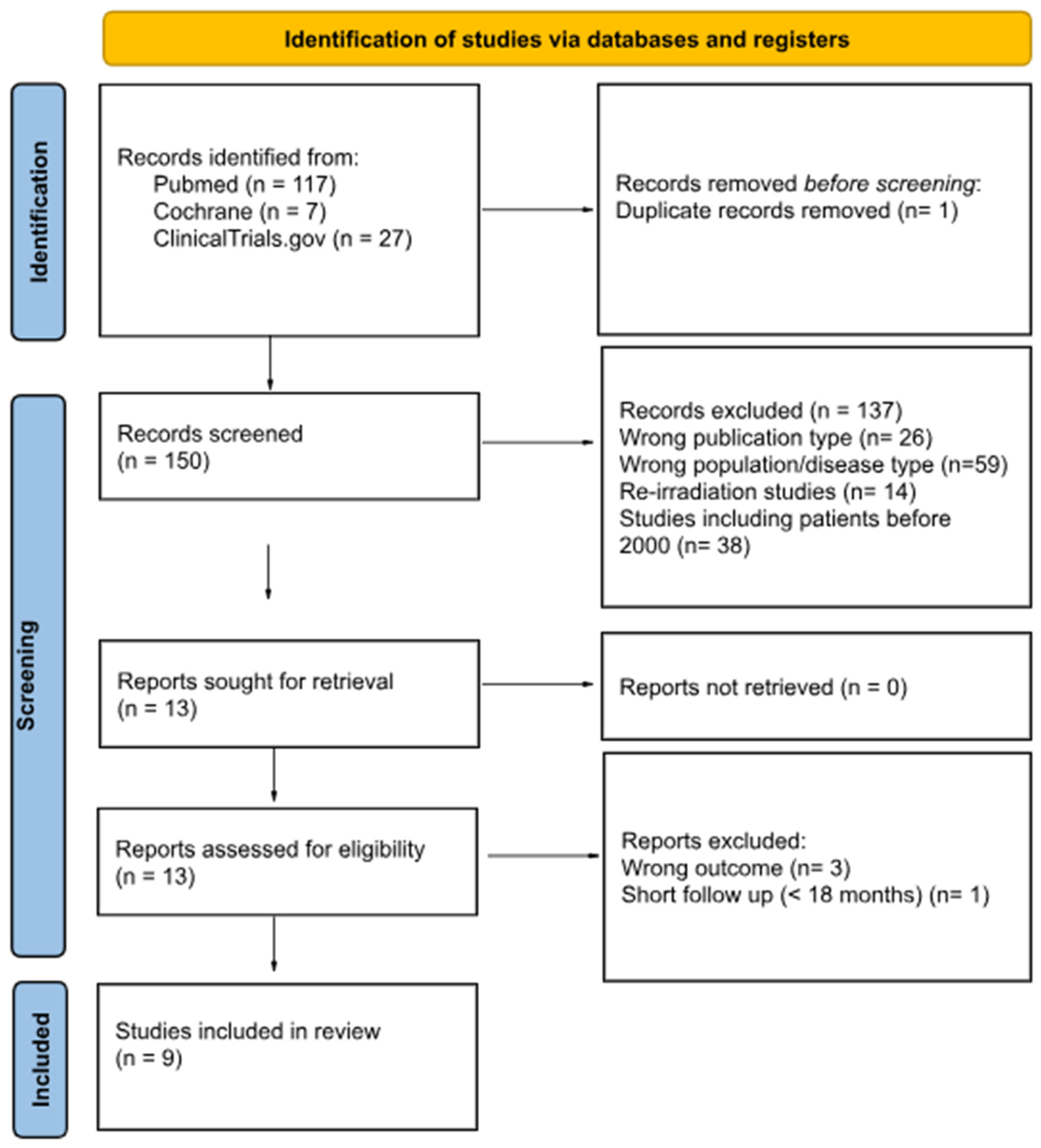
2. Materials and Methods
3. Results
Number and Type of Studies
| Study, Publication Year | Years | Patients (n) | Median Fu (Months) | Pelvic Rec. * | Adj. RT | Stage I | Gr 1–2 | Survival Outcomes | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Lee 2013 [13] | 2003–2011 | 44 | 24 | 14% | 30%, EBRT: 16% | 73% | 73% | 2y LC 96% | G3: 3% ** |
| 2y OS 80% | |||||||||
| 2y DFS 72% | |||||||||
| Vargo 2014 [14] | 2004–2013 | 41 | 18 | 20% | 0% | 83% | 78% | 3y LC 95% | G3: 5% |
| 3y OS 67% | |||||||||
| 3y DFS 68% | |||||||||
| Chapman 2017 [15] | 2000–2010 | 30 | 76.4 | 0% | 0% | 86% | 77% | 5y LRFFS: 87% | G5: 1 pt |
| 5y OS 77% | G3–4: 0 | ||||||||
| 5y PFS 75% | |||||||||
| Arden 2020 [16] | 2004–2018 | 28 | 21 | 18% | 0% | 93% | 82% | 2y LC 93% | G3–5: 0 |
| 2y OS 88% | |||||||||
| 2y DFS 80% | |||||||||
| Sapienza 2020 [17] | 2009–2018 | 30 | 53 | 7% | 3%, VBT: 3% | 63% | 67% | 5y LC 89% | G3: 6% |
| 5y OS 83% | |||||||||
| 5y DFS 69% | |||||||||
| Lindemann 2021 [18] | 2006–2011 | 139 | 79 | 0% | 0% | 75% | NR 1 | 5y DFS 65% | NR 1 |
| 5y OS 68% | |||||||||
| Ørtoft 2024 [20] | 2005–2012 | 141 | 109 | 15% | 0% | NR 1 | NR 1 | NR1 | NR 1 |
| Sherwood 2024 [21] | 2014–2021 | 39 | 22 | 20% | 23%, EBRT: 13% | 66% | 77% | 2y LF 22.6% | G3: 10% |
| 2y OS 85% | |||||||||
| 2y DFS 36.9% | |||||||||
| Klopp 2024 [22] | 2008–2020 | 156 | 62 | 14% | NR 1 | NR 1 | 82% | 3y PFS 73% 2 | G3: 31% 2 |
| 74 RT | G3: 57% 3 | ||||||||
| 82 CRT |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute—Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterince Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 17 February 2025).
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef] [PubMed]
- Sorbe, B.; Nordstrom, B.; Maenpaa, J.; Kuhelj, J.; Kuhelj, D.; Okkan, S.; Delaloye, J.F.; Frankendal, B. Intravaginal brachytherapy in FIGO stage I low-risk endometrial cancer: A controlled randomized study. Int. J. Gynecol. Cancer 2009, 19, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, C.L.; Nout, R.A.; Lybeert, M.L.; Warlam-Rodenhuis, C.C.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; Pras, E.; van de Poll-Franse, L.V.; van Putten, W.L.; et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e631–e638. [Google Scholar] [CrossRef]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology, G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef]
- Wortman, B.G.; Creutzberg, C.L.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.; van der Steen-Banasik, E.M.; Mens, J.W.M.; Slot, A.; Kroese, M.C.S.; et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: Improving patient selection for adjuvant therapy. Br. J. Cancer 2018, 119, 1067–1074. [Google Scholar] [CrossRef]
- Nout, R.A.; Putter, H.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Stenfert Kroese, M.C.; Nijman, H.W.; et al. Five-year quality of life of endometrial cancer patients treated in the randomised Post Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial and comparison with norm data. Eur. J. Cancer 2012, 48, 1638–1648. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; van Putten, W.L.; Koper, P.C.; Lybeert, M.L.; Jobsen, J.J.; Warlam-Rodenhuis, C.C.; De Winter, K.A.; Lutgens, L.C.; van den Bergh, A.C.; van der Steen-Banasik, E.; et al. Survival after relapse in patients with endometrial cancer: Results from a randomized trial. Gynecol. Oncol. 2003, 89, 201–209. [Google Scholar] [CrossRef]
- Bertelsen, K.; Ortoft, G.; Hansen, E.S. Survival of Danish patients with endometrial cancer in the intermediate-risk group not given postoperative radiotherapy: The Danish Endometrial Cancer Study (DEMCA). Int. J. Gynecol. Cancer 2011, 21, 1191–1199. [Google Scholar] [CrossRef]
- Ortoft, G.; Hansen, E.S.; Bertelsen, K. Omitting adjuvant radiotherapy in endometrial cancer increases the rate of locoregional recurrences but has no effect on long-term survival: The Danish Endometrial Cancer Study. Int. J. Gynecol. Cancer 2013, 23, 1429–1437. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Lee, L.J.; Damato, A.L.; Viswanathan, A.N. Clinical outcomes following 3D image-guided brachytherapy for vaginal recurrence of endometrial cancer. Gynecol. Oncol. 2013, 131, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.A.; Kim, H.; Houser, C.J.; Berhane, H.; Sukumvanich, P.; Olawaiye, A.B.; Kelley, J.L.; Edwards, R.P.; Comerci, J.T.; Huang, M.; et al. Definitive salvage for vaginal recurrence of endometrial cancer: The impact of modern intensity-modulated-radiotherapy with image-based HDR brachytherapy and the interplay of the PORTEC 1 risk stratification. Radiother. Oncol. 2014, 113, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.H.; Maghsoudi, K.; Littell, R.D.; Chen, L.M.; Hsu, I.C. Salvage high-dose-rate brachytherapy and external beam radiotherapy for isolated vaginal recurrences of endometrial cancer with no prior adjuvant therapy. Brachytherapy 2017, 16, 1152–1158. [Google Scholar] [CrossRef]
- Arden, J.D.; Gruner, M.F.; Vu, C.C.; Marvin, K.; Ye, H.; Nandalur, S.R.; Al-Wahab, Z.; Gadzinski, J.; Rakowski, J.A.; Field, J.; et al. Outcomes After Salvage Radiation Therapy for Recurrent Endometrial Cancer in Patients With No Prior Adjuvant Therapy: An Institutional Review. Adv. Radiat. Oncol. 2020, 5, 1240–1247. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Ning, M.S.; de la Pena, R.; McNew, L.K.; Jhingran, A.; Georgeon, L.; Rasool, N.; Gomes, M.J.L.; Abu-Isa, E.; Baiocchi, G. Outcomes and toxicity after salvage radiotherapy for vaginal relapse of endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 1535–1541. [Google Scholar] [CrossRef]
- Lindemann, K.; Smogeli, E.; Smastuen, M.C.; Bruheim, K.; Trovik, J.; Nordberg, T.; Kristensen, G.B.; Werner, H.M.J.; Nakken, E. Salvage Radiation for Pelvic Relapse after Surgically Treated Endometrial Cancer. Cancers 2021, 13, 1367. [Google Scholar] [CrossRef]
- Norwegian Directorate of Health. Nasjonalt Handlingsprogram Med Retningslinjer for Gynekologisk Kreft. Available online: https://www.helsedirektoratet.no/retningslinjer/gynekologisk-kreft--handlingsprogram/livmorkreft-endometriecancer/behandling (accessed on 23 June 2025).
- Ørtoft, G.; Fokdal, L.U.; Hogdall, C. Vaginal and pelvic recurrences and salvage treatments in a cohort of Danish endometrial cancer patients not given adjuvant radiotherapy. Int. J. Gynecol. Cancer 2024, 101861, in press. [Google Scholar] [CrossRef]
- Sherwood, M.; Barnes, T.; Chen, H.; Taggar, A.; Paudel, M.; Zhang, L.; Alqaderi, A.; Leung, E. Salvage interstitial brachytherapy for treatment of recurrent endometrial cancers in the vagina: Seven-year single institution experience and review of second recurrence patterns. Brachytherapy 2024, 24, 36–44. [Google Scholar] [CrossRef]
- Klopp, A.H.; Enserro, D.; Powell, M.; Randall, M.; Schink, J.C.; Mannel, R.S.; Holman, L.; Bender, D.; Kushnir, C.L.; Backes, F.; et al. Radiation Therapy With or Without Cisplatin for Local Recurrences of Endometrial Cancer: Results from an NRG Oncology/GOG Prospective Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2024, 42, 2425–2435. [Google Scholar] [CrossRef]
- Sears, J.D.; Greven, K.M.; Hoen, H.M.; Randall, M.E. Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer 1994, 74, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Martinez-Monge, R.; Copeland, L.J.; Vacarello, L.; Lewandowski, G.S. Perineal template interstitial brachytherapy salvage for recurrent endometrial adenocarcinoma metastatic to the vagina. Gynecol. Oncol. 1997, 66, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Jhingran, A.; Burke, T.W.; Eifel, P.J. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1366–1372. [Google Scholar] [CrossRef]
- Petignat, P.; Jolicoeur, M.; Alobaid, A.; Drouin, P.; Gauthier, P.; Provencher, D.; Donath, D.; Van Nguyen, T. Salvage treatment with high-dose-rate brachytherapy for isolated vaginal endometrial cancer recurrence. Gynecol. Oncol. 2006, 101, 445–449. [Google Scholar] [CrossRef]
- Kamrava, M.; Leung, E.; Bachand, F.; Beriwal, S.; Chargari, C.; D’Souza, D.; Erickson, B.; Fokdal, L.; Han, K.; Harkenrider, M.; et al. GEC-ESTRO (ACROP)-ABS-CBG Consensus Brachytherapy Target Definition Guidelines for Recurrent Endometrial and Cervical Tumors in the Vagina. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 654–663. [Google Scholar] [CrossRef]
- Klopp, A.H.; Yeung, A.R.; Deshmukh, S.; Gil, K.M.; Wenzel, L.; Westin, S.N.; Gifford, K.; Gaffney, D.K.; Small, W., Jr.; Thompson, S.; et al. Patient-Reported Toxicity During Pelvic Intensity-Modulated Radiation Therapy: NRG Oncology-RTOG 1203. J. Clin. Oncol. 2018, 36, 2538–2544. [Google Scholar] [CrossRef]
- Tiwari, R.; Narayanan, G.S.; Reddy, V.P.; Vishwanathan, B.; Narayanan, S.; Venugopal, R. Impact of nodal boost irradiation and MR-based brachytherapy on oncologic outcomes in node-positive cervical cancer. Gynecol. Oncol. 2021, 163, 110–116. [Google Scholar] [CrossRef]
- Hart, K.B.; Han, I.; Shamsa, F.; Court, W.S.; Chuba, P.; Deppe, G.; Malone, J.; Christensen, C.; Porter, A.T. Radiation therapy for endometrial cancer in patients treated for postoperative recurrence. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 7–11. [Google Scholar] [CrossRef]
- Ackerman, I.; Malone, S.; Thomas, G.; Franssen, E.; Balogh, J.; Dembo, A. Endometrial carcinoma--relative effectiveness of adjuvant irradiation vs therapy reserved for relapse. Gynecol. Oncol. 1996, 60, 177–183. [Google Scholar] [CrossRef]
- Lin, L.L.; Grigsby, P.W.; Powell, M.A.; Mutch, D.G. Definitive radiotherapy in the management of isolated vaginal recurrences of endometrial cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 500–504. [Google Scholar] [CrossRef]
- Hasbini, A.; Haie-Meder, C.; Morice, P.; Chirat, E.; Duvillard, P.; Lhomme, C.; Delapierre, M.; Gerbaulet, A. Outcome after salvage radiotherapy (brachytherapy +/− external) in patients with a vaginal recurrence from endometrial carcinomas. Radiother. Oncol. 2002, 65, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.P.; Lindegaard, J.C.; Mahantshetty, U.; Tanderup, K.; Jurgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.; Hoskin, P.; Segedin, B.; et al. Risk Factors for Local Failure Following Chemoradiation and Magnetic Resonance Image-Guided Brachytherapy in Locally Advanced Cervical Cancer: Results From the EMBRACE-I Study. J. Clin. Oncol. 2023, 41, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.; Tanderup, K.; Schmid, M.P.; Jurgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, K.; Zaharie, A.; Smet, S.; Spampinato, S.; Chargari, C.; Haie-Meder, C.; Mahantshetty, U.; Segedin, B.; Bruheim, K.; Rai, B.; et al. Association Between the Regular Use of Vaginal Dilators and/or Sexual Activity and Vaginal Morbidity in Locally Advanced Cervical Cancer Survivors: An EMBRACE-I Study Report. Int. J. Radiat. Oncol. Biol. Phys. 2024, 121, 452–464. [Google Scholar] [CrossRef]
- Chargari, C.; Tanderup, K.; Planchamp, F.; Chiva, L.; Humphrey, P.; Sturdza, A.; Tan, L.T.; van der Steen-Banasik, E.; Zapardiel, I.; Nout, R.A.; et al. ESGO/ESTRO quality indicators for radiation therapy of cervical cancer. Radiother. Oncol. 2023, 183, 109589. [Google Scholar] [CrossRef]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novak, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Marth, C.; Moore, R.G.; Bidzinski, M.; Pignata, S.; Ayhan, A.; Rubio, M.J.; Beiner, M.; Hall, M.; Vulsteke, C.; Braicu, E.I.; et al. First-Line Lenvatinib Plus Pembrolizumab Versus Chemotherapy for Advanced Endometrial Cancer: A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2024, 43, 1083–1100. [Google Scholar] [CrossRef]
- van den Heerik, A.; Horeweg, N.; Nout, R.A.; Lutgens, L.; van der Steen-Banasik, E.M.; Westerveld, G.H.; van den Berg, H.A.; Slot, A.; Koppe, F.L.A.; Kommoss, S.; et al. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 2002–2007. [Google Scholar] [CrossRef]

| Study | EQD2 Dose (Gy) D90-CTV (Gy) | IMRT/VMAT | Interstitial IGABT |
|---|---|---|---|
| Lee 2013 [13] | 75.5 D90-CTV: 74.8 | NR 1 | 80% |
| Vargo 2014 [14] | 76 D90-CTV: 76 | 90% | 27% |
| Chapman 2017 [15] | 68.25 D90-CTV: 70.83 | NR 1 | 90% |
| Arden 2020 [16] | 67.6 NR 1 as D90-CTV | 29% | 3% |
| Sapienza 2020 [17] | EQD2 used, NR 1 | 41% | 0% |
| Lindemann, 2021 [18] | 70 NR 1 as D90-CTV | NR 1 | NR 1 |
| Ørtoft 2024 [20] | NR 1 as EQD2 or D90-CTV | 0% | 11% |
| Sherwood 2024 [21] | 76.8 2 D90-CTV: 76.8 | NR 1 | 100% |
| Klopp 2024 [22] | Intended dose 75Gy NR 1 as D90-CTV | NR 1 | 12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragkoudakis, E.; Panoskaltsis, T.; Pavlakis, K.; Grenzelia, M.; Kavoura, E.; Papageorgiou, G.; Georgakopoulos, I.; Kougioumtzopoulou, A.; Kypraiou, E.; Trogkanis, N.; et al. The Impact of Salvage Radiotherapy in Recurrent Endometrial Cancer: A Review Focusing on Early-Stage, Endometrial Cancer Locoregional Relapses. Life 2025, 15, 1013. https://doi.org/10.3390/life15071013
Maragkoudakis E, Panoskaltsis T, Pavlakis K, Grenzelia M, Kavoura E, Papageorgiou G, Georgakopoulos I, Kougioumtzopoulou A, Kypraiou E, Trogkanis N, et al. The Impact of Salvage Radiotherapy in Recurrent Endometrial Cancer: A Review Focusing on Early-Stage, Endometrial Cancer Locoregional Relapses. Life. 2025; 15(7):1013. https://doi.org/10.3390/life15071013
Chicago/Turabian StyleMaragkoudakis, Emmanouil, Theodoros Panoskaltsis, Kitty Pavlakis, Maria Grenzelia, Evangelia Kavoura, Georgios Papageorgiou, Ioannis Georgakopoulos, Andromachi Kougioumtzopoulou, Efrosyni Kypraiou, Nikolaos Trogkanis, and et al. 2025. "The Impact of Salvage Radiotherapy in Recurrent Endometrial Cancer: A Review Focusing on Early-Stage, Endometrial Cancer Locoregional Relapses" Life 15, no. 7: 1013. https://doi.org/10.3390/life15071013
APA StyleMaragkoudakis, E., Panoskaltsis, T., Pavlakis, K., Grenzelia, M., Kavoura, E., Papageorgiou, G., Georgakopoulos, I., Kougioumtzopoulou, A., Kypraiou, E., Trogkanis, N., Maragkoudakis, E., Kouloulias, V., & Zygogianni, A. (2025). The Impact of Salvage Radiotherapy in Recurrent Endometrial Cancer: A Review Focusing on Early-Stage, Endometrial Cancer Locoregional Relapses. Life, 15(7), 1013. https://doi.org/10.3390/life15071013








