5-Aminolevulinic Acid Phosphate as an Immune System Enhancer Along with Vaccination Against SARS-CoV-2 Virus Infection: An Open-Label, Randomized Pilot Study
Abstract
1. Introduction
2. Materials and Methods
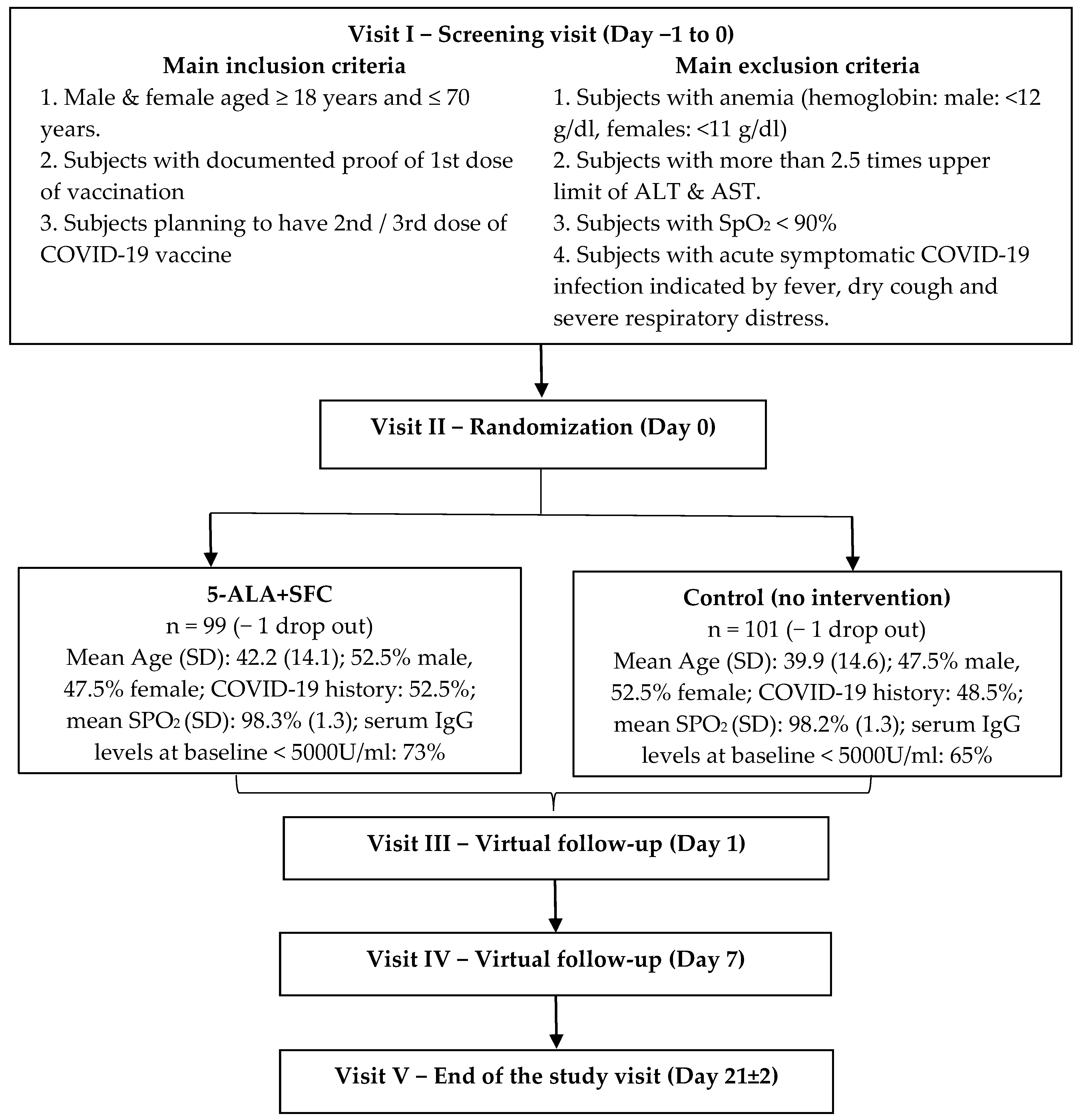
2.1. Study Design and Subject Characteristics
2.2. Prior and Concomitant Therapy
2.3. Measurement of IgG Levels
2.4. Statistical Methods
2.5. Primary Objective and Endpoints
- Number of participants with adverse product reactions as per Common Terminology Criteria for AE grading;
- Number of participants with solicited vaccine-related local and systemic adverse reactions (ARs).
2.6. Secondary Objective and Endpoints
- Absolute change in GMT of IgG levels against spike protein of SARS-CoV-2.
- EQ-5D-5L score.
- VAS for pain and fatigue.
- WHO-5 well-being questionnaire score.
3. Results
3.1. Primary Outcome—Safety: Adverse Product Reactions
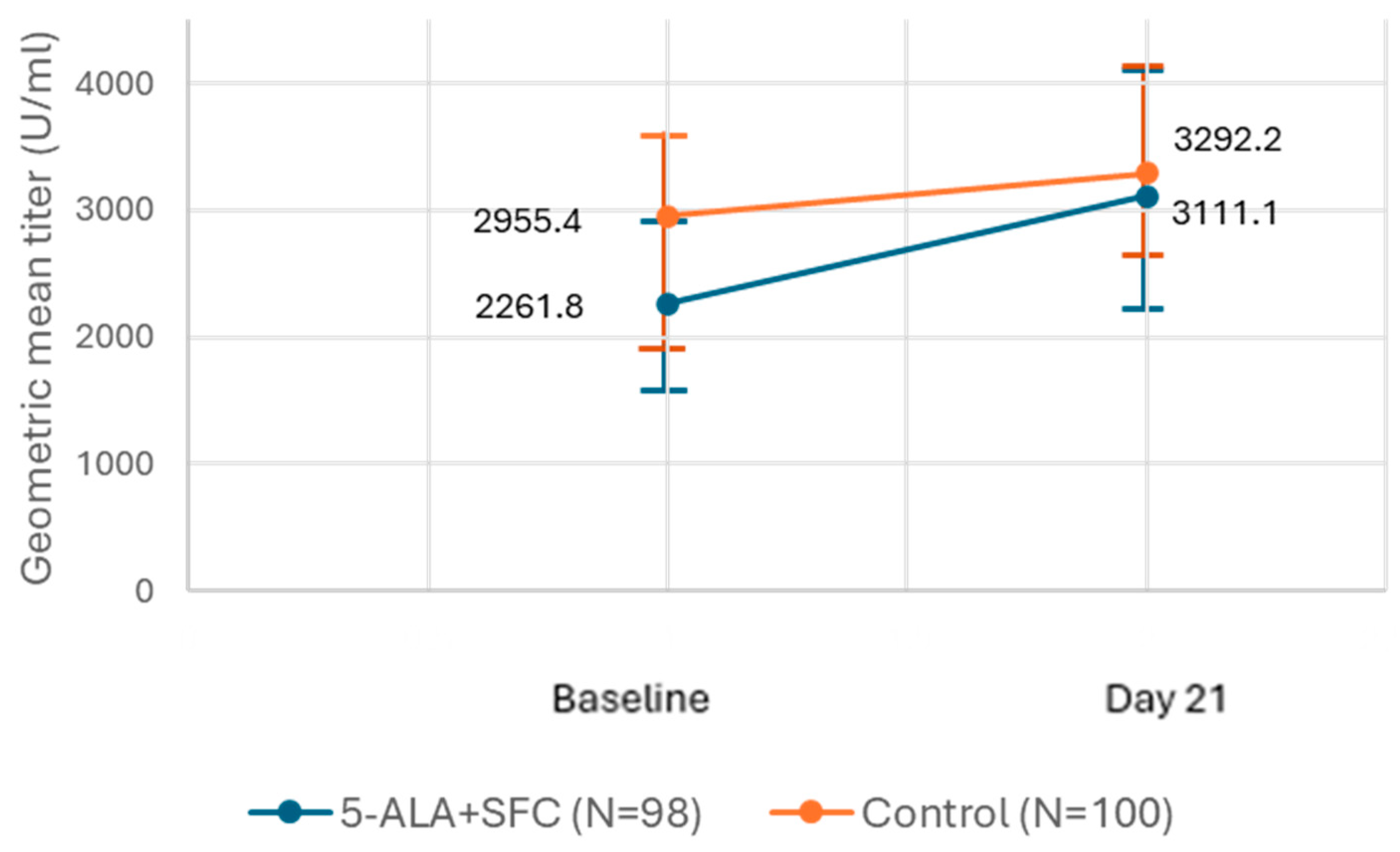
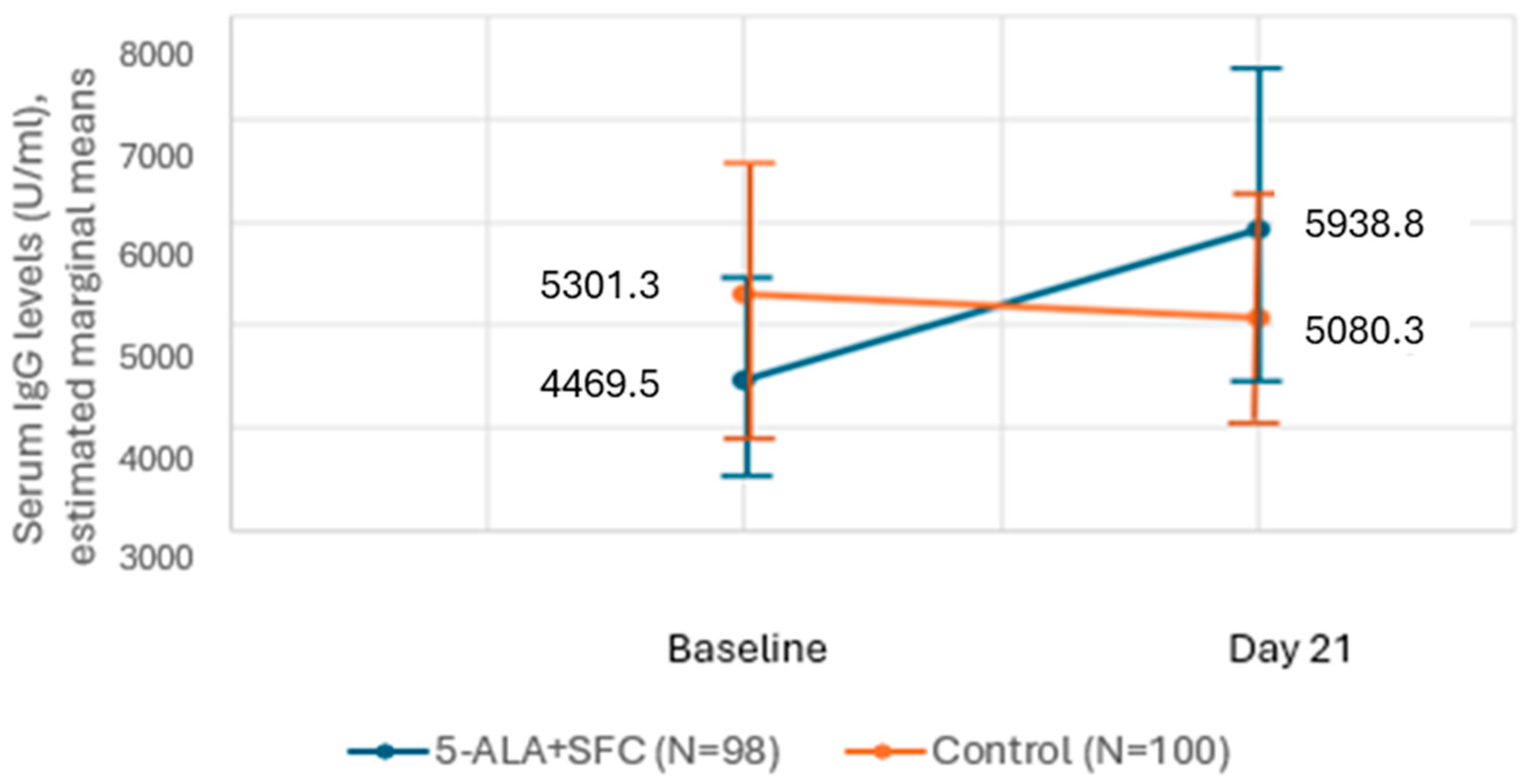
3.2. Secondary Outcome—Efficacy: Change in GMT of IgG Levels Against COVID-19 Spike Protein
Subgroup Analyses
4. Discussion
- −
- A second or third dose of vaccine was allowed and may vary the IgG titer response.
- −
- The vaccine was not limited to one type: the viral vector, Covishield, and the inactivated vaccine, Covaxin, were allowed, which might lead to different responses in vaccine-related AEs and IgG titer.
- −
- Subjects could have suffered from a COVID-19 infection in the past, which would also impact the IgG titer response.
- −
- Subjects aged ≥18 years and ≤70 years were eligible to participate in this study. As age could be decisive for the IgG titer response, an even distribution of subjects to predefined age groups would have been ideal.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ALA | 5-aminolevulinic acid |
| SFC | sodium ferrous citrate |
| AE | adverse event |
| IgG | Immunoglobulin G |
| WHO | World Health Organization |
| PHEIC | Public Health Emergency of International Concern |
| mRNA | messenger ribonucleic acid |
| IEC | Institutional/Independent Ethics Committee |
| WMA | World Medical Association |
| ICH | International Conference on Harmonization |
| GCP | good clinical practice |
| ICMR | Indian Council of Medical Research |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| GMT | geometric mean titer |
| VAS | Visual Analog Scale |
| PR | Pulse Rate |
| BP | Blood Pressure |
| SPO2 | peripheral capillary oxygen saturation |
| RT-PCR | Reverse Transcription-Polymerase Chain Reaction |
| EQ-5D-5L | 5-Dimension Quality of Life Questionnaire from EuroQuol group |
| IP | investigational product |
| SAE | serious adverse event |
| APR | adverse product reaction |
| FAS | Full Analyses Set |
| ITT | intention-to-treat |
| CI | confidence interval |
| GMFR | geometric mean fold rise |
| N | number of participants |
| SD | standard deviation |
| U | unit |
References
- Vasireddy, D.; Atluri, P.; Malayala, S.V.; Vanaparthy, R.; Mohan, G. Review of COVID-19 Vaccines Approved in the United States of America for Emergency Use. J. Clin. Med. Res. 2021, 13, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: Factors Driving New Variants of SARS-CoV-2, Immune Escape, and Resistance to Antiviral Treatments as the End of the COVID-19 Pandemic is Declared. Med. Sci. Monit. 2023, 29, e942960. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, M.M.; Miah, M.; Begum, M.N.; Sarmin, M.; Mahfuz, M.; Hossain, M.E.; Rahman, M.Z.; Chisti, M.J.; Ahmed, T.; et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci. Rep. 2022, 12, 1438. [Google Scholar] [CrossRef] [PubMed]
- Wisnewski, A.V.; Redlich, C.A.; Liu, J.; Kamath, K.; Abad, Q.-A.; Smith, R.F.; Fazen, L.; Santiago, R.; Luna, J.C.; Martinez, B.; et al. Immunogenic amino acid motifs and linear epitopes of COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0252849. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Ogura, S.; Maruyama, K.; Hagiya, Y.; Sugiyama, Y.; Tsuchiya, K.; Takahashi, K.; Abe, F.; Tabata, K.; Okura, I.; Nakajima, M.; et al. The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in mouse liver. BMC Res. Notes 2011, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Fujii, C.; Miyashita, K.; Mitsuishi, M.; Sato, M.; Fujii, K.; Inoue, H.; Hagiwara, A.; Endo, S.; Uto, A.; Ryuzaki, M.; et al. Treatment of sarcopenia and glucose intolerance through mitochondrial activation by 5-aminolevulinic acid. Sci. Rep. 2017, 7, 4013. [Google Scholar] [CrossRef]
- Nozawa, N.; Noguchi, M.; Shinno, K.; Tajima, M.; Aizawa, S.; Saito, T.; Asada, A.; Ishii, T.; Ishizuka, M.; Iijima, K.M.; et al. 5-Aminolevulinic acid and sodium ferrous citrate ameliorate muscle aging and extend healthspan in Drosophila. FEBS Open Bio. 2022, 12, 295–305. [Google Scholar] [CrossRef]
- Hu, X.; Que, W.; Hirano, H.; Wang, Z.; Nozawa, N.; Ishii, T.; Ishizuka, M.; Ito, H.; Takahashi, K.; Nakajima, M.; et al. 5-Aminolevulinic acid/sodium ferrous citrate enhanced the antitumor effects of programmed cell death-ligand 1 blockade by regulation of exhausted T cell metabolism in a melanoma model. Cancer Sci. 2021, 112, 2652–2663. [Google Scholar] [CrossRef]
- Hirose, S.; Isoda, N.; Huynh, L.T.; Kim, T.; Yoshimoto, K.; Tanaka, T.; Inui, K.; Hiono, T.; Sakoda, Y. Antiviral Effects of 5-Aminolevulinic Acid Phosphate against Classical Swine Fever Virus: In Vitro and In Vivo Evaluation. Pathogens 2022, 11, 164. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Sakura, T.; Sakurai, Y.; Kurosaki, Y.; Inaoka, D.K.; Shioda, N.; Yasuda, J.; Kita, K.; Morita, K. Antiviral activity of 5-aminolevulinic acid against variants of severe acute respiratory syndrome coronavirus 2. Trop. Med. Health 2022, 50, 6. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, R. The statistical analysis of immunohaematological data. Blood Transfus. 2008, 6, 37–45. [Google Scholar] [PubMed]
- Darwish, A.; Abdulrahman, A.; Alsalman, J.; Atkin, S.; Murad, M.; Nakajima, M.; Rehani, R.; Ebeling, A.; Stocker, M.; Stummer, W.; et al. Safety and immune-supportive potential of the food supplement 5-aminolevulinic acid phosphate for patients with COVID-19: An open-label, non-randomized pilot study. Bioact. Compd. Health Dis. 2024, 7, 17–35. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Hossain, M.F.; Abdulhakim, J.A.; Alam, M.A.; Ashraf, G.M.; Bungau, S.G.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Aleya, L. nCOVID-19 Pandemic: From Molecular Pathogenesis to Potential Investigational Therapeutics. Front. Cell Dev. Biol. 2020, 10, 616. [Google Scholar] [CrossRef]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Taka, E.; Yilmaz, S.Z.; Golcuk, M.; Kilinc, C.; Aktas, U.; Yildiz, A.; Gur, M. Critical Interactions Between the SARS-CoV-2 Spike Glycoprotein and the Human ACE2 Receptor. J. Phys. Chem. B 2021, 125, 5537–5548. [Google Scholar] [CrossRef] [PubMed]
- Schuijs, M.J.; Hammad, H.; Lambrecht, B.N. Professional and ‘Amateur’ Antigen-Presenting Cells in Type 2 Immunity. Trends Immunol. 2019, 40, 22–34. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. Be aware of SARS-CoV-2 spike protein: There is more than meets the eye. J. Biol. Regul. Homeost. Agents 2021, 35, 833–838. [Google Scholar] [CrossRef]
- Iwasaki, A.; Yang, Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 339–341. [Google Scholar] [CrossRef]
- Goyal, M.; Jain, M.; Patel, N.; Sharma, A. Quantitative estimation of anti-spike SARS-CoV-2 IgG antibody response after covishield vaccination in healthcare workers. J. Indian Acad. Oral Med. Radiol. 2022, 34, 176–179. [Google Scholar] [CrossRef]
- Rudzinski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbot, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in Acute COVID-19 and associations with age and disease severity. Cell 2020, 4, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Zakhartchouk, A.N.; Sharon, C.; Satkunarajah, M.; Auperin, T.; Viswanathan, S.; Mutwiri, G.; Petric, M.; See, R.H.; Brunham, R.C.; Finlay, B.B.; et al. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: Implications for a subunit vaccine. Vaccine 2007, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, H.; Wu, C. The immune responses of HLA-A*0201 restricted SARS-CoV S peptide-specific CD8+ T cells are augmented in varying degrees by CpG ODN, PolyI:C and R848. Vaccine 2011, 29, 6670–6678. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wu, J.; Wei, W.; Du, Y.; Wan, T.; Ma, X.; An, W.; Guo, A.; Miao, C.; Yue, H.; et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 2018, 17, 187–194. [Google Scholar] [CrossRef]
- Zhou, Z.; Post, P.; Chubet, R.; Holtz, K.; McPherson, C.; Petric, M.; Cox, M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine 2006, 24, 3624–3631. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef]
- Al-Saber, F.; Aldosari, W.; Alselaiti, M.; Khalfan, H.; Kaladari, A.; Khan, G.; Harb, G.; Rehani, R.; Kudo, S.; Koda, A.; et al. The safety and tolerability of 5-aminolevulinic acid phosphate with sodium ferrous citrate in patients with type 2 diabetes mellitus in Bahrain. J. Diabetes Res. 2016, 2016, 8294805. [Google Scholar] [CrossRef]
- Rehani, P.R.; Iftikhar, H.; Nakajima, M.; Tanaka, T.; Jabbar, Z.; Rehani, R.N. Safety and mode of action of diabetes medications in comparison with 5-aminolevulinic acid (5-ALA). J. Diabetes Res. 2019, 2019, 4267357. [Google Scholar] [CrossRef]
- Rodriguez, B.L.; Curb, J.D.; Davis, J.; Shintani, T.; Perez, M.H.; ApauLudlum, N.; Johnson, C.; Harrigan, R.C. Use of the dietary supplement 5-aminiolevulinic acid (5-ALA) and its relationship with glucose levels and hemoglobin A1C among individuals with prediabetes. Clin. Transl. Sci. 2012, 5, 314–320. [Google Scholar] [CrossRef] [PubMed]
| Study Day | Screening/Randomization Visit (Day 0) | Virtual Visit (Day 1) | Virtual Visit (Day 7) | End of Study Visit (Day 21 ± 2) |
|---|---|---|---|---|
| Visits | 1 | 3 | 4 | 5 |
| Informed consent | X | |||
| Medical history | X | |||
| Medication history | X | |||
| Study-specific history | X | X | ||
| Demographics data (age, gender) | X | |||
| Anthropometrics data (height and weight) | X | |||
| Vitals (PR, BP, SpO2, and body temperature) | X | X | ||
| Urine pregnancy test | X | X | ||
| Clinical examination | X | X | ||
| Concomitant medication | X | |||
| RT-PCR | X | X | ||
| Blood sample collection (Hemoglobin, ALT and AST) | X | |||
| Inclusion/exclusion criteria | X | |||
| Randomization process | X | |||
| 2nd dose/booster dose | X | |||
| Blood sample collection (IgG) | X | X | ||
| EQ-5D-5L | X | X | X | X |
| VAS (pain and fatigue) | X | X | X | |
| WHO-5 well-being questionnaire | X | X | X | X |
| IP dispensing | X | |||
| IP diary dispensing | X | |||
| Daily diary dispensing | X | X | ||
| IP reconciliation | X | |||
| AE/SAE | X | |||
| ADR | X | |||
| Category | 5-ALA + SFC + Vaccine (n = 99) | Vaccine (n = 101) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| No AE | 2 | 1.0 | 0 | 0.0 | 0.152 |
| AE | 97 | 98.0 | 101 | 100.0 | |
| Odds Ratio (Arm 1/Arm 2), 5.205 (95% CI 0.246–109.81) | |||||
| Preferred Term | 5-ALA + SFC (n = 99) | Control (n = 101) | Total n = 200 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n | n(%) | n | n (%) | n | |||
| Local | Injection site erythema | 0 (0.0%) | 0 | 4 (4.0%) | 4 | 4 (2.0%) | 4 | NE |
| Injection site pain | 96 (97.0%) | 100 | 99 (98.0%) | 101 | 195 (97.5%) | 201 | 0.6812 (F) | |
| Injection site rash | 0 (0.0%) | 0 | 1 (1.0%) | 1 | 1 (0.5%) | 1 | NE | |
| Systemic | Asthenia | 5 (5.1%) | 5 | 2 (2.0%) | 2 | 7 (3.5%) | 7 | 0.2767 (F) |
| Diarrhea | 1 (1.0%) | 1 | 0 (0.0%) | 0 | 1 (0.5%) | 1 | NE | |
| Fatigue | 86 (86.9%) | 106 | 95 (94.1%) | 108 | 181 (90.5%) | 214 | 0.0829 (C) | |
| Headache | 8 (8.1%) | 8 | 7 (6.9%) | 7 | 15 (7.5%) | 15 | 0.7575 (C) | |
| Myalgia | 7 (7.1%) | 7 | 5 (5.0%) | 5 | 12 (6.0%) | 12 | 0.5279 (C) | |
| Nausea | 2 (2.0%) | 2 | 1 (1.0%) | 1 | 3 (1.5%) | 3 | 0.6193 (F) | |
| Pyrexia | 31 (31.3%) | 31 | 26 (25.7%) | 26 | 57 (28.5%) | 57 | 0.3829 (C) | |
| Somnolence | 1 (1.0%) | 1 | 0 (0.0%) | 0 | 1 (0.5%) | 1 | NE | |
| Vomiting | 1 (1.0%) | 1 | 0 (0.0%) | 0 | 1 (0.5%) | 1 | NE | |
| Total | 97 (98.0%) | 262 | 100 (99.0%) | 255 | 197 (98.5%) | 517 | 0.6193 (F) | |
| Preferred Term | 5-ALA + SFC (n = 99) | Control (n = 101) | Total (n = 200) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n | n(%) | n | n (%) | n | |||
| Systemic | Abdominal pain | 0 (0.0%) | 0 | 1 (1.0%) | 1 | 1 (0.5%) | 1 | NE |
| Cough | 1 (1.0%) | 1 | 0 (0.0%) | 0 | 1 (0.5%) | 1 | NE | |
| Dehydration | 0 (0.0%) | 0 | 1 (1.0%) | 1 | 1 (0.5%) | 1 | NE | |
| Diarrhea | 1 (1.0%) | 1 | 1 (1.0%) | 1 | 2 (1.0%) | 2 | >0.9999 (F) | |
| Fatigue | 2 (2.0%) | 2 | 1 (1.0%) | 1 | 3 (1.5%) | 3 | 0.6193 (F) | |
| Headache | 0 (0.0%) | 0 | 3 (3.0%) | 3 | 3 (1.5%) | 3 | NE | |
| Myalgia | 0 (0.0%) | 0 | 4 (4.0%) | 4 | 4 (2.0%) | 4 | NE | |
| Rhinorrhoea | 1 (1.0%) | 1 | 1 (1.0%) | 1 | 2 (1.0%) | 2 | >0.9999 (F) | |
| Somnolence | 0 (0.0%) | 0 | 1 (1.0%) | 1 | 1 (0.5%) | 1 | NE | |
| Vomiting | 1 (1.0%) | 1 | 1 (1.0%) | 1 | 2 (1.0%) | 2 | >0.9999 (F) | |
| Total | 5 (5.1%) | 6 | 12 (11.9%) | 14 | 17 (8.5%) | 20 | 0.0833 (C) | |
| GMFR | 95% CI | p-Value | |
|---|---|---|---|
| 5-ALA + SFC (N = 98) | 1.38 | (0.92–2.06) | 0.135 |
| Control (N = 100) | 1.11 | (0.80–1.55) | 0.953 |
| 5-ALA + SFC (N = 98) | Control (N = 100) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | 95% CI | Min.–Max. | Mean (SD) | Median | 95% CI | Min.–Max. | p-Value | |
| Baseline | 4469.5 (4853.0) | 2500.0 | 3496.6–5442.5 | 7.44–23074 | 5301.3 (6614.1) | 3248.0 | 3988.9–6613.7 | 8.3–42,303 | 0.197 |
| Day 21 | 5938.8 (7528.2) | 3427.5 | 4429.5–7448.1 | 9.5–47525 | 5080.3 (5437.3) | 3898.0 | 4001.4–6159.2 | 10.18–41,266 | 0.692 |
| Change | 1469.3 (7381.1) | 54.5 | −10.6–2949.1 | −22,271.50–36,635.60 | −221.0 (6336.1) | 0.0 | −1478.2–1036.3 | −38,919.0–18,112.0 | 0.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenzen, N.; Rehani, R.; Ebeling, A.; Stocker, M.; Nakajima, M. 5-Aminolevulinic Acid Phosphate as an Immune System Enhancer Along with Vaccination Against SARS-CoV-2 Virus Infection: An Open-Label, Randomized Pilot Study. Life 2025, 15, 953. https://doi.org/10.3390/life15060953
Berenzen N, Rehani R, Ebeling A, Stocker M, Nakajima M. 5-Aminolevulinic Acid Phosphate as an Immune System Enhancer Along with Vaccination Against SARS-CoV-2 Virus Infection: An Open-Label, Randomized Pilot Study. Life. 2025; 15(6):953. https://doi.org/10.3390/life15060953
Chicago/Turabian StyleBerenzen, Norbert, Riyadh Rehani, Andrea Ebeling, Marcus Stocker, and Motowo Nakajima. 2025. "5-Aminolevulinic Acid Phosphate as an Immune System Enhancer Along with Vaccination Against SARS-CoV-2 Virus Infection: An Open-Label, Randomized Pilot Study" Life 15, no. 6: 953. https://doi.org/10.3390/life15060953
APA StyleBerenzen, N., Rehani, R., Ebeling, A., Stocker, M., & Nakajima, M. (2025). 5-Aminolevulinic Acid Phosphate as an Immune System Enhancer Along with Vaccination Against SARS-CoV-2 Virus Infection: An Open-Label, Randomized Pilot Study. Life, 15(6), 953. https://doi.org/10.3390/life15060953







