Healed Perforated Corneal Ulcers in Human
Abstract
1. Introduction
2. Materials and Methods
2.1. Transmission Electron Microscopy
2.2. Immunofluorescence
- Anti-CK14 antibody (1:100; BioLegend, San Diego, CA, USA)
- Anti-CK8/18 antibody (1:1000; Progen, Heidelberg, Germany)
- Anti-vimentin antibody (1:100; BioLegend, San Diego, CA, USA)
- Anti-(α-SMA) antibody (1:1000; Abcam Plc, Cambridge, UK)
- Anti-PCNA antibody (1:100; Abcam Plc, Cambridge, UK)
- Anti-phospho-histone H2AX antibody (1:200; Sigma-Aldrich, Burlington, VT, USA)
- Anti-Iba-1 antibody (1:500; Thermo Fisher Scientific, Waltham, MA, USA)
- Anti-CD45 antibody (1:50; Thermo Fisher Scientific, Waltham, MA, USA).
- Alexa Fluor 488-conjugated anti-mouse IgG antibody
- Alexa Fluor 555-conjugated anti-rabbit IgG antibody
- Alexa Fluor 647-conjugated anti-guinea pig antibody
- Alexa Fluor 647-conjugated anti-chicken antibody (all antibodies from Life Technologies, Carlsbad, CA, USA).
3. Results
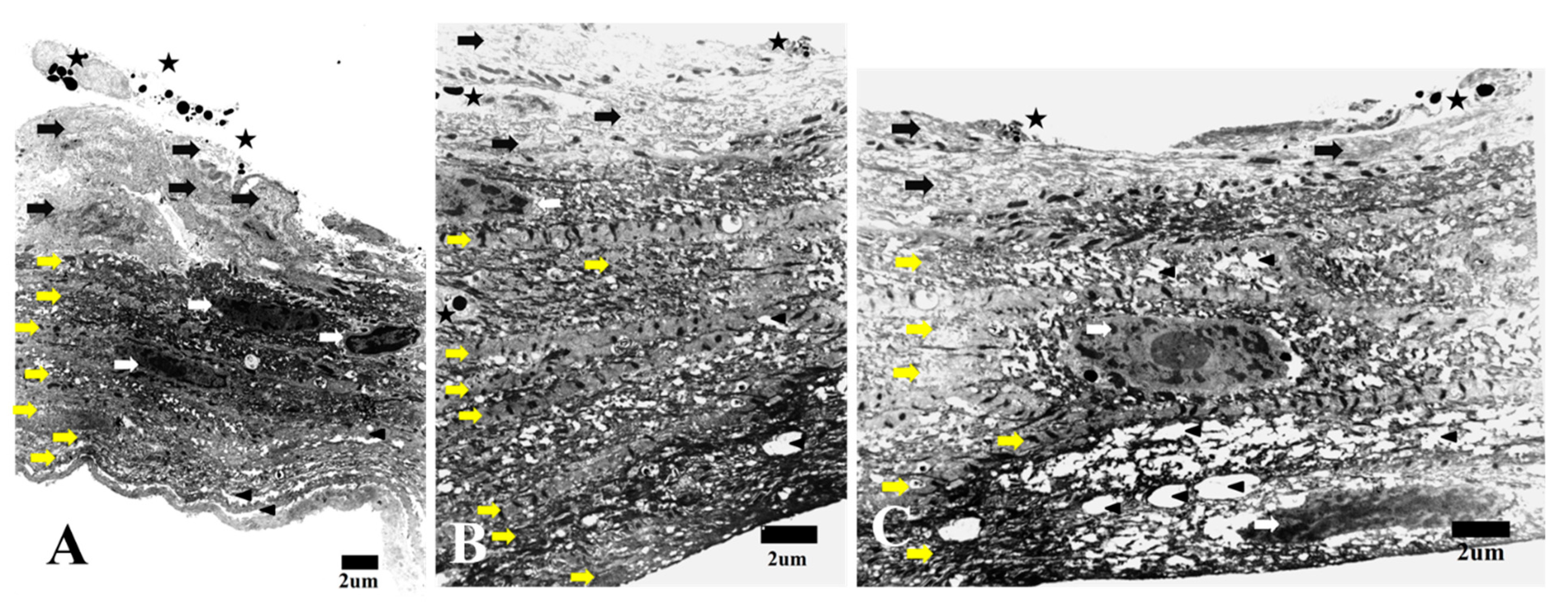
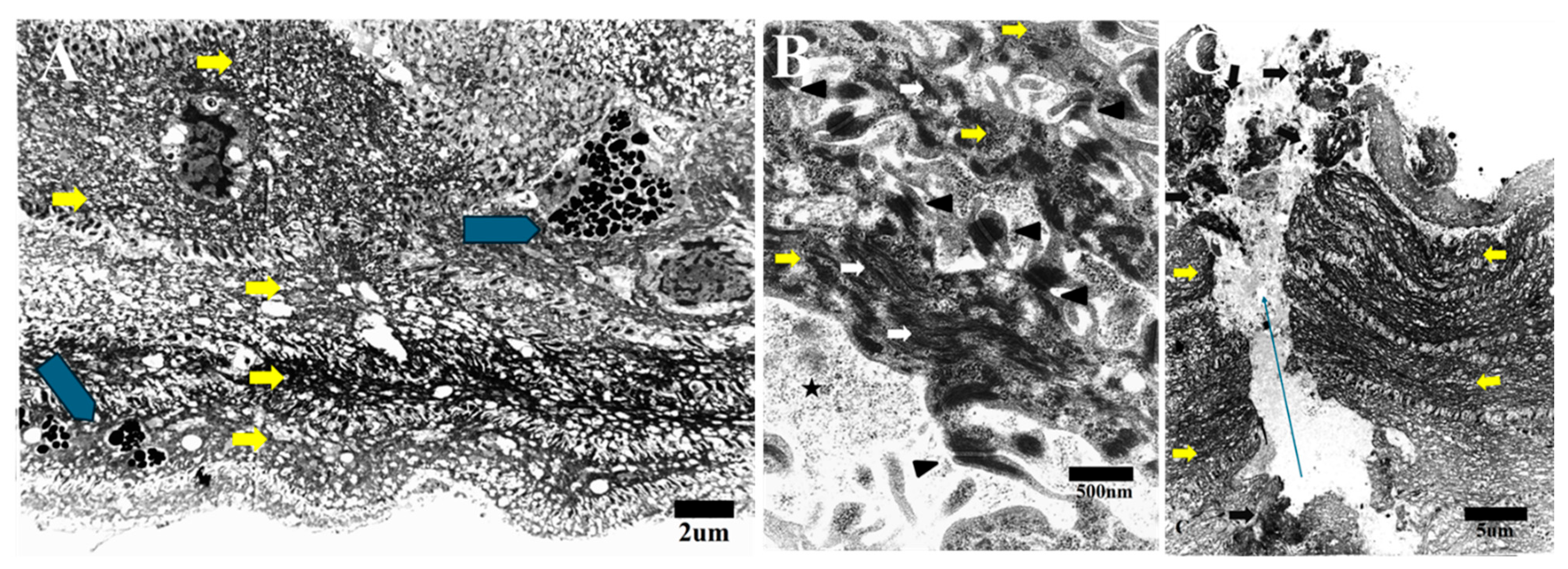
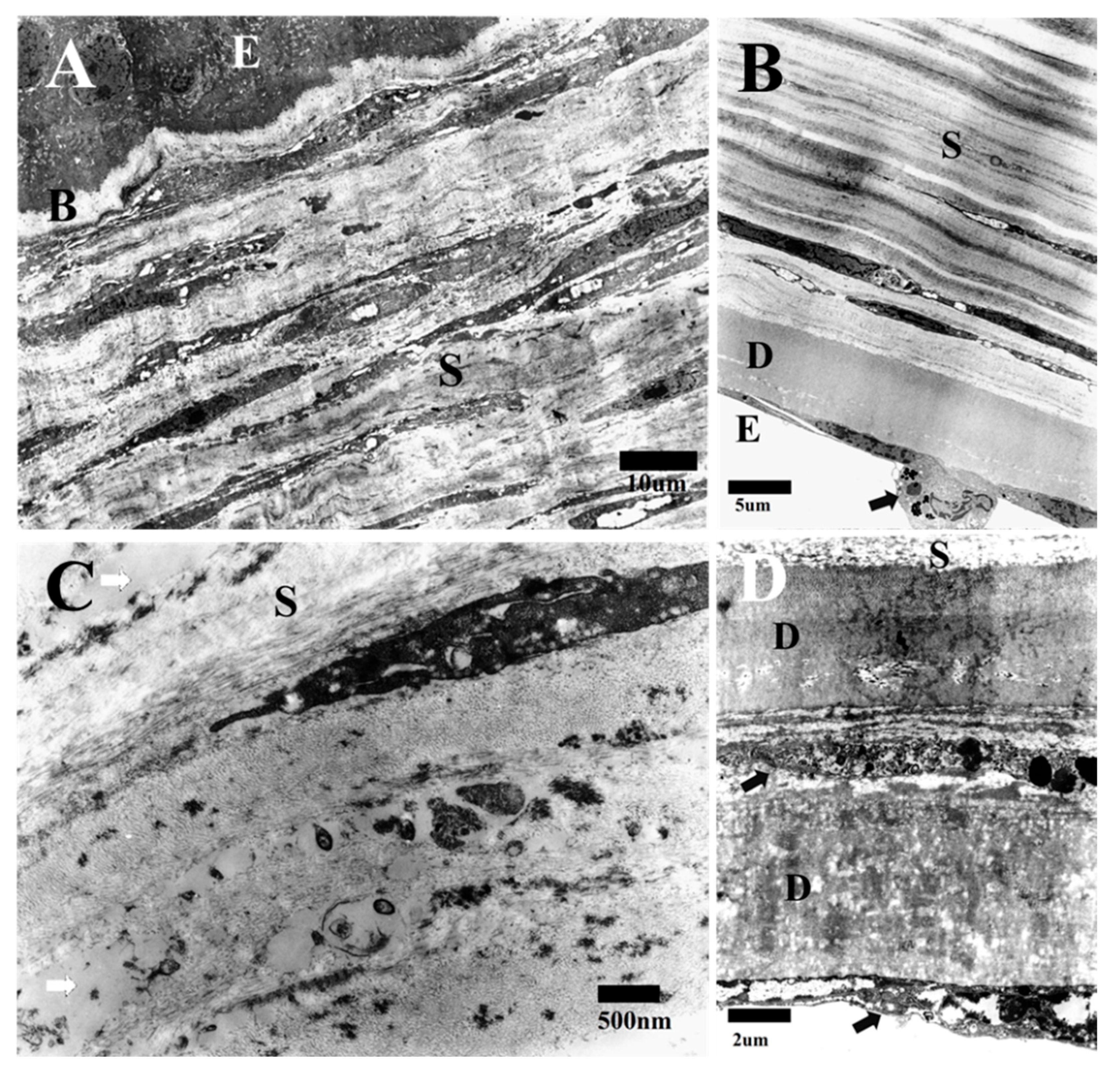
3.1. Transmission Electron Microscopy
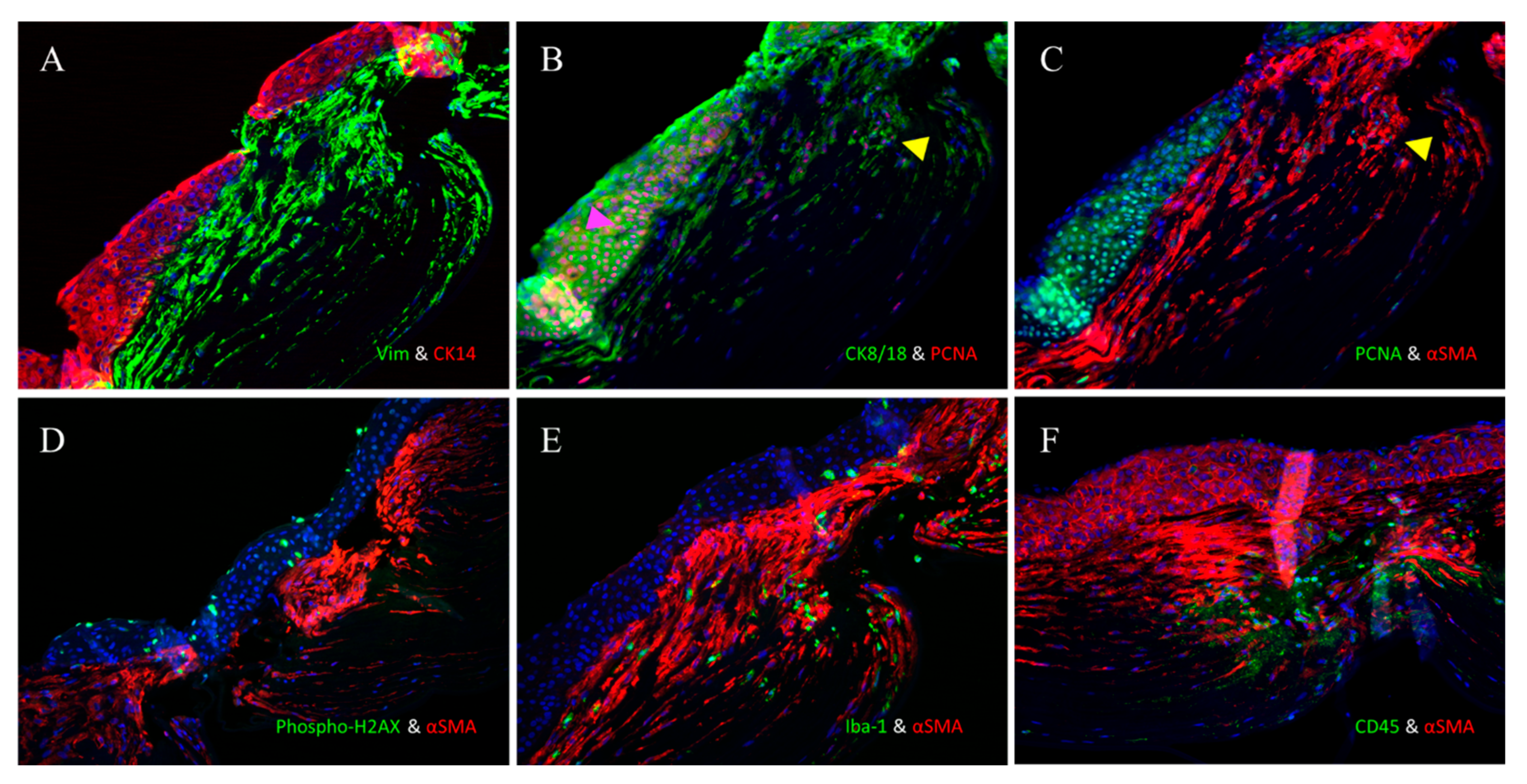
3.2. Immunofluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Krishna, P.V.; Stratis, A.K.; Gopinathan, U. The value of corneal transplantation in reducing blindness. Eye 2005, 19, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.G.; Friedman, D.S.; Lietman, T. Important causes of visual impairment in the world today. JAMA 2003, 290, 2057–2060. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Netto, M.V.; Mohan, R.R.; Ambrosio, R., Jr.; Hutcheon, A.E.; Zieske, J.D.; Wilson, S.E. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therapy. Cornea 2005, 24, 509–522. [Google Scholar] [CrossRef]
- Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Corneal stromal wound healing in refractive surgery: The role of the myofibroblast. Prog. Retin. Eye Res. 1999, 18, 311–356. [Google Scholar] [CrossRef] [PubMed]
- Blalock, T.D.; Duncan, M.R.; Varela, J.C.; Goldstein, N.H.; Tuli, S.S.; Grotengrost, G.R.; Schultz, G.S. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1879–1887. [Google Scholar] [CrossRef]
- Jester, J.V.; Petroll, W.M.; Feng, W.; Essepian, J.; Cavanagh, H.D. Radial keratotomy: I. The wound healing process and measurement of incisional wound gape in two animal models using in vivo, confocal microscopy. Investig. Ophthalmol. Vis. Sci. 1992, 33, 3255–3270. [Google Scholar]
- Jester, J.V.; Huang, J.; Barry-Lane, P.A.; Kao, W.W.; Petroll, W.M.; Cavanagh, H.D. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and f ibronectin assembly. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1959–1967. [Google Scholar]
- Jester, J.V.; Huang, J.; Petroll, W.M.; Cavanagh, H.D. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signalling. Exp. Eye Res. 2002, 75, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guerriero, E.; Sado, Y.; SundarRaj, N. Rho-mediated regulation of TGF-beta1- and FGF-2-induced activation of corneal stromal keratocytes. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3662–3670. [Google Scholar] [CrossRef]
- Etheredge, L.; Kane, B.P.; Hassell, J.R. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Li, C.; Purslow, C.; Yang, Y.; Huang, Z. Evaluation of human corneal ulcer healing process using optical coherence tomography: An in vitro study. In Proceedings of the Optical Elastography and Tissue Biomechanics VI, 108801H, San Francisco, CA, USA, 2–7 February 2019. [Google Scholar] [CrossRef]
- Nowell, C.S.; Radtke, F. Corneal epithelial stem cells and their niche at a glance. J. Cell Sci. 2017, 130, 1021–1025. [Google Scholar] [CrossRef]
- Mohan, R.R.; Kim, W.J.; Wilson, S.E. Modulation of TNF-induced apoptosis in corneal fibroblasts by transcription factor NF-B. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1327–1336. [Google Scholar]
- Ambrósio, R.; Kara-José, N.; Wilson, S.E. Early keratocyte apoptosis after epithelial scrape injury in the humancornea. Exp. Eye Res. 2009, 89, 597–599. [Google Scholar] [CrossRef]
- Zieske, J.D. Extracellular matrix and wound healing. Curr. Opin. Ophthalmol. 2001, 12, 237–241. [Google Scholar] [CrossRef]
- Petroll, W.M.; Kivanany, P.B.; Hagenasr, D.; Graham, E.K. Corneal fibroblast migration patterns during intrastromal wound healing correlate with ECM structure and alignment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7352–7361. [Google Scholar] [CrossRef] [PubMed]
- Miron-Mendoza, M.; Graham, E.; Kivanany, P.; Quiring, J.; Petroll, W.M. The role of thrombin and cell contractility in regulating clustering and collective migration of corneal fibroblasts in different ECM environments. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2079–2090. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Reprint of “Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury”. Exp. Eye Res. 2009, 89, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999, 18, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Petroll, W.M.; Barry, P.A.; Cavanagh, H.D. Expression of alpha-smooth muscle (α-SM) actin during corneal stromal wound healing. Investig. Ophthalmol. Vis. Sci. 1995, 36, 809–819. [Google Scholar] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins’. J. Cell Sci. 1999, 112, 613–622. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Saikia, P.; de Oliveira, R.C.; Lassance, L.; Santhiago, M.R.; Wilson, S.E. Descemet’s Membrane Modulation of Posterior Corneal Fibrosis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1010–1020. [Google Scholar] [CrossRef]
- Barbosa, F.L.; Chaurasia, S.; Cutler, A.; Asosingh, K.; Kaur, H.; Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef]
- Singh, V.; Jaini, R.; Torricelli, A.A.; Santhiago, M.R.; Singh, N.; Ambati, B.K.; Wilson, S.E. TGFb and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp. Eye Res. 2014, 121, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.E.; Stramer, B.M. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea 2005, 24, S2–S11. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Singh, V.; Agrawal, V.; Santhiago, M.R.; Wilson, S.E. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4026–4033. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Singh, V.; Santhiago, M.R.; Wilson, S.E. The corneal epithelial basement membrane: Structure, function, and disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6390–6400. [Google Scholar] [CrossRef] [PubMed]
- Kohnen, G.; Kertschanska, S.; Demir, R.; Kaufmann, P. Placental villous stroma as a model system for myofibroblast differentiation. Histochem. Cell Biol. 1996, 105, 415–429. [Google Scholar] [CrossRef]
- Kivela, T.; Uusitalo, M. Structure, development and function of cytoskeletal elements in non-neuronal cells of the human eye. Prog. Retin. Eye Res. 1998, 17, 385–428. [Google Scholar] [CrossRef] [PubMed]
- Lazarides, E. Desmin and intermediate filaments in muscle cells. Results Probl. Cell Differ. 1980, 11, 124–131. [Google Scholar]
- Andresen, J.L.; Ledet, T.; Ehlers, N. Keratocyte migration and peptide growth factors: The effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-on human keratocyte migration in a collagen gel. Curr. Eye Res. 1997, 16, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Barry-Lane, P.A.; Petroll, W.M.; Olsen, D.R.; Cavanagh, H.D. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF in the rabbit. Cornea 1997, 16, 177–187. [Google Scholar] [CrossRef]
- Gerarduzzi, C.; Di Battista, J.A. Myofibroblast repair mechanisms post-inflammatory response: A fibrotic perspective. Inflamm. Res. 2017, 66, 451–465. [Google Scholar] [CrossRef]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68, 81–93. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, J.G.; Na, M.; Kay, E.P. FGF-2 induced by interleukin-1 beta through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J. Biol. Chem. 2004, 279, 32325–32332. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Sumioka, T.; Saika, S. Endothelial mesenchymal transition: A therapeutic target in retrocorneal membrane. Cornea 2010, 29 (Suppl. S1), S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Petroll, W.M.; Barry-Lane, P.A.; Cavanagh, H.D.; Jester, J.V. ZO-1 reorganization and myofibroblast transformation of corneal endothelial cells after freeze injury in the cat. Exp. Eye Res. 1997, 64, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Lassance, L.; Marino, G.K.; Medeiros, C.S.; Thangavadivel, S.; Wilson, S.E. Fibrocytes migrate to the cornea and differentiate into myofibroblasts during the wound healing response to injury. Exp. Eye Res. 2018, 170, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Cintron, C.; Covington, H.I.; Kublin, C.L. Morphologic analyses of proteoglycans in rabbit corneal scars. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1789–1798. [Google Scholar]
- Jumblatt, M.M.; Willer, S.S. Corneal endothelial repair. Regulation of prostaglandin E2 synthesis. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1294–1301. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, Y.H.; Uematsu, M.; Kusano, M.; Suzuki, K.; Oishi, A. Healed Perforated Corneal Ulcers in Human. Life 2025, 15, 939. https://doi.org/10.3390/life15060939
Mohamed YH, Uematsu M, Kusano M, Suzuki K, Oishi A. Healed Perforated Corneal Ulcers in Human. Life. 2025; 15(6):939. https://doi.org/10.3390/life15060939
Chicago/Turabian StyleMohamed, Yasser Helmy, Masafumi Uematsu, Mao Kusano, Keiji Suzuki, and Akio Oishi. 2025. "Healed Perforated Corneal Ulcers in Human" Life 15, no. 6: 939. https://doi.org/10.3390/life15060939
APA StyleMohamed, Y. H., Uematsu, M., Kusano, M., Suzuki, K., & Oishi, A. (2025). Healed Perforated Corneal Ulcers in Human. Life, 15(6), 939. https://doi.org/10.3390/life15060939






