The Presence of Ejaculatory Bulbs in Vasa Deferentia: A Well-Preserved Trait Among Alpheoid Shrimps (Crustacea, Caridea, Alpheoidea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Species
2.2. Laboratorial Procedures, Macroscopic and Microscopic Analyses and Histology
2.3. Data Analysis
3. Results
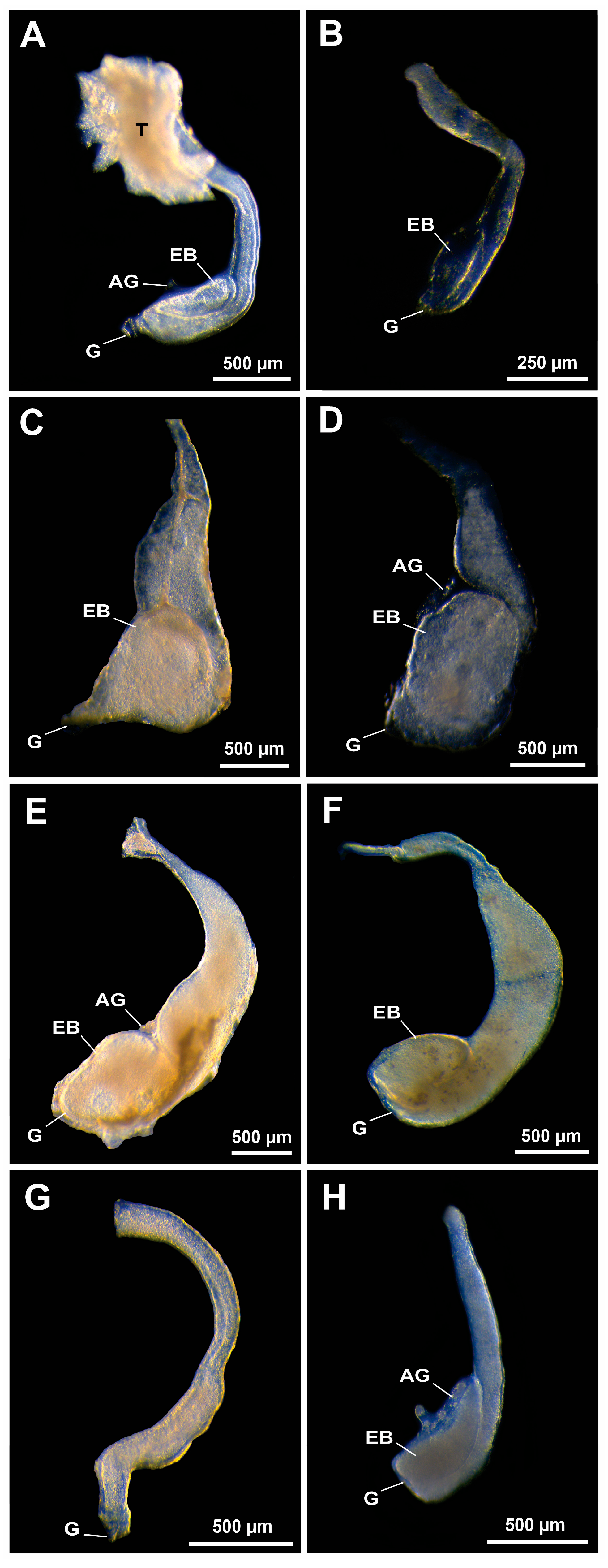
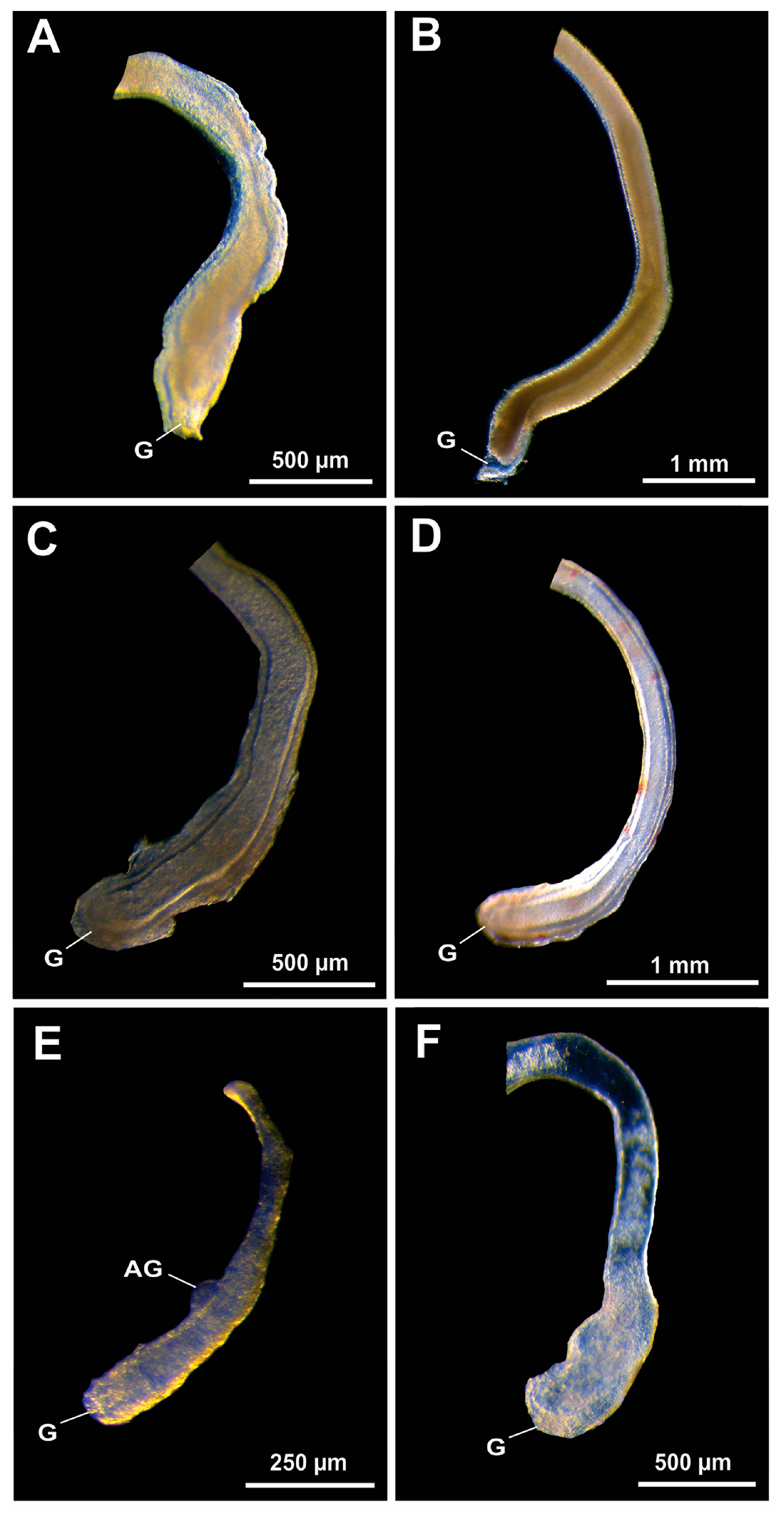
3.1. Presence/Absence of Ejaculatory Bulbs in Gross Morphology
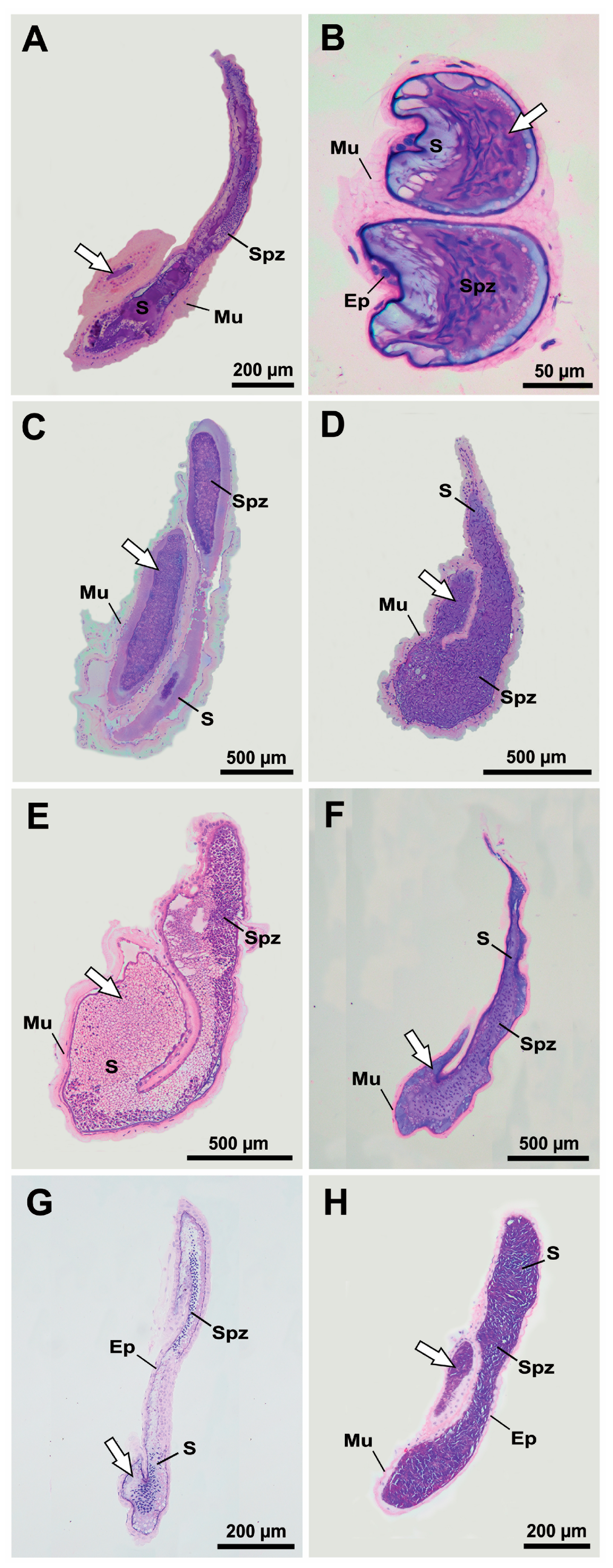
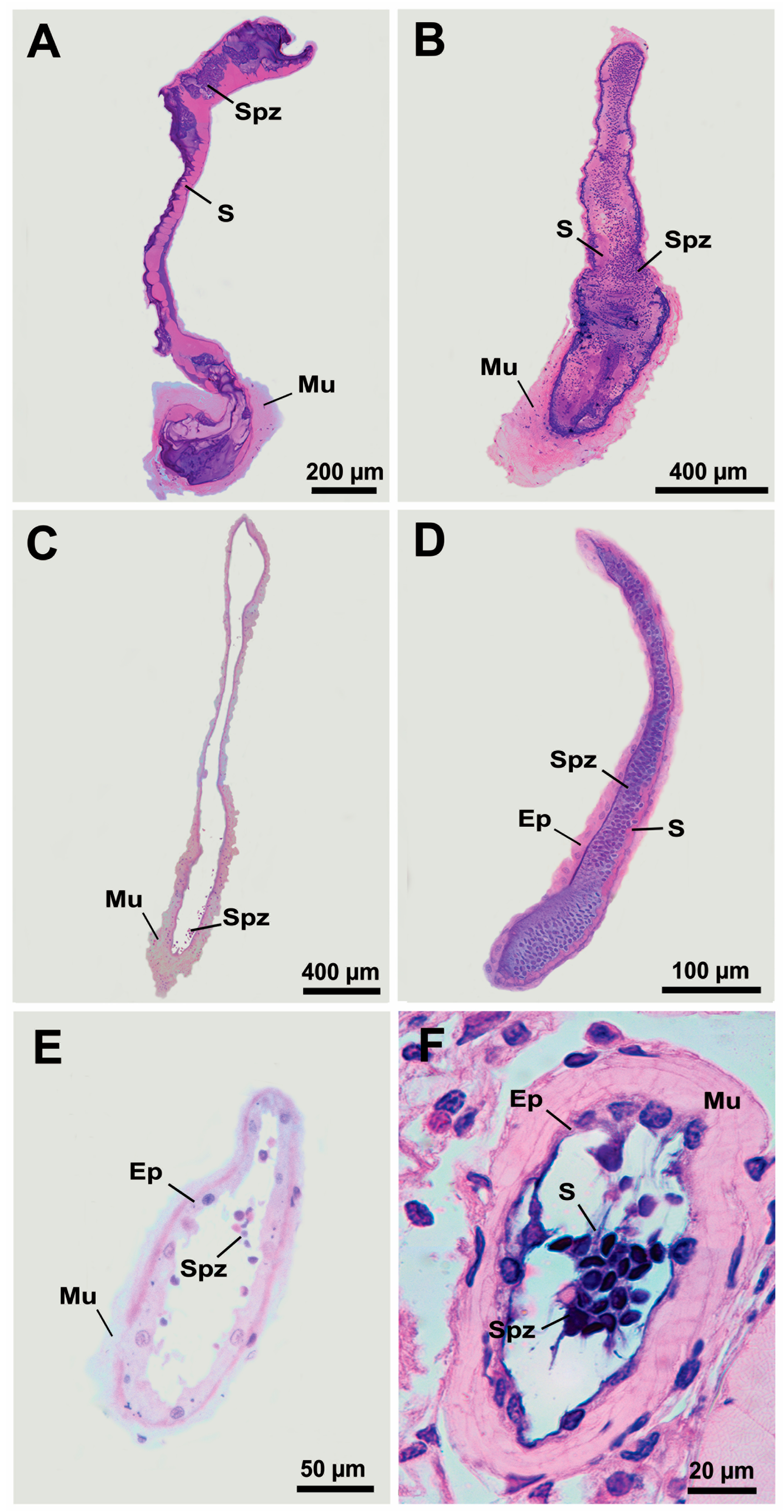
3.2. Histological Confirmation and Structure of Ejaculatory Bulbs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bracken, H.D.; De Grave, S.; Felder, D.L. Phylogeny of the Infraorder Caridea based on mitochondrial and nuclear genes (Crustacea: Decapoda). In Decapod Crustacean Phylogenetics, 1st ed.; Martin, J.W., Crandall, K.A., Felder, D.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 274–300. [Google Scholar]
- De Grave, S.; Fransen, C.H.J.M. Carideorum catalogus: The recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zool. Med. Leiden 2011, 85, 195–588. [Google Scholar]
- Sun, S.; Hui, M.; Wang, M.; Sha, Z. Phylogenetic position of Alvinocarididae (Crustacea: Decapoda: Caridea): New insights into the origin and evolutionary history of the hydrothermal vent alvinocarid shrimps. Deep-Sea Res. I Oceanogr. Res. Pap. 2018, 141, 93–105. [Google Scholar] [CrossRef]
- Bauer, R.T. Shrimps: Their Diversity, Intriguing Adaptations and Varied Lifestyles; Springer Nature: Berlin, Germany, 2023; pp. 1–720. [Google Scholar]
- Terossi, M.; Cardoso, I. How many species of shrimps (Decapoda: Caridea, Dendrobranchiata, Stenopodidea) in Brazil? Diversity, geographic distribution, and history of taxonomic studies based on the Catálogo Taxonômico da Fauna do Brasil (Taxonomic Catalog of the Brazilian Fauna). J. Crust. Biol. 2024, 44, ruae070. [Google Scholar] [CrossRef]
- De Grave, S.; Decock, W.; Dekeyzer, S.; Davie, P.J.; Fransen, C.H.; Boyko, C.B.; Poore, G.C.B.; Macpherson, E.; Ahyong, S.T.; Crandall, K.A.; et al. Benchmarking global biodiversity of decapod crustaceans (Crustacea: Decapoda). J. Crust. Biol. 2023, 43, ruad042. [Google Scholar] [CrossRef]
- WoRMS—World Register of Marine Species. Alpheoidea. Available online: https://www.marinespecies.org/aphia.php?p=browser&id[]=2&id[]=1065&id[]=1066&id[]=845959&id[]=1071&id[]=1086&id[]=1089&id[]=1130&id[]=106670&id[]=106674&id[]=106709#focus (accessed on 16 April 2025).
- De Grave, S.; Li, C.P.; Tsang, L.M.; Chu, K.H.; Chan, T.Y. Unweaving hippolytoid systematics (Crustacea, Decapoda, Hippolytidae): Resurrection of several families. Zool. Scr. 2014, 43, 496–507. [Google Scholar] [CrossRef]
- Poore, G.C.; Ahyong, S.T. Marine Decapod Crustacea: A guide to Families and Genera of the World; CSIRO Publishing: Melbourne, Australia, 2023; pp. 1–916. [Google Scholar]
- Suzuki, H. Taxonomic review of four alpheid shrimps belonging to the genus Athanas with reference to their sexual phenomena. Sci. Rep. Yokohama Natl. Univ. Sect. II 1970, 17, 1–37. [Google Scholar]
- Manjón-Cabeza, M.E.; Cobos, V.; Raso, J.E.G. The reproductive system of Hippolyte niezabitowskii (Decapoda, Caridea). Zoology 2011, 114, 140–149. [Google Scholar] [CrossRef]
- Oliveira, M.V.; Baeza, J.A.; Guéron, R.; Costa-Souza, A.C.; Mariano, R.; Zara, F.J.; Almeida, A.O. Protandric simultaneous hermaphroditism in Salmoneus carvachoi Anker, 2007 (Decapoda: Alpheidae): A new sexual system in alpheid shrimps. Zool. J. Linn. Soc. 2024, 201, zlad137. [Google Scholar] [CrossRef]
- Charniaux-Cotton, H. Hermaphroditism and gynandromorphism in malacostracan Crustacea. In Intersexuality in the Animal Kingdom, 1st ed.; Reinboth, R., Ed.; Springer: New York, NY, USA, 1975; pp. 91–105. [Google Scholar]
- Bauer, R.T. Mating behaviour and spermatophore transfer in the shrimp Heptacarpus pictus (Stimpson) (Decapoda: Caridea: Hippolytidae). J. Nat. Hist. 1976, 10, 415–440. [Google Scholar] [CrossRef]
- Bauer, R.T. Sex change and life history pattern in the shrimp Thor manningi (Decapoda: Caridea): A novel case of partial protandric hermaphroditism. Biol. Bull. 1986, 170, 11–31. [Google Scholar] [CrossRef]
- Onaga, H.; Fiedler, G.C.; Baeza, J.A. Protandric simultaneous hermaphroditism in Parhippolyte misticia (Clark, 1989) (Caridea: Hippolytidae): Implications for the evolution of mixed sexual systems in shrimp. J. Crust. Biol. 2012, 32, 383–394. [Google Scholar] [CrossRef]
- Bauer, R.T.; Holt, G.J. Simultaneous hermaphroditism in the marine shrimp Lysmata wurdemanni (Caridea: Hippolytidae): An undescribed sexual system in the decapod Crustacea. Mar. Biol. 1998, 132, 223–235. [Google Scholar] [CrossRef]
- Bortolini, J.L.; Bauer, R.T. Persistence of reduced androgenic glands after protandric sex change suggests a basis for simultaneous hermaphroditism in a caridean shrimp. Biol. Bull. 2016, 230, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Moraes, I.R.; Antunes, M.; López-Greco, L.S.; Zara, F.J.; Castilho, A.L. Functional reproductive morphology of the snapping shrimp genus Synalpheus Spence Bate, 1888 (Decapoda, Alpheidae). Curr. Zool. 2024, zoae053. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Lin, T. Histological characterization of peppermint shrimp (Lysmata wurdemanni) androgenic gland. J. Ocean Univ. China 2017, 16, 1133–1138. [Google Scholar] [CrossRef]
- Soledade, G.O.; Almeida, A.O. Snapping shrimps of the genus Alpheus Fabricius, 1798 from Brazil (Caridea: Alpheidae): Updated checklist and key for identification. Nauplius 2013, 21, 89–122. [Google Scholar] [CrossRef]
- Pachelle, P.P.; Carvalho, L.; Alves, D.F.; Anker, A. A revision of the Brazilian species of Lysmata Risso, 1816 (Decapoda: Caridea: Lysmatidae), with discussion of the morphological characters used in their identification. Zootaxa 2020, 4789, zootaxa-4789. [Google Scholar] [CrossRef]
- Williams, A.B. Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida; Smithsonian Institution Press: Washington, DC, USA, 1984; pp. 1–550. [Google Scholar]
- Paschoal, L.R.P.; Zara, F.J. Is there a trade-off between sperm production and sexual weaponry in Macrobrachium amazonicum (Heller, 1862)? Zoology 2022, 153, 126029. [Google Scholar] [CrossRef]
- Nogueira, C.S.; Antunes, M.; Zara, F.J.; Costa, R.C. Male reproductive system of the freshwater prawn Macrobrachium brasiliense (Decapoda: Palaemonidae): Notes on spermatophore formation and sperm count. Tissue Cell 2023, 81, 102008. [Google Scholar] [CrossRef]
- Baeza, J.A. Protandric simultaneous hermaphroditism in the shrimps Lysmata bahia and Lysmata intermedia. Invert. Biol. 2008, 127, 181–188. [Google Scholar] [CrossRef]
- Baeza, J.A. Observations on the sexual system and the natural history of the semi-terrestrial shrimp Merguia rhizophorae (Rathbun, 1900). Invert. Biol. 2010, 129, 266–276. [Google Scholar] [CrossRef]
- Braga, A.A.; López Greco, L.S.; Santos, D.C.; Fransozo, A. Morphological evidence for protandric simultaneous hermaphroditism in the caridean Exhippolysmata oplophoroides. J. Crust. Biol. 2009, 29, 34–41. [Google Scholar] [CrossRef]
- Chow, S.; Ogasawara, Y.; Taki, Y. Male reproductive system and fertilization of the palaemonid shrimp Macrobrachium rosenbergii. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 177–183. [Google Scholar] [CrossRef]
- Paschoal, L.R.P.; Zara, F.J. The androgenic gland in male morphotypes of the Amazon River prawn Macrobrachium amazonicum (Heller, 1862). Gen. Comp. Endocrinol. 2019, 275, 6–14. [Google Scholar] [CrossRef]
- Nogueira, C.S.; Zara, F.J.; Costa, R.C. Male reproductive systems of Macrobrachium pantanalense Dos Santos, Hayd & Anger, 2013 and M. amazonicum (Heller, 1862) (Decapoda: Caridea: Palaemonidae): New insights into the morphology of the spermatozoa of decapod crustaceans. J. Crust. Biol. 2025, 45, ruaf003. [Google Scholar]
- Tomas, A.L.; Garcia-Bento, M.A.; Mutti, L.D.; Zara, F.J.; L’opez-Greco, L.S. New insights in the male anatomy, spermatophore formation, and sperm structure in Atyidae: The red cherry shrimp Neocaridina davidi. Invertebr. Biol. 2019, 138, 17–28. [Google Scholar] [CrossRef]
- Machado, M.; Salti, F.C.; Bertini, G.; Zara, F.J.; Negreiros-Fransozo, M.L. Is Potimirim potimirim (Crustacea, Decapoda, Atyidae) a protandric hermaphrodite species? Behavioral and morphological aspects of the reproductive system. Arthropod Struct. Dev. 2021, 63, 101060. [Google Scholar] [CrossRef]
- Paschoal, L.R.P.; Nogueira, C.S.; Zara, F.J. Exploring New Territories: New Records and Occurrence Confirmation of Two Caridean Shrimps in Brazil. Arthropoda 2025, 3, 5. [Google Scholar] [CrossRef]
- Hoffman, D.L. The development of the androgenic glands of a protandric shrimp. Biol. Bull. 1969, 137, 286–296. [Google Scholar] [CrossRef]
- Bergstrom, B.I. The biology of Pandalus. Adv. Mar. Biol. 2000, 38, 55–245. [Google Scholar]
- Okumura, T.; Nikaido, H.; Yoshida, K.; Kotaniguchi, M.; Tsuno, Y.; Seto, Y.; Watanabe, T. Changes in gonadal development, androgenic gland cell structure, and hemolymph vitellogenin levels during male phase and sex change in laboratory-maintained protandric shrimp, Pandalus hypsinotus (Crustacea: Caridea: Pandalidae). Mar. Biol. 2005, 148, 347–361. [Google Scholar] [CrossRef]
- WoRMS—World Register of Marine Species. Pandaloidea. Available online: https://www.marinespecies.org/aphia.php?p=browser&id[]=2&id[]=1065&id[]=1066&id[]=845959&id[]=1071&id[]=1086&id[]=1089&id[]=1130&id[]=106670&id[]=106674&id[]=106716#focus (accessed on 16 April 2025).


| Superfamily | Family | Species | Sampling Methods | Environments | Municipality (State) | Macro | Micro | Presence of EB |
|---|---|---|---|---|---|---|---|---|
| Alpheoidea Rafinesque, 1815 | Alpheidae Rafinesque, 1815 | Alpheus armillatus H. Milne Edwards, 1837 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Absent |
| Alpheus brasileiro Anker, 2012 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | No | Absent | ||
| Alpheus buckupi Almeida, Terossi, Araújo-Silva and Mantelatto, 2013 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Absent | ||
| Alpheus carlae Anker, 2012 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Absent | ||
| Alpheus estuariensis Christoffersen, 1984 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | No | Absent | ||
| Alpheus formosus Gibbes, 1850 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Absent | ||
| Alpheus intrinsecus Spence Bate, 1888 | trawling (daytime) | marine (in shore) | Ubatuba (São Paulo) | Yes | No | Absent | ||
| Alpheus nuttingi (Schmitt, 1924) | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | Yes | Absent | ||
| Alpheus cf. packardii Kingsley, 1880 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | Yes | Absent | ||
| Alpheus thomasi Hendrix and Gore, 1973 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Absent | ||
| Athanas dimorphus Ortmann, 1894 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | No | Absent | ||
| Athanas nitescens (Leach, 1814) | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | Yes | Absent | ||
| Potamalpheops tyrymembe Soledade, Santos and Almeida, 2014 | suction pump | mangrove | Maraú (Bahia) | Yes | Yes | Absent | ||
| Salmoneus ortmanni (Rankin, 1898) | manual (hand net) | marine (in shore) | São Sebastião (São Paulo) | Yes | No | Absent | ||
| Synalpheus apioceros Coutière, 1909 | scuba diving | marine (in shore) | Ubatuba (São Paulo) | No | No | Absent | ||
| Synalpheus brevicarpus (Herrick, 1891) | scuba diving | marine (in shore) | Ubatuba (São Paulo) | No | Yes | Absent | ||
| Synalpheus fritzmuelleri Coutière, 1909 | scuba diving | marine (in shore) | Ubatuba (São Paulo) | Yes | No | Absent | ||
| Synalpheus minus (Say, 1818) | scuba diving | marine (in shore) | Ubatuba (São Paulo) | No | Yes | Absent | ||
| Synalpheus ubatuba Mantelatto, França, Cunha and Almeida, 2023 | scuba diving | marine (in shore) | Ubatuba (São Paulo) | No | No | Absent | ||
| Hippolytidae Spence Bate, 1888 | Hippolyte obliquimanus Dana, 1852 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | Yes | Present | |
| Latreutes baueri Terossi, Almeida and Mantelatto, 2019 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | Yes | Present | ||
| Lysmatidae Dana, 1852 | Exhippolysmata oplophoroides (Holthuis, 1948) | trawling (daytime) | marine (in shore) | Ubatuba (São Paulo) | No | No | Present | |
| Lysmata ankeri Rhyne and Lin, 2006 | scuba diving | marine (in shore) | Ubatuba (São Paulo) | No | No | Present | ||
| Lysmata bahia Rhyne and Lin, 2006 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Present | ||
| Lysmata dispar Hayashi, 2007 | artificial refuge | rocky shore | Recife (Pernambuco) | No | Yes | Present | ||
| Lysmata grabhami (Gordon, 1935) | Aquarism | - | - | No | Yes | Present | ||
| Lysmata intermedia (Kingsley, 1878) | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Present | ||
| Lysmata jundalini Rhyne, Calado and dos Santos, 2012 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | No | Present | ||
| Lysmata rauli Laubenheimer and Rhyne, 2010 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | No | Present | ||
| Lysmata uncicornis Holthuis and Maurin, 1952 | manual (hand net) | rocky shore | Ubatuba (São Paulo) | Yes | No | Present | ||
| Lysmata wurdemanni (Gibbes, 1850) | manual (hand net) | rocky shore | Ubatuba (São Paulo) | No | No | Present | ||
| Merguiidae Christoffersen, 1990 | Merguia rhizophorae (Rathbun, 1900) | manual (hand net) | mangrove | Recife (Pernambuco) | Yes | Yes | Present | |
| Ogyrididae Holthuis, 1955 | Ogyrides alphaerostris (Kingsley, 1880) | trawling (nighttime) | marine (in shore) | Ubatuba (São Paulo) | No | Yes | Present | |
| Ogyrides hayi Williams, 1981 | suction pump | sand beach | São Sebastião (São Paulo) | Yes | Yes | Present | ||
| Thoridae Kingsley, 1878 | Thor manningi Chace, 1972 | hand net | rocky shore | Ubatuba (São Paulo) | Yes | Yes | Present | |
| Atyoidea De Haan, 1849 | Atyidae De Haan, 1849 | Atya scabra (Leach, 1816) | manual (sieve) | river | Ubatuba (São Paulo) | No | Yes | Absent |
| Caridina pareparensis De Man, 1892 | aquarism | - | - | Yes | Yes | Absent | ||
| Nematocarcinoidea Smith, 1884 | Rhynchocinetidae Ortmann, 1890 | Cinetorhynchus erythrostictus Okuno, 1997 | scuba diving/ manual (hand net) | rocky shore | Maraú (Bahia) | No | Yes | Absent |
| Cinetorhynchus rigens (Gordon, 1936) | scuba diving/ manual (hand net) | rocky shore | Maraú (Bahia) | Yes | No | Absent | ||
| Palaemonoidea Rafinesque, 1815 | Euryrhynchidae Holthuis, 1950 | Euryrhynchus amazoniensis Tiefenbacher, 1978 | manual (sieve) | river | Manaus (Amazonas) | Yes | Yes | Absent |
| Palaemonidae Rafinesque, 1815 | Macrobrachium olfersii (Wiegmann, 1836) | manual (sieve) | river | Ubatuba (São Paulo) | No | Yes | Absent | |
| Pseudopalaemon amazonensis Ramos-Porto, 1979 | manual (sieve) | river | Manaus (Amazonas) | Yes | No | Absent | ||
| Typton distinctus Chace, 1972 | scuba diving | marine (in shore) | Ubatuba (São Paulo) | Yes | Yes | Absent | ||
| Processoidea Ortmann, 1896 | Processidae Ortmann, 1896 | Ambidexter cochensis Rodríguez and Lira, 2022 | hand net | rocky shore | Ubatuba (São Paulo) | Yes | Yes | Absent |
| Processa hemphilli Manning and Chace, 1971 | trawling (nighttime) | Marine (in shore) | Ubatuba (São Paulo) | No | Yes | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschoal, L.R.P.; Nogueira, C.S.; Zara, F.J. The Presence of Ejaculatory Bulbs in Vasa Deferentia: A Well-Preserved Trait Among Alpheoid Shrimps (Crustacea, Caridea, Alpheoidea). Life 2025, 15, 940. https://doi.org/10.3390/life15060940
Paschoal LRP, Nogueira CS, Zara FJ. The Presence of Ejaculatory Bulbs in Vasa Deferentia: A Well-Preserved Trait Among Alpheoid Shrimps (Crustacea, Caridea, Alpheoidea). Life. 2025; 15(6):940. https://doi.org/10.3390/life15060940
Chicago/Turabian StylePaschoal, Lucas Rezende Penido, Caio Santos Nogueira, and Fernando José Zara. 2025. "The Presence of Ejaculatory Bulbs in Vasa Deferentia: A Well-Preserved Trait Among Alpheoid Shrimps (Crustacea, Caridea, Alpheoidea)" Life 15, no. 6: 940. https://doi.org/10.3390/life15060940
APA StylePaschoal, L. R. P., Nogueira, C. S., & Zara, F. J. (2025). The Presence of Ejaculatory Bulbs in Vasa Deferentia: A Well-Preserved Trait Among Alpheoid Shrimps (Crustacea, Caridea, Alpheoidea). Life, 15(6), 940. https://doi.org/10.3390/life15060940






