Ultrasound of the Gallbladder—An Update on Measurements, Reference Values, Variants and Frequent Pathologies: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Indications for Sonographic Assessment of the Gallbladder
2.5. Examination Technique
2.6. Prerequisites for Optimum Measurement
2.6.1. Patient Preparation (Scheduled Examination)
2.6.2. Patient Position
- Supine position.
- A 15–30° left lateral oblique position.
- Seated or standing position.
2.6.3. Transducer Type and Initial Position
3. Reference Values and Recommendations
3.1. Gallbladder Size (Length and Width)
3.2. Gallbladder Volume
3.3. Gallbladder Wall
3.4. Factors Influencing Interpretation
4. Clinical Relevance of Common Pathological Findings
4.1. Diffuse Gallbladder Wall Thickening
4.2. Focal Gallbladder Wall Thickening
4.3. Gallbladder Polyps
4.4. Gallstones
4.5. Gallbladder Hydrops
5. Congenital Changes and Their Clinical Relevance
6. Future Perspectives, Open Questions
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakker, A.; Wijers, M.; Jonker, V.; Akkerman, S. The use, nature and purposes of measurement in intermediate-level occupations. ZDM 2011, 43, 737–746. [Google Scholar] [CrossRef]
- Wüstner, M.; Radzina, M.; Calliada, F.; Cantisani, V.; Havre, R.F.; Jenderka, K.-V.; Kabaalioğlu, A.; Kocian, M.; Kollmann, C.; Künzel, J.; et al. Professional Standards in Medical Ultrasound—EFSUMB Position Paper (Long Version)—General Aspects. Ultraschall Med. 2022, 43, e36–e48. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Lucius, C.; Möller, K.; Jenssen, C.; Zervides, C.; Gschmack, A.M.; Dong, Y.; Srivastava, D.; Dietrich, C.F. Pancreatic ultrasound: An update of measurements, reference values, and variations of the pancreas. Ultrasound Int. Open 2024, 10, a23899085. [Google Scholar] [CrossRef] [PubMed]
- Sienz, M.; Ignee, A.; Dietrich, C.F. Reference values in abdominal ultrasound—Liver and liver vessels. Z. Gastroenterol. 2010, 48, 1141–1152. [Google Scholar] [CrossRef]
- Sienz, M.; Ignee, A.; Dietrich, C.F. Reference values in abdominal ultrasound—Biliopancreatic system and spleen. Z. Gastroenterol. 2011, 49, 845–870. [Google Scholar] [CrossRef]
- Sienz, M.; Ignee, A.; Dietrich, C.F. Sonography today: Reference values in abdominal ultrasound: Aorta, inferior vena cava, kidneys. Z. Gastroenterol. 2012, 50, 293–315. [Google Scholar]
- Lucius, C.; Meier, J.; Gschmack, A.; Zervides, C.; Jenssen, C.; Dong, Y.; Graumann, O.; Petry, M.; Dietrich, C.F. Ultrasound of the spleen—An update on measurements, reference values, and influencing factors. A systematic review. Med. Ultrason. 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, X.-F.; Cheng, J.; Xu, X.-L.; Cao, J.-Y.; Dong, Y.; Dietrich, C.F. Normal value of virtual touch imaging quantification elastography in measurements of pancreas. Clin. Hemorheol. Microcirc. 2024, 87, 427–436. [Google Scholar] [CrossRef]
- Mathis, J.; Dong, Y.; Abendstein, B.; Hollerweger, A.; Jenssen, C.; Westerway, S.; Dietrich, C.F. Normative values of the internal genital organs of the female pelvis in transvaginal and transabdominal ultrasound. Med. Ultrason. 2022, 24, 290–299. [Google Scholar] [CrossRef]
- Srivastava, S.; Dighe, M.; Möller, K.; Chammas, M.C.; Dong, Y.; Cui, X.-W.C.; Dietrich, C.F. Ultrasound measurements and normal findings in the thyroid gland. Med. Ultrason. 2024. [Google Scholar] [CrossRef]
- Möller, K.; Saborio, M.; Gottschall, H.; Blaivas, M.; Borges, A.C.; Morf, S.; Möller, B.; Dietrich, C.F. The Perception of the Diaphragm with Ultrasound: Always There Yet Overlooked? Life 2025, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Fischer, P.; Gilja, O.H.; Gottschall, H.; Jenssen, C.; Hollerweger, A.; Lucius, C.; Meier, J.; Rogler, G.; Misselwitz, B.; et al. Gastrointestinal Ultrasound: Measurements and Normal Findings—What Do You Need to Know? Dig. Dis. 2025, 43, 300–335. [Google Scholar] [CrossRef]
- Jenssen, C.; Lorentzen, T.; Dietrich, C.F.; Lee, J.Y.; Chaubal, N.; Choi, B.I.; Rosenberg, J.; Gutt, C.; Nolsøe, C.P. Incidental Findings of Gallbladder and Bile Ducts—Management Strategies: General Aspects, Gallbladder Polyps and Gallbladder Wall Thickening—A World Federation of Ultrasound in Medicine and Biology (WFUMB) Position Paper. Ultrasound Med. Biol. 2022, 48, 2355–2378. [Google Scholar] [CrossRef]
- Kratzer, W.; Fritz, V.; Mason, R.A.; Haenle, M.M.; Kaechele, V. Factors affecting liver size: A sonographic survey of 2080 subjects. J. Ultrasound Med. 2003, 22, 1155–1161. [Google Scholar] [CrossRef]
- Kratzer, W.; Haenle, M.M.; A Mason, R.; von Tirpitz, C.; Kaechele, V. Prevalence of cholelithiasis in patients with chronic inflammatory bowel disease. World J. Gastroenterol. 2005, 11, 6170–6175. [Google Scholar] [CrossRef]
- Kratzer, W.; Kron, M.; Hay, B. Prevalence of cholecystolithiasis in South Germany—An ultrasound study of 2,498 persons of a rural population. Z. Gastroenterol. 1999, 37, 1157–1162. [Google Scholar]
- Kratzer, W.; Mason, R.A.; Kächele, V. Prevalence of gallstones in sonographic surveys worldwide. J. Clin. Ultrasound 1999, 27, 1–7. [Google Scholar] [CrossRef]
- Kratzer, W.; Walcher, T.; Arnold, F.; Akinli, A.; Mason, R.; Denzer, C.; Böhm, B.; Imhof, A.; Hänle, M. Gallstone prevalence and risk factors for gallstone disease in an urban population of children and adolescents. Z. Gastroenterol. 2010, 48, 683–687. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Atkinson, N.S.; Lee, W.J.; Kling, K.; Neumayr, A.; Braden, B.; Richter, J.; Akpata, R.; Southisavath, P.; Schreiber-Dietrich, D.; et al. Never seen before? Opisthorchiasis and Clonorchiasis. Z. Gastroenterol. 2018, 56, 1513–1520. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Kabaalioglu, A.D.N.A.N. Fasciolosis. Z. Gastroenterol. 2015, 53, 285–290. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bekkali, N.L.; Burmeister, S.; Dong, Y.; Everett, S.M.; Hocke, M.; Ignee, A.; On, W.; Hebbar, S.; Oppong, K.; et al. Controversies in ERCP: Indications and preparation. Endosc. Ultrasound 2022, 11, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Bekkali, N.L.; Burmeister, S.; Dong, Y.; Everett, S.M.; Hocke, M.; Ignee, A.; On, W.; Hebbar, S.; Oppong, K.; et al. Controversies in ERCP: Technical aspects. Endosc. Ultrasound 2022, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Braden, B.; Burmeister, S.; Aabakken, L.; Arciadacono, P.G.; Bhutani, M.S.; Götzberger, M.; Healey, A.J.; Hocke, M.; Hollerbach, S.; et al. How to perform EUS-guided biliary drainage. Endosc. Ultrasound 2022, 11, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Braden, B.; Jenssen, C. Interventional endoscopic ultrasound. Curr. Opin. Gastroenterol. 2021, 37, 449–461. [Google Scholar] [CrossRef]
- Braden, B.; Gupta, V.; Dietrich, C. Therapeutic EUS: New tools, new devices, new applications. Endosc. Ultrasound 2019, 8, 370–381. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Lorentzen, T.; Appelbaum, L.; Buscarini, E.; Cantisani, V.; Correas, J.M.; Cui, X.W.; D’Onofrio, M.; Gilja, O.H.; Hocke, M.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part III—Abdominal Treatment Procedures (Long Version). Ultraschall Med. 2016, 37, E1–E32. [Google Scholar] [CrossRef]
- Ignee, A.; Cui, X.; Schuessler, G.; Dietrich, C.F. Percutaneous transhepatic cholangiography and drainage using extravascular contrast enhanced ultrasound. Z. Gastroenterol. 2015, 53, 385–390. [Google Scholar] [CrossRef]
- Ignee, A.; Baum, U.; Schuessler, G.; Dietrich, C.F. Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD). Endoscopy 2009, 41, 725–726. [Google Scholar] [CrossRef]
- Goetzberger, M.; Nuessler, N.; Braden, B.; Dietrich, C.F.; Mueller, T. Acute Cholecystitis in high-risk surgical patients: Sonographic and endoscopic treatment options. Z. Gastroenterol. 2021, 59, 983–990. [Google Scholar]
- Fusaroli, P.; Jenssen, C.; Hocke, M.; Burmester, E.; Buscarini, E.; Havre, R.F.; Ignee, A.; Saftoiu, A.; Vilmann, P.; Nolsøe, C.P.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part V—EUS-Guided Therapeutic Interventions (short version). Ultraschall Med. 2016, 37, 412–420. [Google Scholar] [CrossRef]
- Jenssen, C.; Hocke, M.; Fusaroli, P.; Gilja, O.H.; Buscarini, E.; Havre, R.F.; Ignee, A.; Saftoiu, A.; Vilmann, P.; Burmester, E.; et al. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV—EUS-guided Interventions: General aspects and EUS-guided sampling (Long Version). Ultraschall Med. 2016, 37, E33–E76. [Google Scholar] [CrossRef] [PubMed]
- Matcuk, G.R., Jr.; Grant, E.G.; Ralls, P.W. Ultrasound measurements of the bile ducts and gallbladder: Normal ranges and effects of age, sex, cholecystectomy, and pathologic states. Ultrasound Q. 2014, 30, 41–48. [Google Scholar] [CrossRef]
- A Ikhuoriah, T.; Olatunji, O.; Adeyinka, B.; Oboh, D. Sonographic Evaluation of the Gallbladder in Adult Patients With Type 2 Diabetes Mellitus. Cureus 2022, 14, e23920. [Google Scholar] [CrossRef]
- Adeyekun, A.; Ikubor, J. Ultrasonographic assessment of gallbladder dimensions in healthy adults in benin city. West Afr. J. Radiol. 2013, 20, 4–8. [Google Scholar] [CrossRef]
- Maconi, G.; Hausken, T.; Dietrich, C.F.; Pallotta, N.; Sporea, I.; Nurnberg, D.; Dirks, K.; Romanini, L.; Serra, C.; Braden, B.; et al. Gastrointestinal Ultrasound in Functional Disorders of the Gastrointestinal Tract—EFSUMB Consensus Statement. Ultrasound Int. Open 2021, 7, E14–E24. [Google Scholar] [CrossRef]
- Dietrich, C.F. Examination technique videos. In EFSUMB Course Book; Dietrich, C.F., Ed.; European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB): London, UK, 2014; Available online: https://efsumb.org/examination-technique-videos-english/ (accessed on 12 April 2025).
- Dietrich, C.F.; Chichakli, M.; Hirche, T.O.; Bargon, J.; Leitzmann, P.; Wagner, T.O.; Lembcke, B. Sonographic findings of the hepatobiliary-pancreatic system in adult patients with cystic fibrosis. J. Ultrasound Med. 2002, 21, 409–416; quiz 417. [Google Scholar] [CrossRef]
- Baran, E.; D’Ascenzo, M.V.; Bosia, J.D.; Montaña, P. Abdominal ultrasound findings in adult patients with cystic fibrosis. Rev. Gastroenterol. Mex. (Engl. Ed.) 2024, 89, 19–24. [Google Scholar] [CrossRef]
- Dodds, W.; Groh, W.; Darweesh, R.; Lawson, T.; Kishk, S.; Kern, M. Sonographic measurement of gallbladder volume. Am. J. Roentgenol. 1985, 145, 1009–1011. [Google Scholar] [CrossRef]
- Stolk, M.F.; Van Erpecum, K.J.; Henegouwen, G.P.V.B.; Kesselring, O.F.; Hopman, W.P. Gallbladder volume and contraction measured by sum-of-cylinders method compared with ellipsoid and area-length methods. Acta Radiol. 1990, 31, 591–596. [Google Scholar] [CrossRef]
- Damião, A.O.; Sipahi, A.M.; Vezozzo, D.P.; Gonçalves, P.L.; A Laudanna, A. Reproducibility of the ultrasound method for measurement of gallbladder volume. Rev. Hosp. Clin. 1996, 51, 151–153. [Google Scholar]
- Everson, G.T.; Braverman, D.Z.; Johnson, M.L.; Kern, F. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology 1980, 79, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.R.; Lamps, L.W. Gallbladder. In Diagnostic Pathology: Normal Histology, 2nd ed.; Lindberg, M.R., Lamps, L.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 268–271. [Google Scholar]
- Zissin, R.; Osadchy, A.; Shapiro-Feinberg, M.; Gayer, G. CT of a thickened-wall gall bladder. Br. J. Radiol. 2003, 76, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Tahnia, N.J.; Hossain, M.S.; Khan, S.J.; Hossain, S. Comparison of Ultrasonographic Evaluation of Gallbladder Volume in Type II Diabetic Patients with Non-diabetic Healthy Subjects. J. Curr. Adv. Med. Res. 2021, 8, 114–118. [Google Scholar] [CrossRef]
- Kogha, N.; Ikubor, J.E.; Emuoghenerue, E.O.; Abolodje, E.; Nwajei, I.A.; Agboge, R.E. Influence of Sociodemographic and Anthropometric Factors on Gallbladder Volume in Pregnancy in a Tertiary Hospital in Nigeria. Oman Med. J. 2022, 37, e434. [Google Scholar] [CrossRef] [PubMed]
- Gether, I.M.; Andersen, E.S.; Foghsgaard, S.; Ellegaard, A.; Kelstrup, L.; Sonne, D.P.; Brønden, A.; Gillum, M.P.; Holst, J.J.; Hartmann, B.; et al. Increased gallbladder emptying and reduced GLP-1 response in pregnancy with and without gestational diabetes mellitus. Diabetes Obes. Metab. 2025, 27, 697–709. [Google Scholar] [CrossRef]
- Tai, M.; Chen, L.; He, Y.; Wang, F.; Tian, Z. Ultrasonographic evaluation of the gallbladder motor function in the diagnosis and prognosis of intrahepatic cholestasis of pregnancy. BMC Pregnancy Childbirth 2024, 24, 17. [Google Scholar] [CrossRef]
- Chapman, B.A.; Chapman, T.M.; Frampton, C.M.; Chisholm, R.J.; Allan, R.B.; Wilson, I.R.; Burt, M.J. Gallbladder volume: Comparison of diabetics and controls. Dig. Dis. Sci. 1998, 43, 344–348. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Bojuwoye, B.J.; Olokoba, L.B.; Wahab, K.W.; Salami, A.K.; Braimoh, K.T.; Inikori, A.K. The relationship between gallstone disease and gall bladder volume. Niger. J. Clin. Pract. 2008, 11, 89–93. [Google Scholar]
- Pauletzki, J.; Cicala, M.; Holl, J.; Sauerbruch, T.; Schafmayer, A.; Paumgartner, G. Correlation between gall bladder fasting volume and postprandial emptying in patients with gall stones and healthy controls. Gut 1993, 34, 1443–1447. [Google Scholar] [CrossRef]
- Kishk, S.; Darweesh, R.; Dodds, W.; Lawson, T.; Stewart, E.; Kern, M.; Hassanein, E. Sonographic evaluation of resting gallbladder volume and postprandial emptying in patients with gallstones. Am. J. Roentgenol. 1987, 148, 875–879. [Google Scholar] [CrossRef]
- Jazrawi, R.P.; Pazzi, P.; Petroni, M.L.; Prandini, N.; Paul, C.; Adam, J.A.; Gullini, S.; Northfield, T.C. Postprandial gallbladder motor function: Refilling and turnover of bile in health and in cholelithiasis. Gastroenterology 1995, 109, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Van Erpecum, K.J.; Henegouwen, G.P.V.B.; Stolk, M.F.; Hopman, W.P.; Jansen, J.B.; Lamers, C.B. Fasting gallbladder volume, postprandial emptying and cholecystokinin release in gallstone patients and normal subjects. J. Hepatol. 1992, 14, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Fraquelli, M.; Pagliarulo, M.; Colucci, A.; Paggi, S.; Conte, D. Gallbladder motility in obesity, diabetes mellitus and coeliac disease. Dig. Liver Dis. 2003, 35 (Suppl. S3), S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Horsager, J.; Tiroke, L.H.; Skjærbæk, C.; Knudsen, K.; Fedorova, T.D.; Okkels, N.; Borghammer, P. Fasting gallbladder volume is increased in patients with Parkinson’s disease. Park. Relat Disord 2021, 87, 56–60. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Braden, B. Sonographic assessments of gastrointestinal and biliary functions. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 353–367. [Google Scholar] [CrossRef]
- Lange, A.H.; Hansen, N.L.; Pedersen, M.G.; Nerild, H.H.; Rehfeld, J.F.; Hartmann, B.; Holst, J.J.; Ellegaard, A.-M.; Knop, F.K. Exogenous Glucagon-like Peptide 2 Counteracts Exogenous Cholecystokinin-induced Gallbladder Contraction in Healthy Men. J. Clin. Endocrinol. Metab. 2024, 110, 123–129. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.W.; Kim, H.C.; Park, S.J.; Yang, D.M. Gallbladder contraction at CT and sonography secondary to bowel preparation for colonoscopy. Abdom. Radiol. 2020, 45, 161–167. [Google Scholar] [CrossRef]
- Nazaroglu, H.; Meric, K.; Ozmen, C.A.; Bukte, Y.; Akay, H.O. The effects of paramagnetic contrast agents on the gallbladder volume. Diagn. Interv. Radiol. 2010, 16, 97–98. [Google Scholar] [CrossRef]
- Park, Y.S.; Yoon, H.; Kang, S.Y.; Jo, I.J.; Woo, S.; Lee, G.; Park, J.E.; Kim, T.; Lee, S.U.; Hwang, S.Y.; et al. Use of Gallbladder Width Measurement by Computed Tomography in the Diagnosis of Acute Cholecystitis. Diagnostics 2022, 12, 721. [Google Scholar] [CrossRef]
- Brook, O.R.; Kane, R.A.; Tyagi, G.; Siewert, B.; Kruskal, J.B. Lessons learned from quality assurance: Errors in the diagnosis of acute cholecystitis on ultrasound and CT. Am. J. Roentgenol. 2011, 196, 597–604. [Google Scholar] [CrossRef]
- Matsui, Y.; Hirooka, S.; Kotsuka, M.; Yamaki, S.; Kosaka, H.; Yamamoto, T.; Satoi, S. Prognosis in patients with gallbladder edema misdiagnosed as cholecystitis. JSLS J. Soc. Laparoendosc. Surg. 2019, 23, e2019.00022. [Google Scholar] [CrossRef] [PubMed]
- Schuster, K.M.; Schroeppel, T.J.; O’Connor, R.; Enniss, T.M.; Cripps, M.; Cullinane, D.C.; Kaafarani, H.M.; Crandall, M.; Puri, R.; Tominaga, G.T. Imaging acute cholecystitis, one test is enough. Am. J. Surg. 2023, 226, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.R.; Brahm, G.L.; Fung, C.; Sebastian, S.; Kirkpatrick, I.D.C. Recommendations for the Management of Incidental Hepatobiliary Findings in Adults: Endorsement and Adaptation of the 2017 and 2013 ACR Incidental Findings Committee White Papers by the Canadian Association of Radiologists Incidental Findings Working Group. Can. Assoc. Radiol. J. 2020, 71, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Vriesman, A.C.v.B.; Engelbrecht, M.R.; Smithuis, R.H.M.; Puylaert, J.B.C.M. Diffuse gallbladder wall thickening: Differential diagnosis. Am. J. Roentgenol. 2007, 188, 495–501. [Google Scholar] [CrossRef]
- Gupta, P.; Marodia, Y.; Bansal, A.; Kalra, N.; Kumar-M, P.; Sharma, V.; Dutta, U.; Sandhu, M.S. Imaging-based algorithmic approach to gallbladder wall thickening. World J. Gastroenterol. 2020, 26, 6163–6181. [Google Scholar] [CrossRef]
- Mohakud, S.; Mishra, T.S.; Naik, S.; Muduly, D.; Patra, S.; Bag, N.D.; Kar, M.; Divya, M.; Patel, R.K.; Tripathy, T.P. Differentiating carcinoma from benign causes of nonspecific gall bladder wall thickening: A prospective observational study on the role of multiparametric MRI and proposition of an MpMRI-based criteria. J. Cancer Res. Ther. 2025, 21, 64–70. [Google Scholar] [CrossRef]
- Verma, A.; Kumar, I.; Yadav, Y.; Kumar, S.; Puneet; Shukla, R.C.; Verma, A. Utility of contrast-enhanced ultrasound in differentiation between benign mural lesions and adenocarcinoma of gallbladder. J. Med. Ultrasound 2020, 28, 143–150. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, B.; Cao, Q.; Zhang, Q.; Qiu, Y.; Yang, D.; Yu, L.; Wang, W.-P. Incidentally detected focal fundal gallbladder wall thickening: Differentiation contrast enhanced ultrasound features with high-resolution linear transducers. Clin. Hemorheol. Microcirc. 2020, 74, 315–325. [Google Scholar] [CrossRef]
- Moon, J.; Shin, Y.C.; Heo, T.-G.; Choi, P.W.; Kim, J.I.; Jun, H.; Jung, S.M.; Um, E. Differentiation of gallbladder adenomyomatosis from early-stage gallbladder cancer before surgery. Ann. Hepato-Biliary-Pancreat. Surg. 2019, 23, 334–338. [Google Scholar] [CrossRef]
- Oh, S.H.; Han, H.Y.; Kim, H.J. Comet tail artifact on ultrasonography: Is it a reliable finding of benign gallbladder diseases? Ultrasonography 2019, 38, 221–230. [Google Scholar] [CrossRef]
- Hammad, A.Y.; Miura, J.T.; Turaga, K.K.; Johnston, F.M.; Hohenwalter, M.D.; Gamblin, T.C. A literature review of radiological findings to guide the diagnosis of gallbladder adenomyomatosis. HPB 2016, 18, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Gupta, P.; Kalage, D.; Soundararajan, R.; Kumar-M, P.; Dutta, U. Grayscale ultrasonography findings for characterization of gallbladder wall thickening in non-acute setting: A systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Dutta, U.; Rana, P.; Singhal, M.; Gulati, A.; Kalra, N.; Soundararajan, R.; Kalage, D.; Chhabra, M.; Sharma, V.; et al. Gallbladder reporting and data system (GB-RADS) for risk stratification of gallbladder wall thickening on ultrasonography: An international expert consensus. Abdom. Radiol. 2022, 47, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Riddell, Z.C.; Corallo, C.; Albazaz, R.; Foley, K.G. Gallbladder polyps and adenomyomatosis. Br. J. Radiol. 2023, 96, 20220115. [Google Scholar] [CrossRef]
- Cocco, G.; Basilico, R.; Pizzi, A.D.; Cocco, N.; Boccatonda, A.; D’ardes, D.; Fabiani, S.; Anzoletti, N.; D’alessandro, P.; Vallone, G.; et al. Gallbladder polyps ultrasound: What the sonographer needs to know. J. Ultrasound 2021, 24, 131–142. [Google Scholar] [CrossRef]
- Wennmacker, S.Z.; Lamberts, M.P.; Di Martino, M.; Drenth, J.P.; Gurusamy, K.S.; van Laarhoven, C.J. Transabdominal ultrasound and endoscopic ultrasound for diagnosis of gallbladder polyps. Cochrane Database Syst. Rev. 2018, 2018, CD012233. [Google Scholar] [CrossRef]
- Anderson, M.A.; Mercaldo, S.; Cao, J.; Mroueh, N.; Furtado, F.S.; Cochran, R.L.; Chung, R.; Goiffon, R.J.; Sertic, M.; Pierce, T.T.; et al. Society of Radiologists in Ultrasound Consensus Conference Recommendations for Incidental Gallbladder Polyp Management: Interreader Agreement Among 10 Radiologists. Am. J. Roentgenol. 2024, 222, e2330720. [Google Scholar] [CrossRef]
- Jo, I.H.; Paik, C.N.; Ahn, H.G.; You, D.D.; Han, J.H.; A Kim, H. Predicting Neoplastic Gallbladder Polyps: The Role of Current Surgical Indications and Preoperative Images. Korean J. Gastroenterol. 2025, 85, 52–63. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.Y.; Baek, J.H.; Eun, H.W.; Kim, Y.J.; Han, J.K.; Choi, B.I. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. Am. J. Roentgenol. 2015, 204, W150–W159. [Google Scholar] [CrossRef]
- Cocco, G.; Delli Pizzi, A.; Basilico, R.; Fabiani, S.; Taraschi, A.L.; Pascucci, L.; Boccatonda, A.; Catalano, O.; Schiavone, C. Imaging of gallbladder metastasis. Insights Imaging 2021, 12, 100. [Google Scholar] [CrossRef]
- Choi, T.W.; Kim, J.H.; Park, S.J.; Ahn, S.J.; Joo, I.; Han, J.K. Risk stratification of gallbladder polyps larger than 10 mm using high-resolution ultrasonography and texture analysis. Eur. Radiol. 2018, 28, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Torabi Sagvand, B.; Edwards, K.; Shen, B. Frequency, Risk Factors, and Outcome of Gallbladder Polyps in Patients With Primary Sclerosing Cholangitis: A Case-Control Study. Hepatol. Commun. 2018, 2, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.G.; Lahaye, M.J.; Thoeni, R.F.; Soltes, M.; Dewhurst, C.; Barbu, S.T.; Vashist, Y.K.; Rafaelsen, S.R.; Arvanitakis, M.; Perinel, J.; et al. Management and follow-up of gallbladder polyps: Updated joint guidelines between the ESGAR, EAES, EFISDS and ESGE. Eur. Radiol. 2022, 32, 3358–3368. [Google Scholar] [CrossRef]
- Li, Q.; Dou, M.; Liu, H.; Jia, P.; Wang, X.; Geng, X.; Zhang, Y.; Yang, R.; Li, J.; Yang, W.; et al. Prediction of neoplastic gallbladder polyps in patients with different age level based on preoperative ultrasound: A multi-center retrospective real-world study. BMC Gastroenterol. 2024, 24, 146. [Google Scholar] [CrossRef]
- Sebastian, S.; Araujo, C.; Neitlich, J.D.; Berland, L.L. Managing incidental findings on abdominal and pelvic CT and MRI, Part 4: White paper of the ACR Incidental Findings Committee II on gallbladder and biliary findings. J. Am. Coll. Radiol. 2013, 10, 953–956. [Google Scholar] [CrossRef]
- Lee, K.-C.; Kim, J.-K.; Kim, D.-K. Comparison of the Size Measurement of Gallbladder Polyps by Three Different Radiologists in Abdominal Ultrasonography. Tomography 2024, 10, 1031–1041. [Google Scholar] [CrossRef]
- Kim, N.H.; Kang, J.H.; Kim, H.J. Impact of nonalcoholic fatty liver disease on the risk of gallbladder polyps in lean and non-obese individuals: A cohort study. Hepatobiliary Pancreat. Dis. Int. 2024, 23, 573–578. [Google Scholar] [CrossRef]
- Vo-Phamhi, J.M.; Tiyarattanachai, T.; Matuszczak, M.; Shen, L.; Kim, S.; Kamaya, A. Follow-up Imaging and Surgical Costs Associated with Different Guidelines for Management of Incidentally Detected Gallbladder Polyps. Acad. Radiol. 2025, 32, 757–766. [Google Scholar] [CrossRef]
- Gutt, C.; Jenssen, C.; Barreiros, A.P.; Götze, T.O.; Stokes, C.S.; Jansen, P.L.; Neubrand, M.; Lammert, F. Updated S3-Guideline for Prophylaxis, Diagnosis and Treatment of Gallstones. German Society for Digestive and Metabolic Diseases (DGVS) and German Society for Surgery of the Alimentary Tract (DGAV)—AWMF Registry 021/008. Z. Gastroenterol. 2018, 56, 912–966. [Google Scholar]
- Lammert, F.; Gurusamy, K.; Ko, C.W.; Miquel, J.F.; Méndez-Sánchez, N.; Portincasa, P.; Van Erpecum, K.J.; Van Laarhoven, C.J.; Wang, D.Q.H. Gallstones. Nat. Rev. Dis. Primers 2016, 2, 16024. [Google Scholar] [CrossRef]
- Cariati, A.; Piromalli, E.; Cetta, F. Gallbladder cancers: Associated conditions, histological types, prognosis, and prevention. Eur. J. Gastroenterol. Hepatol. 2014, 26, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Kokilavani, J.; Indiran, V. Phrygian cap. Abdom. Radiol. 2018, 43, 1264–1265. [Google Scholar] [CrossRef] [PubMed]
- Van Kamp, M.J.S.; Bouman, D.E.; Steenvoorde, P.; Klaase, J.M. A Phrygian Cap. Case Rep. Gastroenterol. 2013, 7, 347–351. [Google Scholar] [CrossRef]
- Boyden, E.A. The accessory gall? bladder? An embryological and comparative study of aberrant biliary vesicles occurring in man and the domestic mammals. Am. J. Anat. 1926, 38, 177–231. [Google Scholar] [CrossRef]
- Adamski, J.; Mohan, D.; Waasdorp, C. Pseudo-duplication of the Gallbladder. Clin. Pr. Cases Emerg. Med. 2020, 4, 103–104. [Google Scholar] [CrossRef]
- Rafailidis, V.; Varelas, S.; Kotsidis, N.; Rafailidis, D. Two congenital anomalies in one: An ectopic gallbladder with phrygian cap deformity. Case Rep. Radiol. 2014, 2014, 246476. [Google Scholar] [CrossRef]
- Nasreen, S.; Parveen, S. Ultrasound imaging of gallbladder variants. J. Anat. Soc. India 2016, 65, 118–122. [Google Scholar] [CrossRef]
- van Eijck, F.C.; van Veen, R.N.; Kleinrensink, G.J.; Lange, J.F. Hartmann’s gallbladder pouch revisited 60 years later. Surg. Endosc. 2007, 21, 1122–1125. [Google Scholar] [CrossRef]
- Nadeem, G. A study of the clinico-anatomical variations in the shape and size of gallbladder. J. Morphol. Sci. 2016, 33, 062–067. [Google Scholar] [CrossRef]
- Abdullah, N.M.; Mohamed, A.K.Q.; Akkila, S.S. A Cadaveric Study on the Prevalence of Hartmann Pouch of Gallbladder with Relation to Gallstones in Basrah City. Indian J. Forensic Med. Toxicol. 2020, 14, 332–338. [Google Scholar]
- Khan, K.S.; Sajid, M.A.; McMahon, R.K.; Mahmud, S.; Nassar, A.H.M. Hartmann’s pouch stones and laparoscopic cholecystectomy: The challenges and the solutions. JSLS J. Soc. Laparosc. Robot. Surg. 2020, 24, e2020-00043. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.B.; Aithal, A.P.; Padavinangadi, A.; Prabhu, G. Double pouched, sigmoid gallbladder that can cause a diagnostic dilemma to radiologists: A case report. Anat. Cell Biol. 2018, 51, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.-H.; Putra, J. Type 1 Choledochal Cyst with Ectopic Pancreas and Septate Gallbladder. Fetal Pediatr. Pathol. 2022, 41, 334–337. [Google Scholar] [CrossRef]
- Hsieh, Y.M.; Hsieh, Y.L.; Wang, N.L.; Wu, P.S.; Weng, S.C. Multiseptate gallbladder: A case report and literature review. Medicine 2021, 100, e27992. [Google Scholar] [CrossRef] [PubMed]
- Ghei, R.; Perice, L.; Odashima, K. Incidental Diagnosis of Honeycomb Gallbladder on Point-of-Care Ultrasound. Cureus 2022, 14, e32650. [Google Scholar] [CrossRef]
- Revzin, M.V.; Scoutt, L.; Smitaman, E.; Israel, G.M. The gallbladder: Uncommon gallbladder conditions and unusual presentations of the common gallbladder pathological processes. Abdom. Imaging 2015, 40, 385–399. [Google Scholar] [CrossRef]
- Kramer, A.J.; Bregman, A.; A Zeddies, C.; Guynn, V.L. Gallbladder diverticulum: A case report and review of the literature. Am. Surg. 1998, 64, 298. [Google Scholar]
- Chin, N.W.; Chapman, I. Carcinoma in a true diverticulum of the gallbladder. Am. J. Gastroenterol. 1988, 83, 667–669. [Google Scholar]
- Gross, R.E. Congenital anomalies of the gallbladder: A review of one. hundred and forty-eight cases, with report of a double gallbladder. Arch. Surg. 1936, 32, 131–162. [Google Scholar] [CrossRef]
- Lee, T.H.; Park, S.-H.; Park, J.-Y.; Lee, C.-K.; Chung, I.-K.; Kim, H.S.; Kim, S.-J. Gallbladder pseudodiverticulosis mimicking a multiseptate gallbladder with stones. Gut Liver 2009, 3, 134–136. [Google Scholar] [CrossRef]
- Reddy, R. True Diverticulum of the Gallbladder in Acute Calculous Cholangitis: A Rare Presentation. Dubai Med. J. 2023, 6, 216–218. [Google Scholar] [CrossRef]
- Rajguru, J.; Jain, S.; Khare, S.; Fulzele, R.R.; Ghai, R. Embryological Basis and Clinical Correlation of the Rare Congenital Anomaly of the Human Gall Bladder:—“The Diverticulum”—A Morphological Study. J. Clin. Diagn. Res. 2013, 7, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Talib, V.; Khan, A.S.; Dawani, S.; Ahmed, H. A case of an absent gall bladder presenting as biliary colic in a tertiary care hospital in Karachi. J. Pak. Med. Assoc. 2019, 69, 731–733. [Google Scholar]
- Winters, M.; Clar, D.T.; Van Fossen, K. Intraoperative Diagnosis of Gallbladder Agenesis. Am. Surg. 2023, 89, 2844–2846. [Google Scholar] [CrossRef]
- Tagliaferri, E.; Bergmann, H.; Hammans, S.; Azizi, A.; Stüber, E.; Seidlmayer, C. Agenesis of the Gallbladder: Role of Clinical Suspicion and Magnetic Resonance to Avoid Unnecessary Surgery. Case Rep. Gastroenterol. 2017, 10, 819–825. [Google Scholar] [CrossRef]
- Bani-Hani, K.E. Agenesis of the gallbladder: Difficulties in management. J. Gastroenterol. Hepatol. 2005, 20, 671–675. [Google Scholar] [CrossRef]
- Pipia, I.; Kenchadze, G.; Demetrashvili, Z.; Nemsadze, G.; Jamburia, L.; Zamtaradze, T.; Abiatari, I. Gallbladder agenesis: A case report and review of the literature. Int. J. Surg. Case Rep. 2018, 53, 235–237. [Google Scholar] [CrossRef]
- Toouli, J.; Geenen, J.; Hogan, W.; Dodds, W.; Arndorfer, R. Sphincter of Oddi motor activity: A comparison between patients with common bile duct stones and controls. Gastroenterology 1982, 82, 111–117. [Google Scholar] [CrossRef]
- Whittle, C.; Skoknic, V.; Maldonado, I.; Schiappacasse, G.; Pose, G. Multimodality Imaging of Congenital Variants in the Gallbladder: Pictorial Essay. Ultrasound Q. 2019, 35, 195–199. [Google Scholar] [CrossRef]
- Thapar, P.; Masurkar, V.; Philip, R.; Rokade, M.; Dalvi, A. Rudimentary Gallbladder Mimicking Choledochal Cyst. Indian J. Surg. 2015, 77 (Suppl. S2), 726–728. [Google Scholar] [CrossRef][Green Version]
- Botsford, A.; McKay, K.; Hartery, A.; Hapgood, C. MRCP imaging of duplicate gallbladder: A case report and review of the literature. Surg. Radiol. Anat. 2015, 37, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Gigot, J.F.; Van Beers, B.; Goncette, L.; Etienne, J.; Collard, A.; Jadoul, P.; Therasse, A.; Otte, J.B.; Kestens, P.J. Laparoscopic treatment of gallbladder duplication: A plea for removal of both gallbladders. Surg. Endosc. 1997, 11, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.Q.; Liang, Q.; Li, E.Z.; Gong, J.L.; Fan, J.M.; Wang, P. 3D reconstruction of a gallbladder duplication to guide LC: A case report and literature review. Medicine 2023, 102, e33054. [Google Scholar] [CrossRef]
- Picchi, E.; Leomanni, P.; Dell’olio, V.B.; Pucci, N.; Di Giuliano, F.; Ferrazzoli, V.; Minosse, S.; Rho, M.; Chiocchi, M.; Garaci, F.; et al. Triple gallbladder: Radiological review. Clin. J. Gastroenterol. 2023, 16, 629–640. [Google Scholar] [CrossRef]
- Pereira, R.; Singh, T.; Avramovic, J.; Baker, S.; Eslick, G.D.; Cox, M.R. Left-sided gallbladder: A systematic review of a rare biliary anomaly. ANZ J. Surg. 2019, 89, 1392–1397. [Google Scholar] [CrossRef]
- Roli, I.; Colli, F.; Mullineris, B.; Esposito, S.; Piccoli, M. Left sided gallbladder: A case report during laparoscopic cholecystectomy for acute cholecystitis. Int. J. Surg. Case Rep. 2020, 77, S34–S36. [Google Scholar] [CrossRef]
- Hsu, S.L.; Chen, T.Y.; Huang, T.L.; Sun, C.K.; Concejero, A.M.; Tsang, L.L.C.; Cheng, Y.F. Left-sided gallbladder: Its clinical significance and imaging presentations. World J. Gastroenterol. 2007, 13, 6404–6409. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Dent, D.M.; Mervis, B.; Kottler, R.E. Cholecystitis in an intrahepatic gallbladder. A case report. S. Afr. Med. J. 1982, 62, 1042–1043. [Google Scholar]
- Hibbs, H.; Ahmad, U. Inverted liver with suprahepatic, anteriorly displaced gallbladder. J. La. State Med. Soc. 2010, 162, 150–152. [Google Scholar]
- Steiner, E.; Youssefzadeh, S. The suprahepatic gallbladder and liver inversion: Their imaging via US and CT. Rofo 1993, 159, 102–103. [Google Scholar] [CrossRef]
- Sheu, B.-S.; Lin, X.-Z.; Chen, C.-Y.; Chow, N.-H.; Lin, P.-W.; Tsai, H.-M. Suprahepatic gallbladder and right lobe anomaly of the liver in patients with biliary cancers. Dig. Dis. Sci. 1995, 40, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Patricio, G.S.; G, L.M.F.; Jorge, S.H.; Caicedo, H.R.; Cardona, A.F.V.; Cardona, C.D.G. Floating gallbladder in exomphalos minor an exceptional condition that should be considered, a case report. Int. J. Surg. Case Rep. 2022, 92, 106809. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Chau, G.-Y.; Su, C.-W.; Wu, J.-C. Wandering abdominal pain due to a floating gallbladder. Dig. Liver Dis. 2013, 45, e13. [Google Scholar] [CrossRef] [PubMed]
- Ueo, T.; Yazumi, S.; Okuyama, S.; Okada, Y.; Oono, T.; Watanabe, M.; Umehara, Y.; Honjo, H.; Mitumoto, Y.; Mori, T.; et al. Acute cholecystitis due to strangulation of a floating gallbladder by the lesser omentum. Abdom. Imaging 2007, 32, 348–350. [Google Scholar] [CrossRef]
- Lyons, K.P.; Challa, S.; Abrahm, D.; Kennelly, B.M. Floating gallbladder: A questionable prelude to torsion: A case report. Clin. Nucl. Med. 2000, 25, 182–183. [Google Scholar] [CrossRef]
- Nayak, S.; Shetty, S.; Vasudeva, S. Complete enclosure of gall bladder inside the lesser omentum—A rare anomaly. Morphologie 2022, 106, 206–208. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Y.; Yu, C.; Liu, J.; Duan, X.; Weng, Z.; Chen, D.; Liang, Q.; Fang, Q.; Zhou, J.; et al. Ensembled deep learning model outperforms human experts in diagnosing biliary atresia from sonographic gallbladder images. Nat. Commun. 2021, 12, 1259. [Google Scholar] [CrossRef]
- Jang, S.I.; Kim, Y.J.; Kim, E.J.; Kang, H.; Shon, S.J.; Seol, Y.J.; Lee, D.K.; Kim, K.G.; Cho, J.H. Diagnostic performance of endoscopic ultrasound-artificial intelligence using deep learning analysis of gallbladder polypoid lesions. J. Gastroenterol. Hepatol. 2021, 36, 3548–3555. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, C.; Zeng, J.; Jiang, Y.; Sun, R.; Li, C.; Li, J.; Xing, C.; Tan, B.; Liu, K.; et al. Establishing a preoperative predictive model for gallbladder adenoma and cholesterol polyps based on machine learning: A multicentre retrospective study. World J. Surg. Oncol. 2025, 23, 27. [Google Scholar] [CrossRef]
- He, Z.; Yang, S.; Cao, J.; Gao, H.; Peng, C. Predicting Neoplastic Polyp in Patients With Gallbladder Polyps Using Interpretable Machine Learning Models: Retrospective Cohort Study. Cancer Med. 2025, 14, e70739. [Google Scholar] [CrossRef]

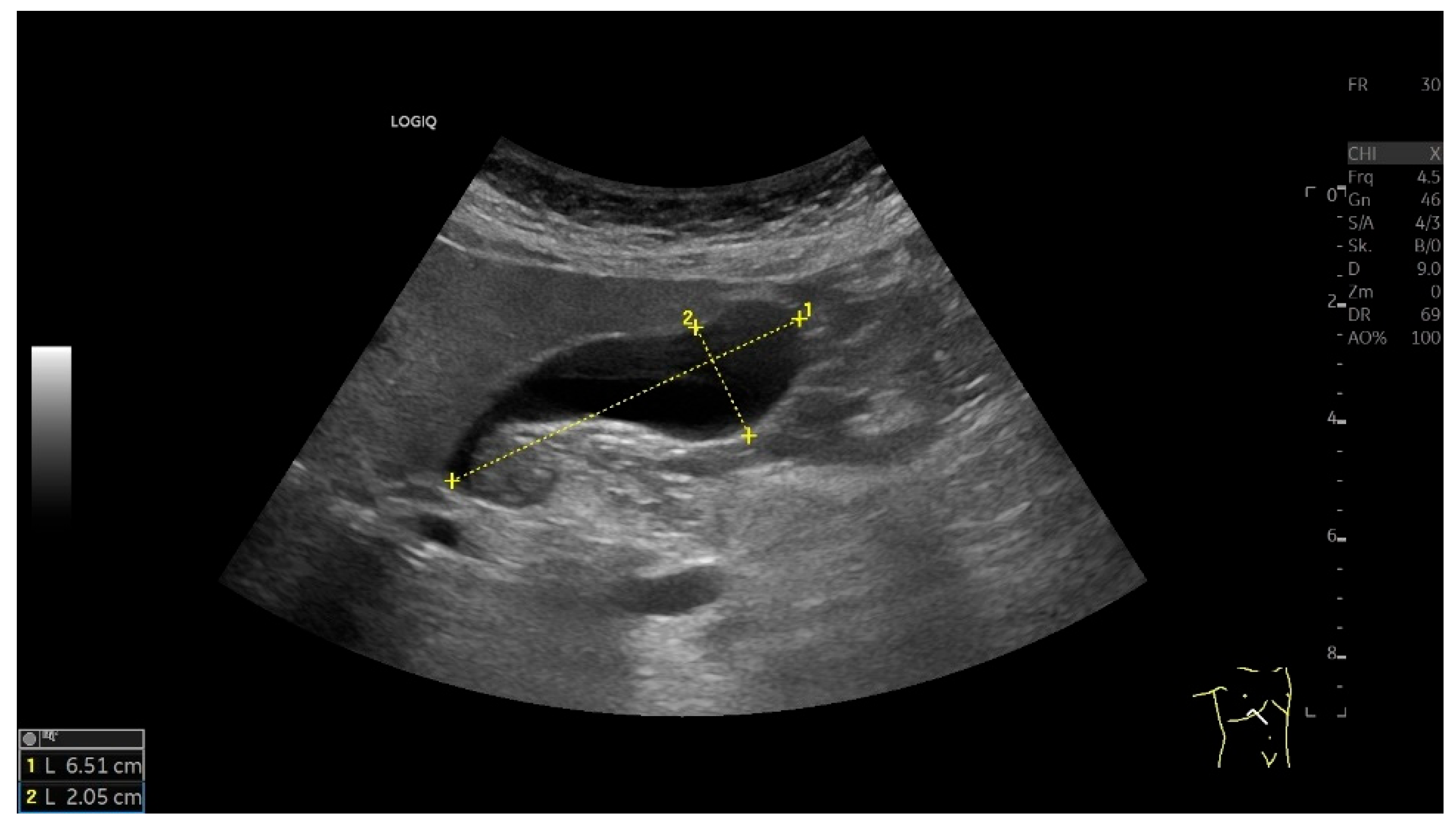


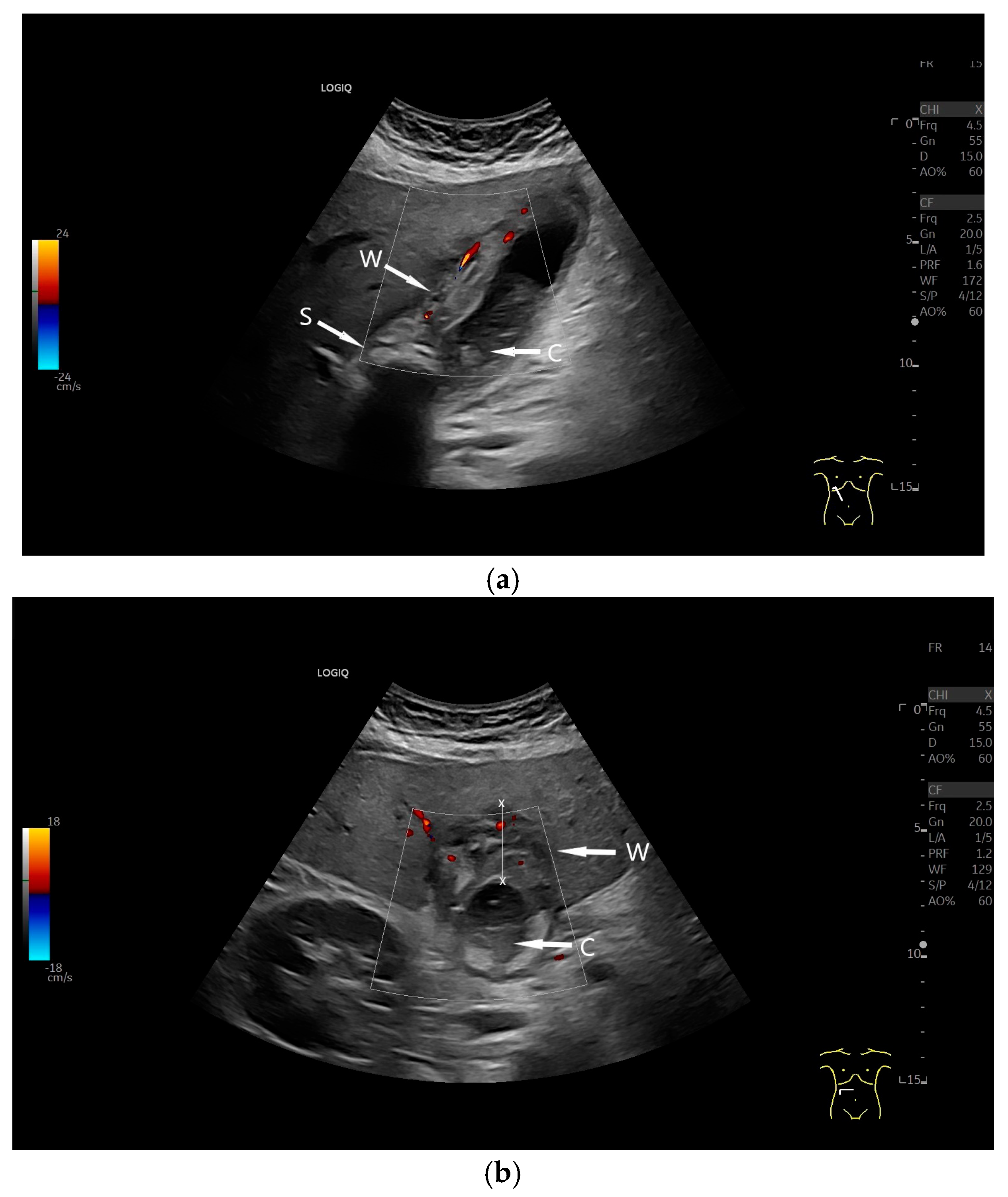
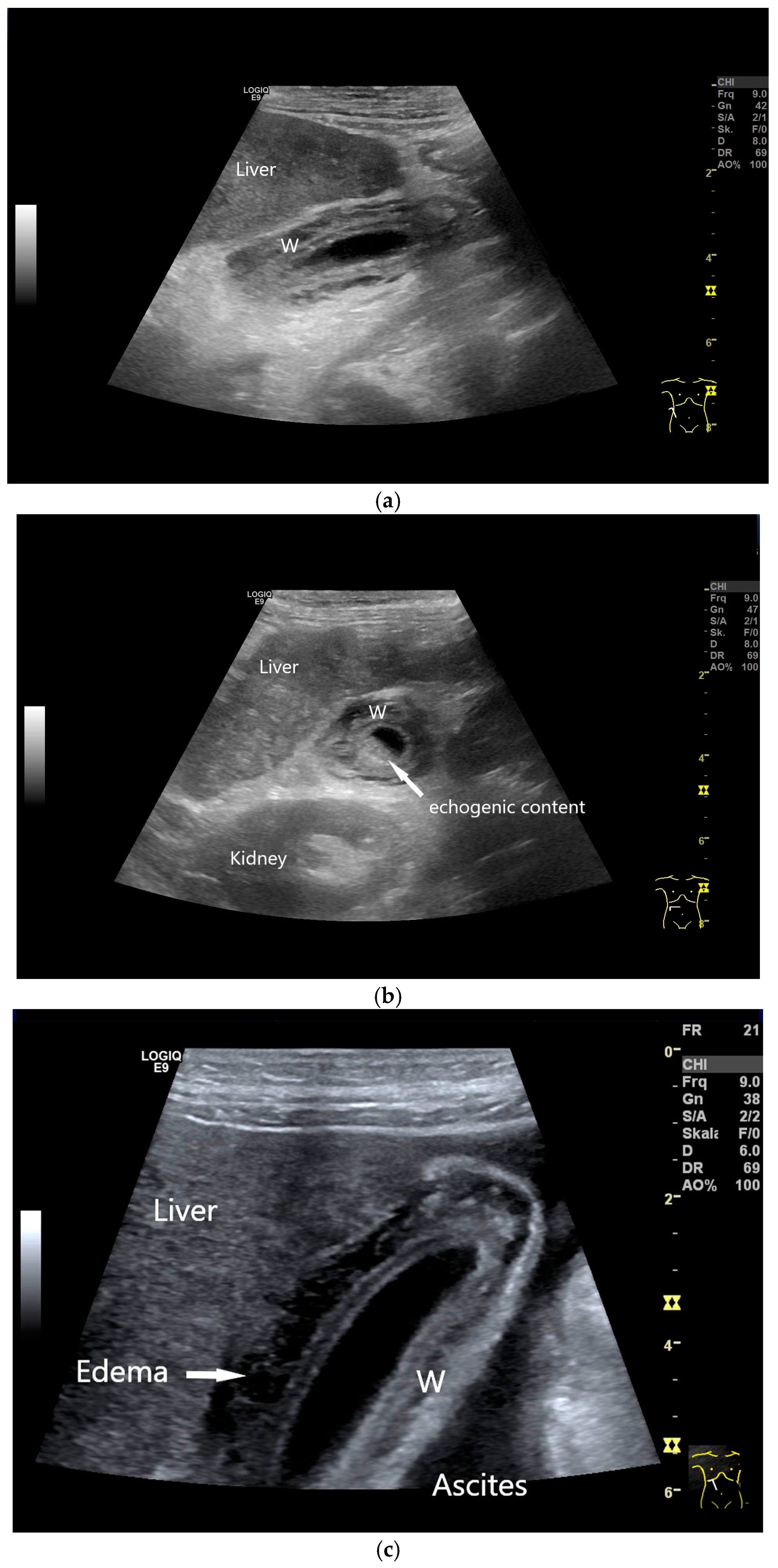
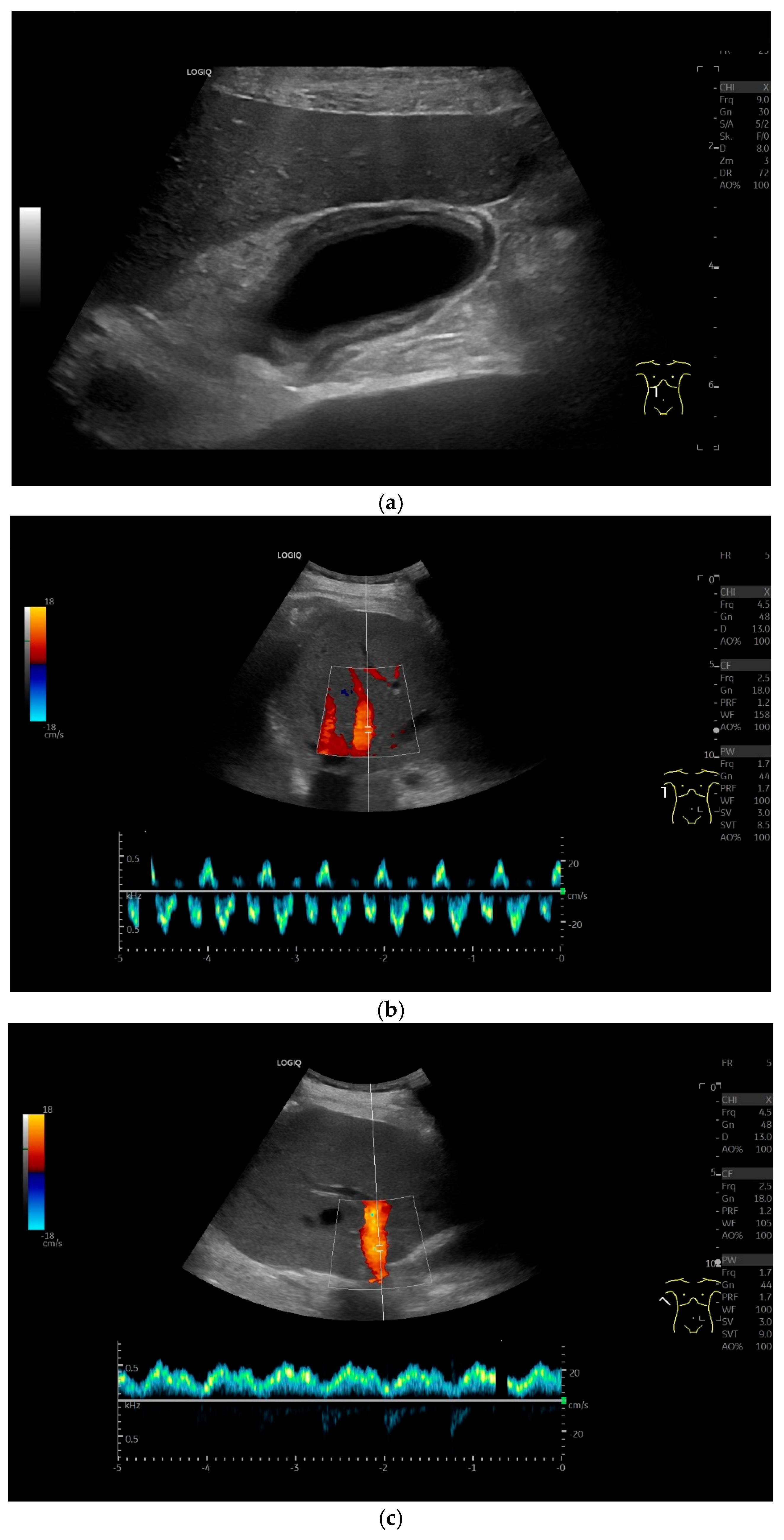


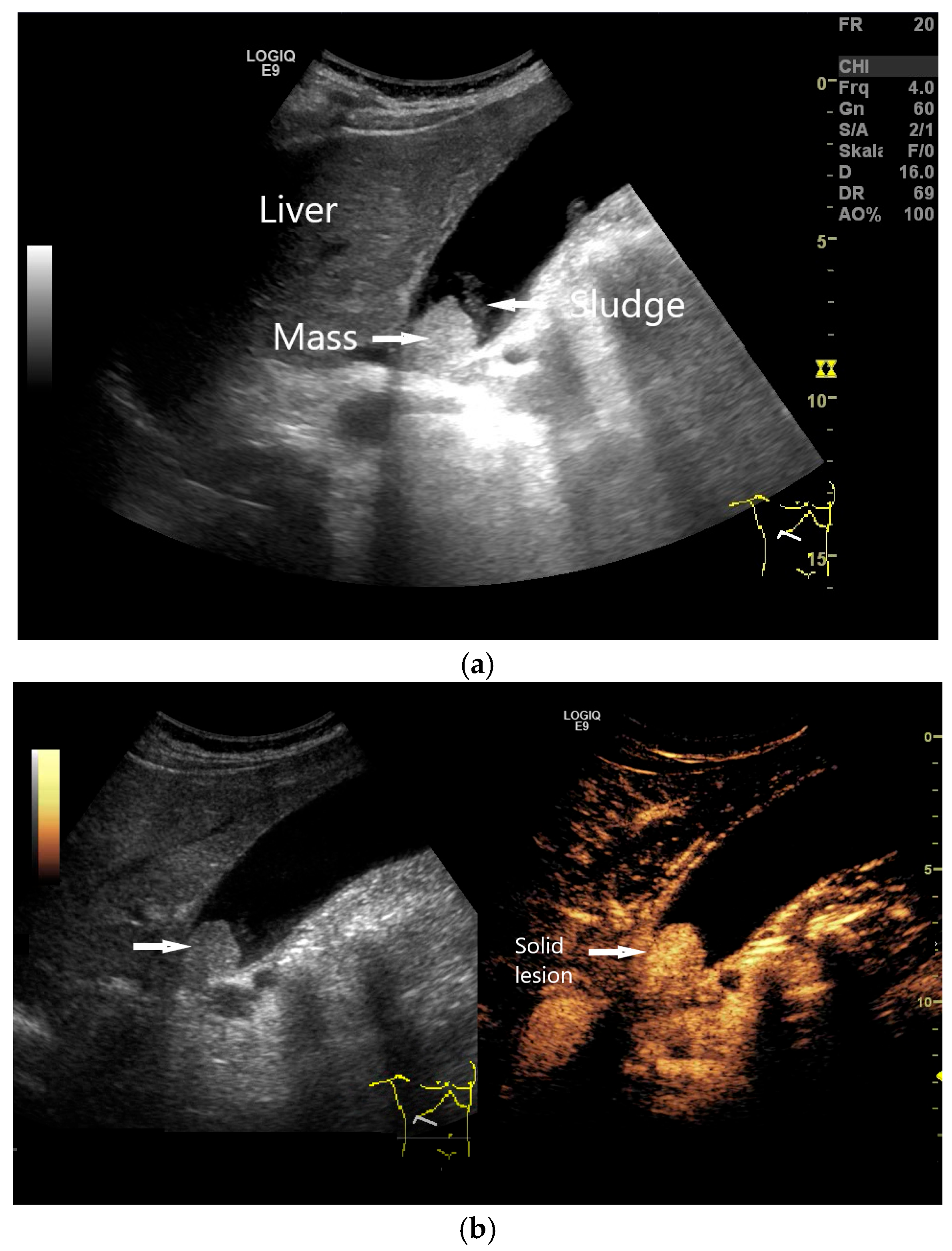
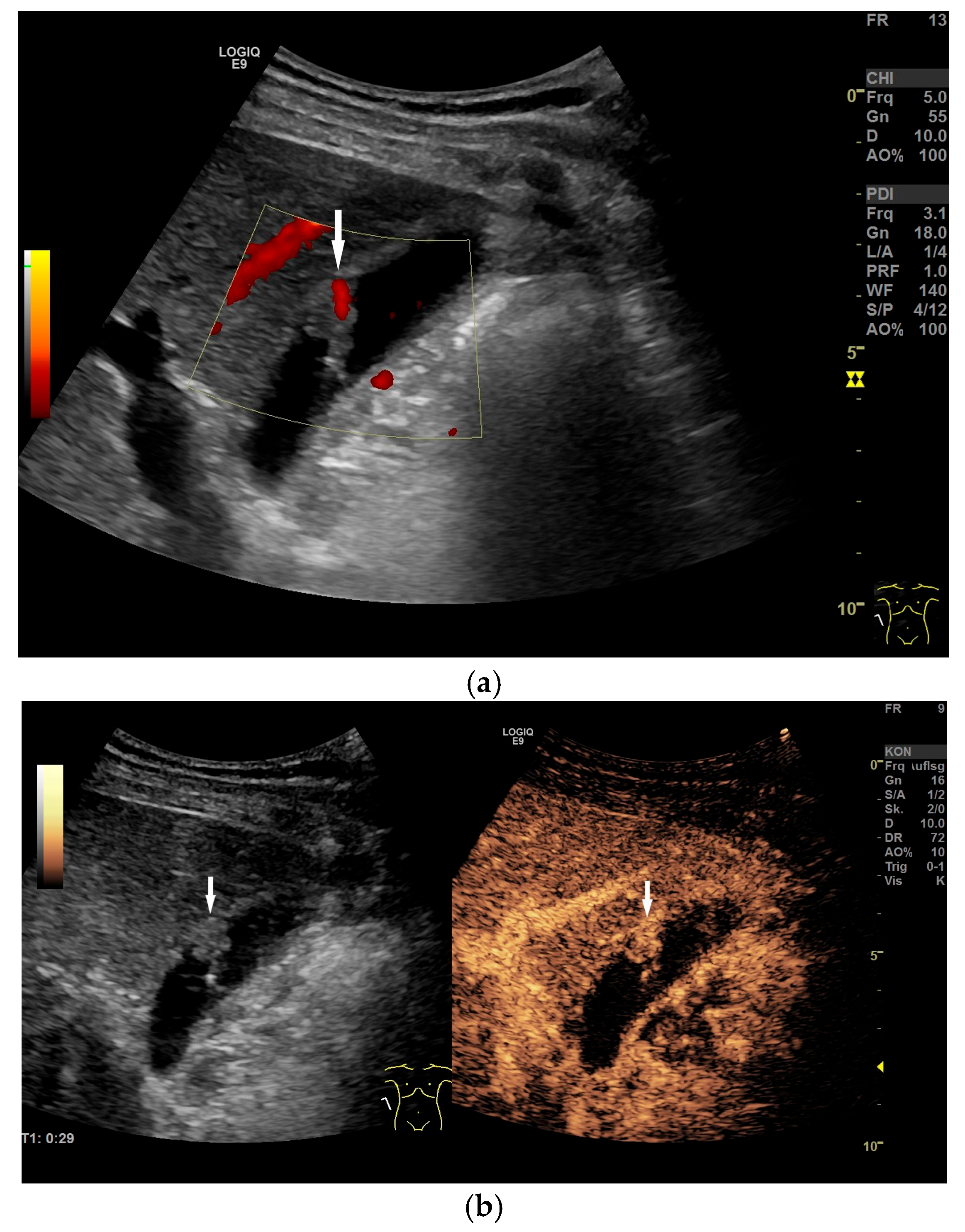

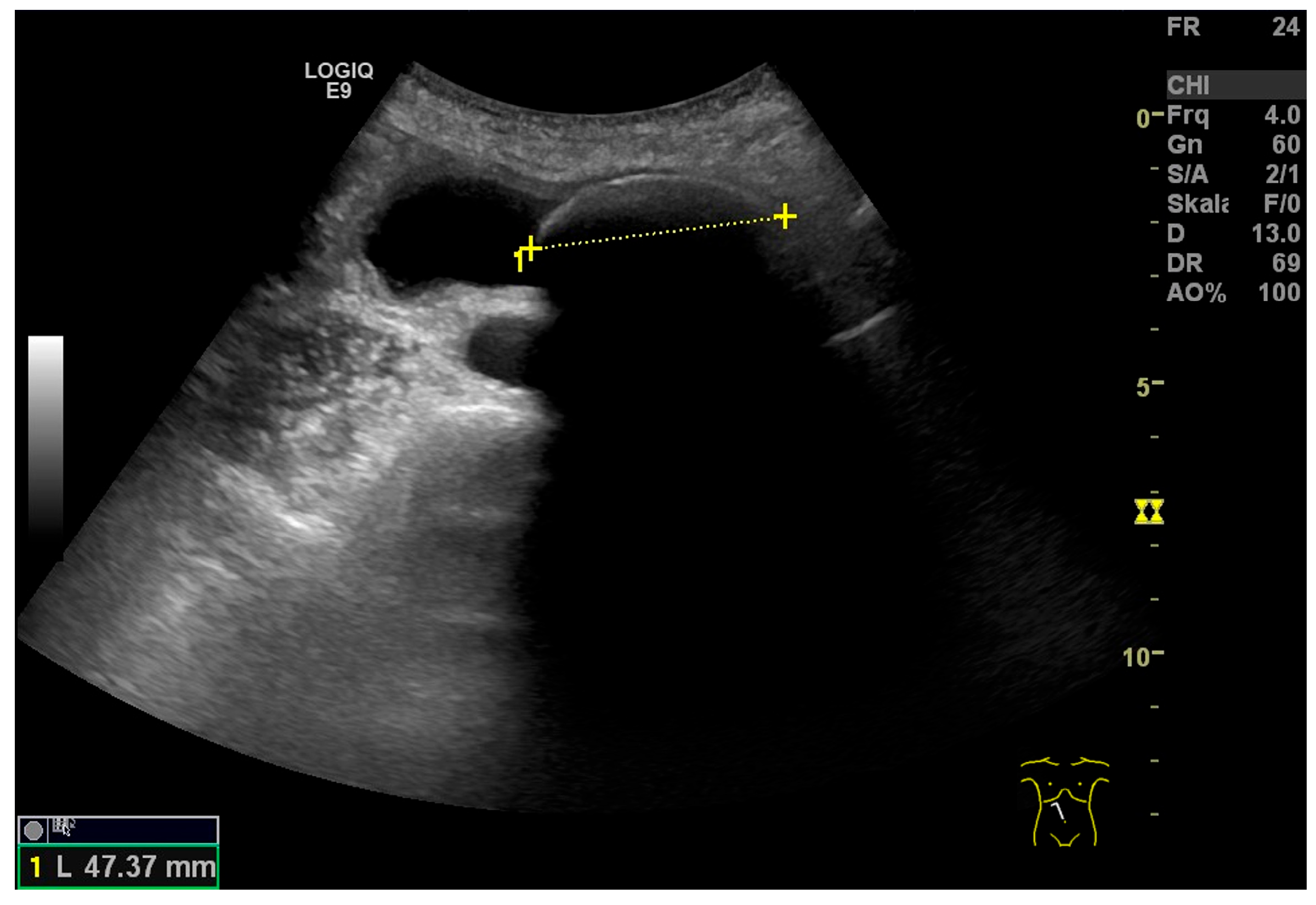




| Anatomical Structure | What Should I Do? |
|---|---|
| Gallbladder | Imaging of the GB in its maximum longitudinal extent in the longitudinal section at the level of the medio-clavicular line. Be aware of the infundibulum and scan it thoroughly. Use various transducer positions and body positions (supine, left-sided, also standing). Measurement of the following:
|
| Measured GB Structures | Reference Values |
|---|---|
| Length × width × depth | <10 × 4 × 4 cm [5,32,33,34]. |
| Wall thickness | <3 mm [5,32,33,34] |
| Influencing Factor | GB Volume | GB Wall | GB Ejection Fraction |
|---|---|---|---|
| Postprandial state | ↓ | ↑ | |
| Type 2 diabetes | ↑ | ↑ | ↓ |
| High BMI | ↑ | ||
| High BMI and pregnancy | ↑ | ||
| Several drugs (e.g., NSAID, GLP2-agonists) | ↑ | ||
| Same-day colonoscopy, urography, or other contrast agents | ↓ | ||
| Gender, ethnicity, and age | -- | -- | -- |
| Indication | Anatomical Structure |
|---|---|
| Routine examination |
|
| Defined clinical indications |
|
| Gallbladder Shape—Congenital Anomalies | ||
|---|---|---|
| Nature of Changes | Description | Meaning |
| Phrygian cap [94,95,96,97,98,99] (Figure 17) | - GB is angled in the area of the fundus—either by folding or a septum. - Most common abnormal form with 1–7% prevalence. - Pseudo-duplication of the GB can occur in the presence of a Phrygian cap with an incidence of 0.025%. | - Can be missed if the GB is not assessed in several planes. - Can potentially lead to misdiagnosis of thickened GB wall or mistaken as liver lesion. - No significance unless gallstones are hiding there. |
| Hartmann’s gallbladder pouch [100,101,102,103] | - Hartmann’s pouch is an outpouching of the GB at the transition of the GB to the cystic duct. Prevalence varies from 4.7 to 52%. - Common finding in normal and pathologic GBs. | - Significantly associated with cholecystolithiasis. - Hartmann’s pouch stones encountered during laparoscopic cholecystectomy may hinder the safe dissection of the cystic pedicle. |
| Sigmoid gallbladder/Constriction with two pouches [13,104] | Described as two pouches with a narrow isthmus in between, like two GB in a line. | Differential diagnosis of a cystic lesion/tumor. Clinical relevance for surgery. |
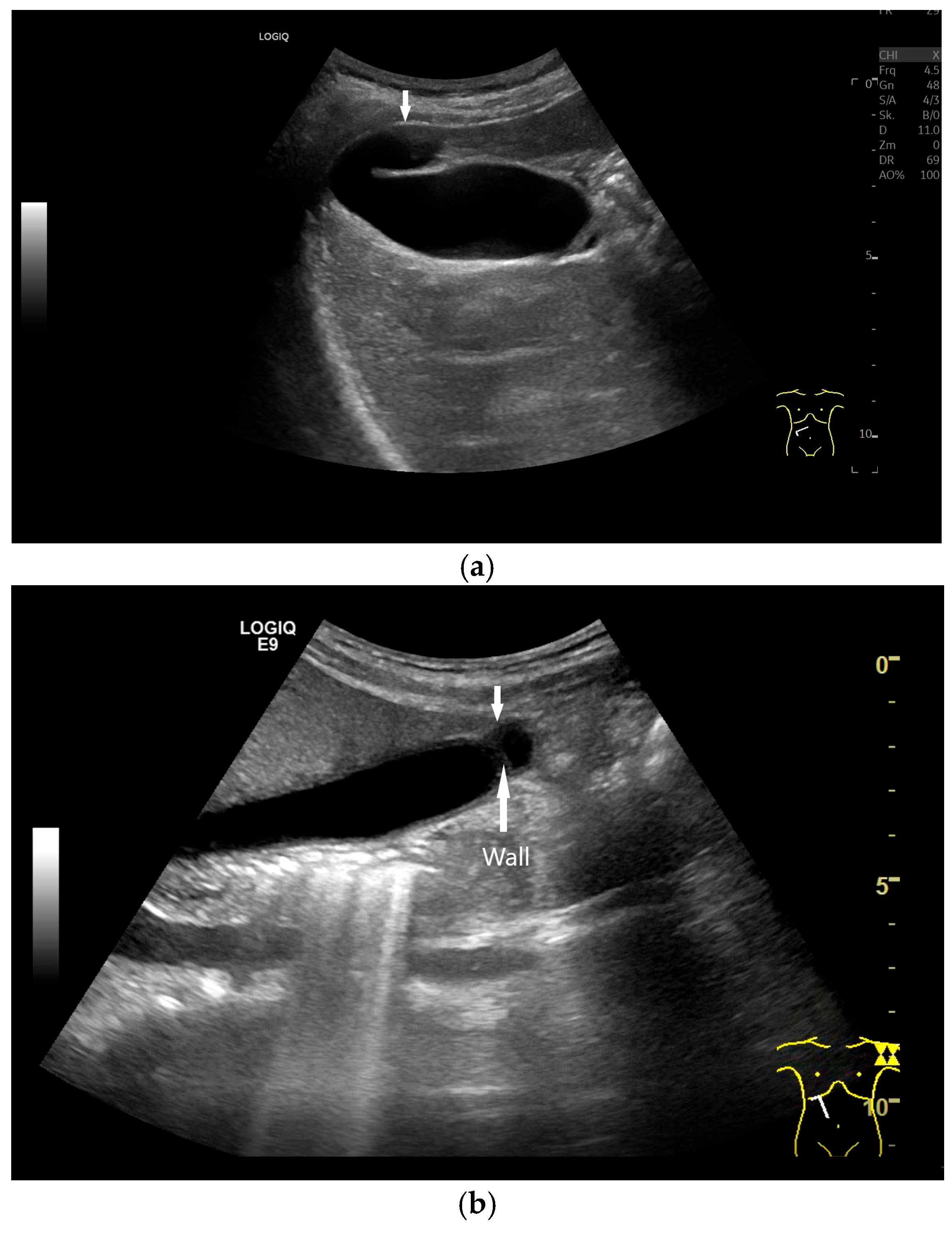
| Multiseptated gallbladder [105,106,107,108] (Figure 18) | - Multiple septa of various sizes. “Honeycomb-like” appearance. - Rare and benign anomaly with <150 cases reported. | Differential diagnosis of multicystic tumor, lymphangiosis, xanthogranulomatous cholecystitis |
| Diverticula [13,109,110,111,112,113,114] | - Congenital or acquired. - Prevalence 0.001–0.2%. | - Differentiate true diverticula (all layers involved) and pseudodiverticula (secondary after partial perforation. - Risk of inflammation due to bile stasis and sludge formation. |
| Gallbladder Anomalies of Number and Size | ||
|---|---|---|
| Nature of Changes | Description | Meaning |
| Agenesia [13,115,116,117,118,119,120] | Non-displayable GB. Prevalence of 0.01–0.3% with a male-to-female ratio of 1:3. The incidence during autopsy was reported to be 0.035–0.3%. | Misdiagnosis of a shrunken GB and unnecessary surgery due to adjacent intestinal air that may be mistaken for concrements. |
| Hypoplasia/Micro-gallbladder [121,122] | Incomplete development of the embryonal GB bud. Very small GB. | Associate conditions such as cystic fibrosis, biliary atresia, cholangitis, neonatal hepatitis are reported. Differential diagnoses are postprandial contraction, chronic cholecystitis, choledochal cyst. Symptomatic patients benefit from laparoscopic cholecystectomy. |
| Duplication (Partial or complete) [96,99,101,121,123,124,125] | A duplicated GB may present bilobed, Y-shaped or V-shaped. Bilobed GBs have two completely divided cavities. Prevalence of 0.02–2%. Only 50% of cases with GB duplication are detected pre-operatively on conventional imaging. | Differential diagnoses are angled GB, choledochal cyst, Phrygian cap, GB diverticulum, adenomyomatosis. Diagnosis is easier when gallstones are present. Cholecystitis can affect one or both lumina. |
| Vesica fellea triplex [126] | Triple gallbladder resulting from incomplete regression of rudimentary bile ducts. It is a very rare condition: Between 1958 and 2022, only 21 cases were identified and published. | Increased risk of gallbladder metaplasia, dysplasia, and adenocarcinoma. There is an association between gastric and duodenal metaplasia with the potential for adenocarcinoma development. |
| Gallbladder Location—Congenital Anomalies | ||
|---|---|---|
| Nature of Changes | Description | Meaning |
| Left-sided gallbladder [121,127,128,129] | The GB is located on the left side of the ligamentum teres. There are three anatomic variants:
| Often not detected until surgery. Differentiated surgical techniques. Higher incidence of common bile duct injury at cholecystectomy due to anomalies of the bile duct, portal vein, and other structures. |
| Intrahepatic gallbladder [121,130] | Completely surrounded by liver parenchyma, often with biliary stasis and cholelithiasis. | Acute cholecystitis may represent as hepatic abscess secondary to GB perforation. Preoperative diagnosis is important to avoid biliary injuries. |
| Suprahepatic gallbladder position [131,132,133] | - Positioned on lateral liver margin or subdiaphragmal. - Overlay by lung artifacts possible. | Association with other congenital changes in the right lobe of the liver is possible. |
| Floating gallbladder [134,135,136,137] | The gallbladder is suspended from the mesentery and can move freely. The gallbladder changes position during repositioning. | - Torsion with acute pain symptoms is possible. - Risk for acute cholecystitis. |
| Inside the lesser omentum [138] | Enclosed in the right free margin of the lesser omentum. | Possible complications in laparoscopic cholecystectomy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucius, C.; Braden, B.; Jenssen, C.; Möller, K.; Sienz, M.; Zervides, C.; Essig, M.W.; Dietrich, C.F. Ultrasound of the Gallbladder—An Update on Measurements, Reference Values, Variants and Frequent Pathologies: A Scoping Review. Life 2025, 15, 941. https://doi.org/10.3390/life15060941
Lucius C, Braden B, Jenssen C, Möller K, Sienz M, Zervides C, Essig MW, Dietrich CF. Ultrasound of the Gallbladder—An Update on Measurements, Reference Values, Variants and Frequent Pathologies: A Scoping Review. Life. 2025; 15(6):941. https://doi.org/10.3390/life15060941
Chicago/Turabian StyleLucius, Claudia, Barbara Braden, Christian Jenssen, Kathleen Möller, Michael Sienz, Constantinos Zervides, Manfred Walter Essig, and Christoph Frank Dietrich. 2025. "Ultrasound of the Gallbladder—An Update on Measurements, Reference Values, Variants and Frequent Pathologies: A Scoping Review" Life 15, no. 6: 941. https://doi.org/10.3390/life15060941
APA StyleLucius, C., Braden, B., Jenssen, C., Möller, K., Sienz, M., Zervides, C., Essig, M. W., & Dietrich, C. F. (2025). Ultrasound of the Gallbladder—An Update on Measurements, Reference Values, Variants and Frequent Pathologies: A Scoping Review. Life, 15(6), 941. https://doi.org/10.3390/life15060941







