1. Introduction
Sepsis is one of the most critical matters in current medicine due to the complexity of its pathophysiology and therapeutic approaches. It consists of life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is a subset of sepsis with circulatory and cellular/metabolic dysfunction associated with a higher risk of mortality.
Shock is defined as a condition in which there is an insufficient delivery of oxygen, where an imbalance between oxygen consumption and demand occurs. Septic shock is characterized by hypotension that is persistent after fluid resuscitation with signs of hypoperfusion and organ failure. Septic shock is distributive, and the main pathological mechanism is the formation of shunts [
1,
2,
3].
Owing to missed diagnoses, epidemiologic studies deal with the “treated incidence” of sepsis cases, rather than the actual incidence. It is recorded in 2% of patients admitted to hospitals, half of which are treated in intensive care units, accounting for 10% of all ICU admissions [
4,
5,
6,
7,
8]. Sepsis can also induce septic cardiomyopathy, with de novo acute heart failure, due to myocardial depression, contributing to a sepsis mortality rate of 30%. The prevalence of septic cardiomyopathy among septic patients widely varies (from 20% to 60%), and it is the main cause of the need for inotrope administration in septic shock [
3].
The host response to sepsis consists of both pro-inflammatory and anti-inflammatory immunosuppressive reactions.
The direction, extent, and duration of these reactions are determined by both host factors (genetic factors, age, coexisting illnesses, and medications) and pathogen factors (microbial load and virulence). From a hemodynamic point of view, alterations lead to a significant decrease in vascular tone; a hypovolemic component resulting from relative or central hypovolemia; and fluid losses due to vascular leak, a variable degree of myocardial dysfunction, dysregulation of the regional blood flow distribution, and microvascular alterations. Perfusion abnormalities often persist despite achieving resuscitation targets, leading to the development of organ dysfunction [
6,
7,
8,
9,
10]. The orthogonal polarization spectral imaging technique is employed to study microcirculation in humans to investigate the sublingual microcirculation, which is altered in patients with sepsis [
11].
A vasomotion disequilibrium occurs in sepsis, characterized by non-specific systemic vasodilatation, pulmonary vasoconstriction, and increases in capillary permeability. This paralysis of the peripheral vascular system leads to impairment in the regulation and distribution of peripheral blood flow [
7,
8].
Moreover, sepsis is associated with a loss of endothelium structure, increasing vascular permeability. This dysregulation of endothelial cells is also associated with a procoagulant and pro-inflammatory state, with the secretion of adhesion molecules, contributing to the alterations in perfusion and an impaired sensitivity to vasodilating and vasoconstrictive substances. In addition, the glycocalyx is degraded; the severity of its breakdown is associated with poor clinical outcomes.
An intact and functional microcirculatory system is essential for adequate oxygen delivery in the peripheral tissues [
9]. Therefore, an impaired microcirculation damages the organ perfusion despite the macrocirculation being preserved and the parameters staying within the normal range, as conventional hemodynamic resuscitation may not correct microcirculatory alterations [
8,
12].
Keeping an optimal peripheral perfusion minimizes hypoxia-induced cellular damage. A non-invasive assessment of muscular microcirculation can be performed with the laser Doppler technique.
In this context, inodilators could play a pivotal role in restoring microcirculation function, whereas vasopressor administration alone could detrimentally affect the oxygen consumption/delivery balance. Leading to both cardiac output augmentation and peripheral vasodilation, inodilatory agents can increase tissue oxygenation, which is fundamental for restoring organ function [
13,
14].
Among inodilatory agents, levosimendan leads to the sensitization of troponin C to calcium in cardiac muscle, exerting a positive inotropic effect without increasing myocardial oxygen consumption [
15]. Furthermore, the drug opens adenosine triphosphate-sensitive potassium (K
ATP) channels in vascular smooth muscle cells and induces the vasodilation of the pulmonary, coronary, and peripheral arteries and venous circulation. Levosimendan also opens mitochondrial K channels and exerts an organ-protective effect. At higher doses, it also acts as a phosphodiesterase type 3 (PDE3) inhibitor [
16]. Levosimendan does not stimulate adrenergic receptors, causes little increase in myocardial oxygen consumption, and may, contrarily, have a cardioprotective effect. Furthermore, it improves diastolic function (a key element of septic cardiomyopathy) to a better extent than dobutamine and improves ventriculoarterial coupling. Levosimendan infusion has been demonstrated to improve microcirculation perfusion and restore the muscular lactate/pyruvate ratio, leading to aerobic metabolism. Binding to the K
ATP channels located in mitochondria, the drug can protect mitochondria from oxidative injury, preventing calcium overload and thereby reducing mitochondrial ROS generation [
16]. Thanks to its vasodilatory and pleiotropic effects, levosimendan enhances both convection and diffusion (increased perfused vessel density) in microcirculation, reducing the oxygen diffusion distance and improving cellular oxygen delivery and availability [
17,
18,
19]. Nevertheless, most studies, including the Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis (LeoPARDS) trial, have shown that adding levosimendan to standard management does not result in less severe organ dysfunction or mortality. Moreover, the ICU stay duration is not reduced, although levosimendan is related to a higher reduction in lactate levels and improvement in cardiac function. There is no evidence of the superiority of levosimendan over dobutamine in patients with sepsis and septic shock [
20,
21,
22].
However, studies show heterogeneity in their mortality data, which hinders their interpretation. Moreover, the individual clinical trials are small and employ different treatment protocols. We can, therefore, affirm that levosimendan offers no advantage over dobutamine in septic shock patients in terms of mortality and the length of ICU stays, even though it improves the metabolic state and cardiac function [
22].
Aim of the Study
The aim of this study was to assess the effects of levosimendan administration on peripheral perfusion associated with the infusion of norepinephrine in the prodromic phases of sepsis. We compared them with the variations in microcirculation perfusion occurring with norepinephrine associated with dobutamine. Given levosimendan’s mechanisms of action, we believe it could restore peripheral perfusion and induce a greater vasodilatory effect than dobutamine.
2. Materials and Methods
We enrolled 16 patients admitted to intensive care units for severe sepsis who underwent the early treatment and fluid resuscitation indicated by the international guidelines of the Surviving Sepsis Campaign of 2021. We monitored the adequate volemia with the PiCCO system. The patients were euvolemic and maintained their sinus rhythm, not presenting arrhythmic phenomena. All enrolled patients had been intubated and mechanically ventilated. Informed consent for their inclusion in this study was obtained from all subjects involved. We excluded patients with pulmonary sepsis, with a previous diagnosis of peripheral arteriopathy, and affected by Raynaud’s phenomenon.
The patients were then divided into 2 groups.
Group A comprised 8 patients treated with levosimendan (with a bolus of 12 mcg/kg followed by a continuous infusion of 0.1 mcg/kg/min for 24 h) and a continuous infusion of noradrenaline, with a maximum dosage of 0.4 mcg/kg/min. The patients included in this group had an indication for levosimendan treatment because they showed a reduced cardiac output (
Table 1).
Group B included 8 patients treated with 5 mcg/kg/min of dobutamine and noradrenaline at the same dosage as the previous group (
Table 2).
The two groups were homogeneous concerning diagnosis, age, and sex. As a main indication of levosimendan is a depressed EF, the main difference between the two groups was the cardiological disease. Nevertheless, our primary endpoint was the assessment of microcirculation activity caused by the drugs’ administration to compare the two groups.
We employed either dobutamine or levosimendan, focusing on their dilatory function in microcirculation. Although all patients had systolic dysfunction, it was documented before the onset of the septic shock in the levosimendan group, whereas it was only induced by septic shock in the dobutamine group. Patients did not receive any other vasopressor or inotropic support besides the mentioned drugs.
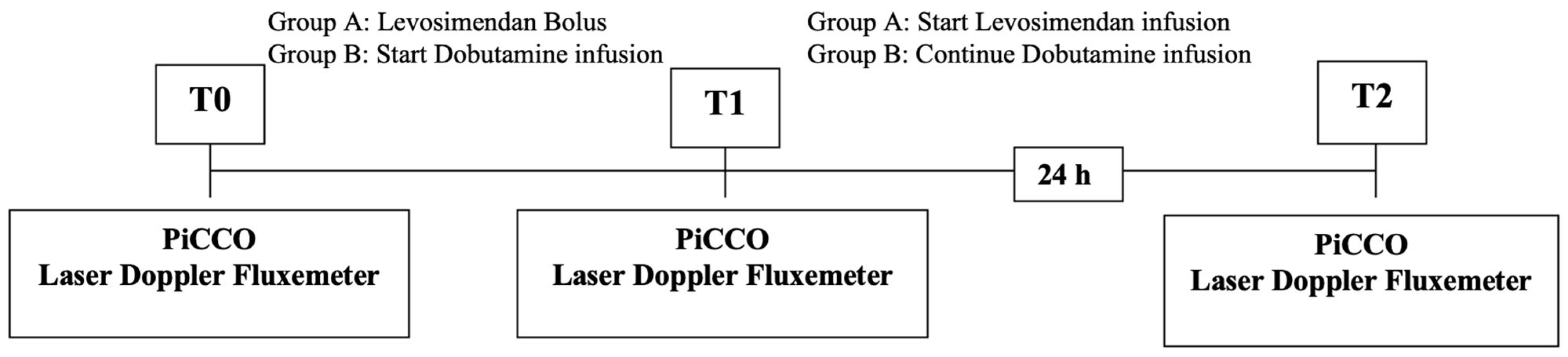
The outcomes of the two treatments were analyzed by detecting the variations in the hemodynamic parameters measured with the PiCCO system and assessing the peripheral blood flow with Periflux, a laser Doppler fluxmeter (
Figure 1).
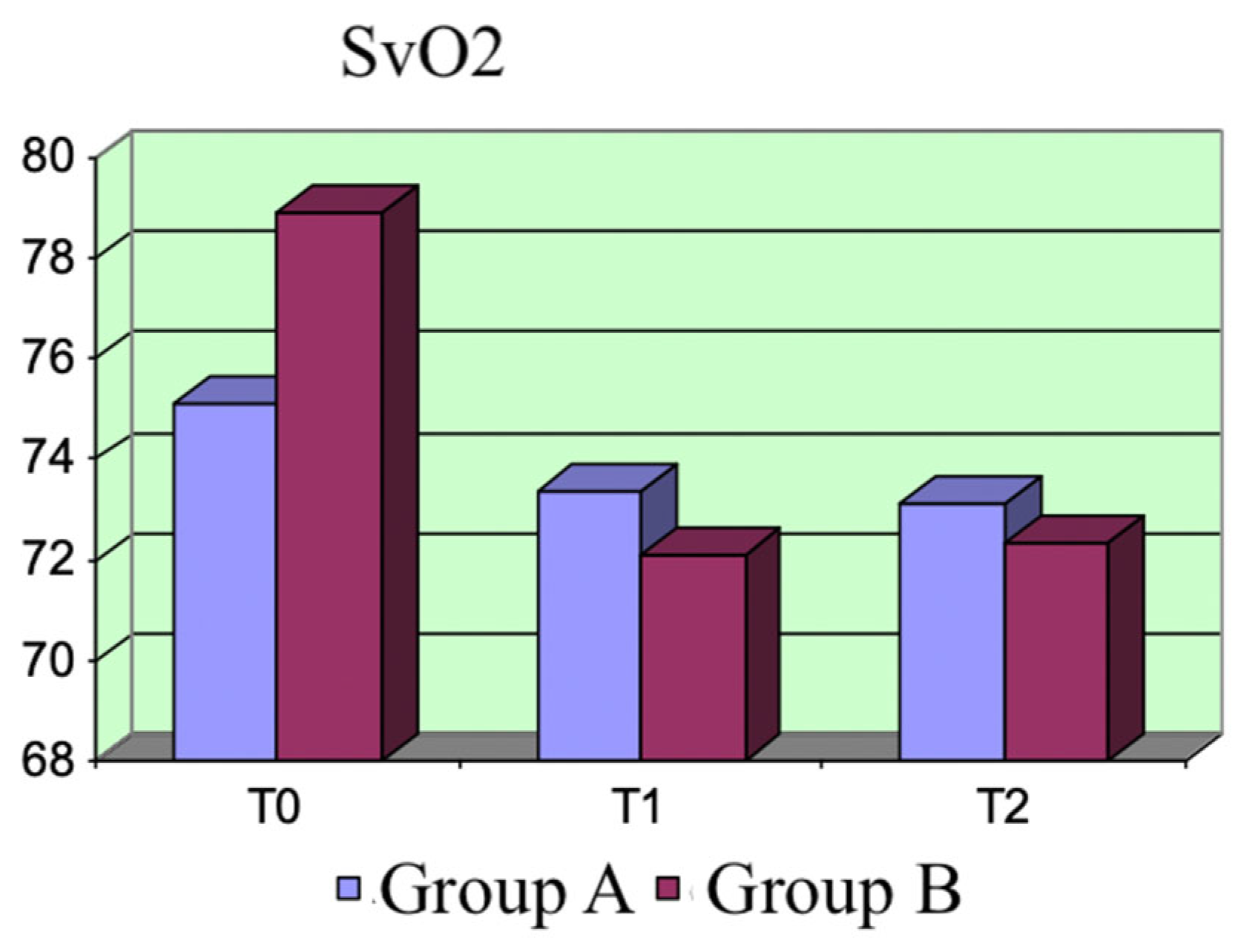
The hemodynamic parameters (PAM, CI, SvO
2, lactate, and RVSI) were measured for each patient at the beginning, T
0 (=basal), at T
1 (=after the administration of the bolus of levosimendan in Group A patients and after starting the dobutamine infusion in Group B patients), and at T
2 (=after 24 h) using the PiCCO monitor (pulse index continuous cardiac output) [
23].
The peripheral blood flow assessment was performed with the laser Doppler fluxmeter PERIFLUX PF 4001 MASTER
®, manufactured by PERIMED, Järfälla, Sweden, a laser diode system producing non-collimated light at 780 nm, emitting a maximal radiation of 0.8 mW, with a range of measurement of Doppler variation from 20 Hz to 24 kHz. All data were collected employing the same kind of probe (PF 408), maintaining the same setting during the time (0.2 s), and keeping the same measurement scale. The environmental temperature was kept between 25 and 26 °C during all the measurement times. The measurements were performed in all patients at times T
0, T
1, and T
2 on the upper limb (proximally on the deltoid muscle and distally on the extensor carpi radialis longus muscle) and the lower limb (proximally on the vastus lateralis and distally on the anterior tibialis muscle). The Periflux system allows the measurement of cutaneous microcirculation by using the Doppler effect. The probe emits a laser light beam toward the skin. The beam is reflected and divided into two components when it hits the body: the first consists of the laser light, which hits the static objects and is reflected with the same frequency as the incident ray; the second component is reflected after the interaction with the moving objects, in this case, red blood cells. Its frequency varies according to the Doppler effect, as analyzed with the probe. The product of multiplication between the velocity (v) and concentration of moving red blood cells (CMBCs) represents the flow, described as the perfusion unit (PU) [
24,
25] (
Figure 2).
4. Discussion
Microvascular dysfunction is an important element of the pathophysiology of septic shock; it may occur even in the presence of a normal systemic oxygen supply and an optimal mean arterial pressure [
20]. In this context, cells are in a state of energetic failure associated with poor outcomes and organ dysfunction. Microcirculatory alterations are characterized by the presence of well-perfused areas close to non-perfused areas, leading to the distributive pattern typical of shock [
8,
26].
Given the reduction in hematic lactate concentrations, our findings suggest that levosimendan plays a pivotal role in restoring aerobic metabolism. Binding to the KATP channels located in mitochondria, the drug can protect mitochondria from oxidative injury, preventing calcium overload, thereby reducing mitochondrial ROS generation.
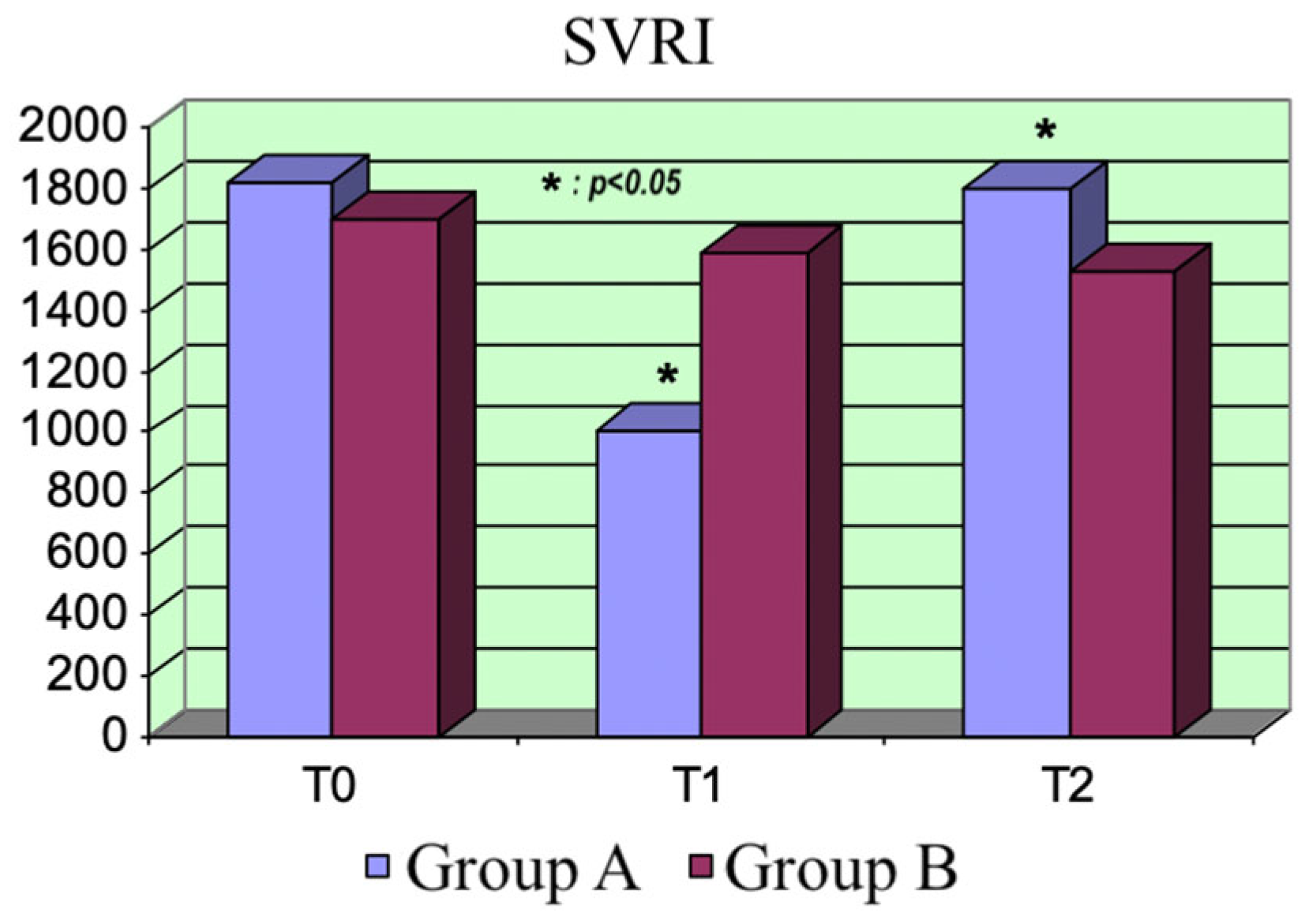
Furthermore, the drug opens adenosine triphosphate-sensitive potassium (KATP) channels in vascular smooth muscle cells, inducing vasodilation. Our data showed this effect, whereby a significant increase in peripheral perfusion occurred. At higher doses, the drug also acts as a phosphodiesterase type 3 (PDE3) inhibitor. This mechanism could be responsible for the drop in peripheral resistances after administering the bolus of levosimendan at T1.
The structural and functional modifications of microcirculation that occur in sepsis are considered a pivotal element in the genesis of multiorgan dysfunction. The development of a targeted therapy is needed, given the lack of treatment indications for impaired microcirculation in sepsis.
Therefore, it would be worthwhile to monitor and optimize microcirculation. In this framework, the dose, timing, and duration of therapy with inodilators should be explored and defined, as limiting microcirculation damage may reduce mortality, which remains high.
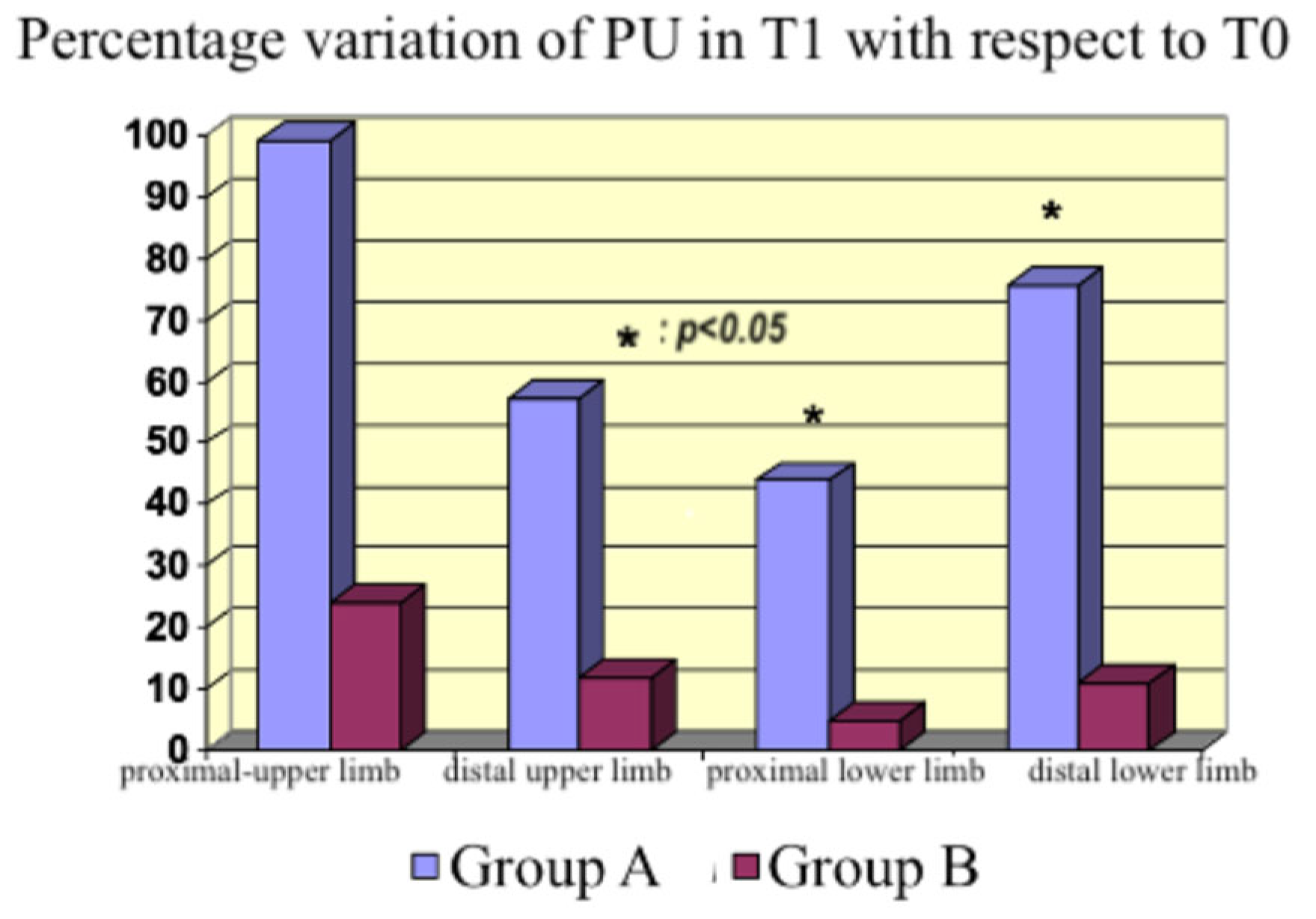
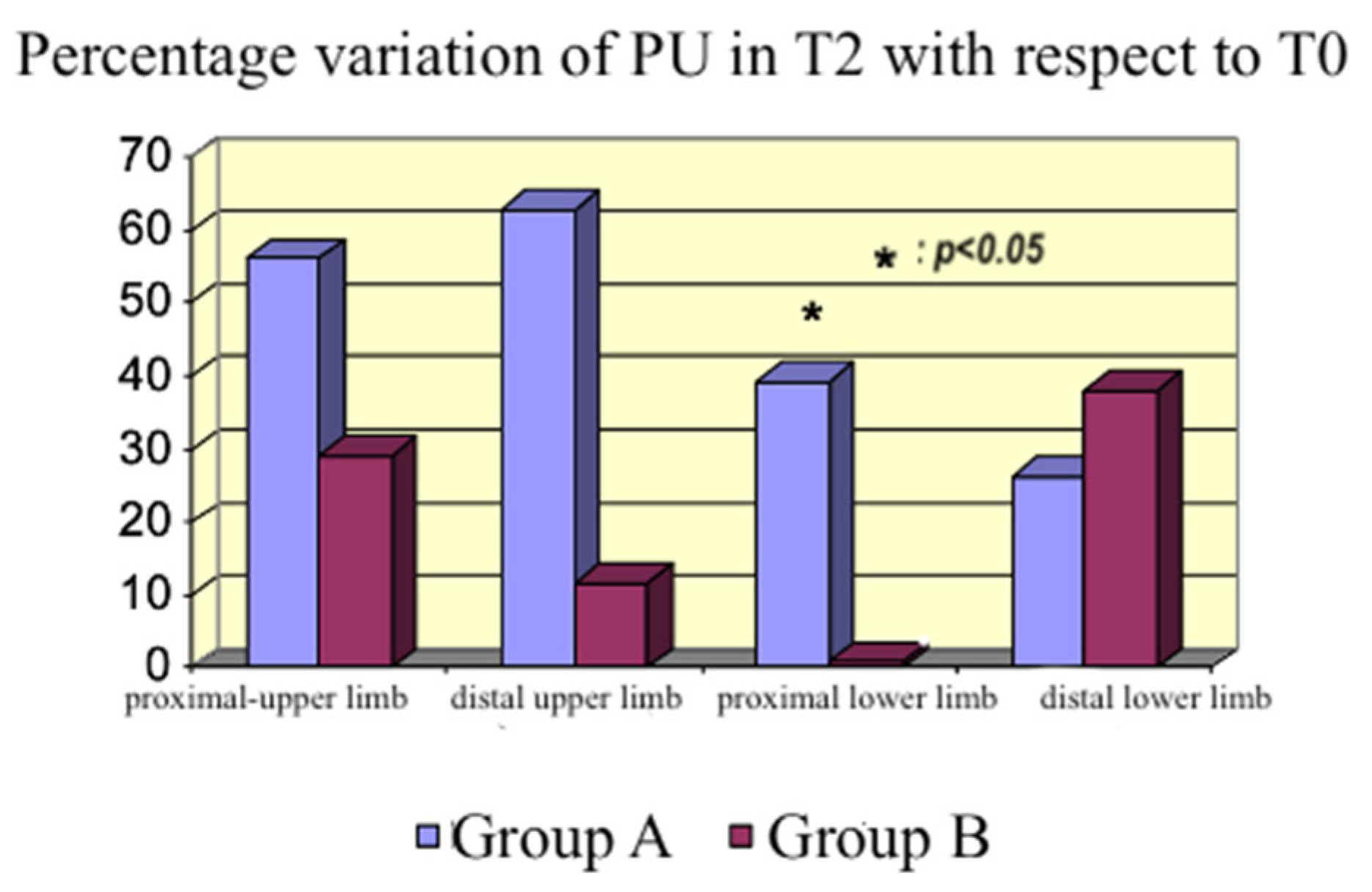
In the context of impaired microcirculation and inadequate tissue oxygenation, inodilators improve peripheral perfusion [
27,
28]. An increase occurred with both drugs in our study, which was higher with levosimendan although not statistically significant at every site studied with the probe. Therefore, we cannot affirm that the flow increased in all body areas; however, we detected an increasing tendency in peripheral blood flow. The discrepancy between the measurements in the distal lower limb (
Figure 8), where the dobutamine group had a higher PU% variation than the levosimendan group compared with the other sites, could be explained by the inhomogeneous endothelial damage induced by sepsis, producing a regional variability in peripheral perfusion.
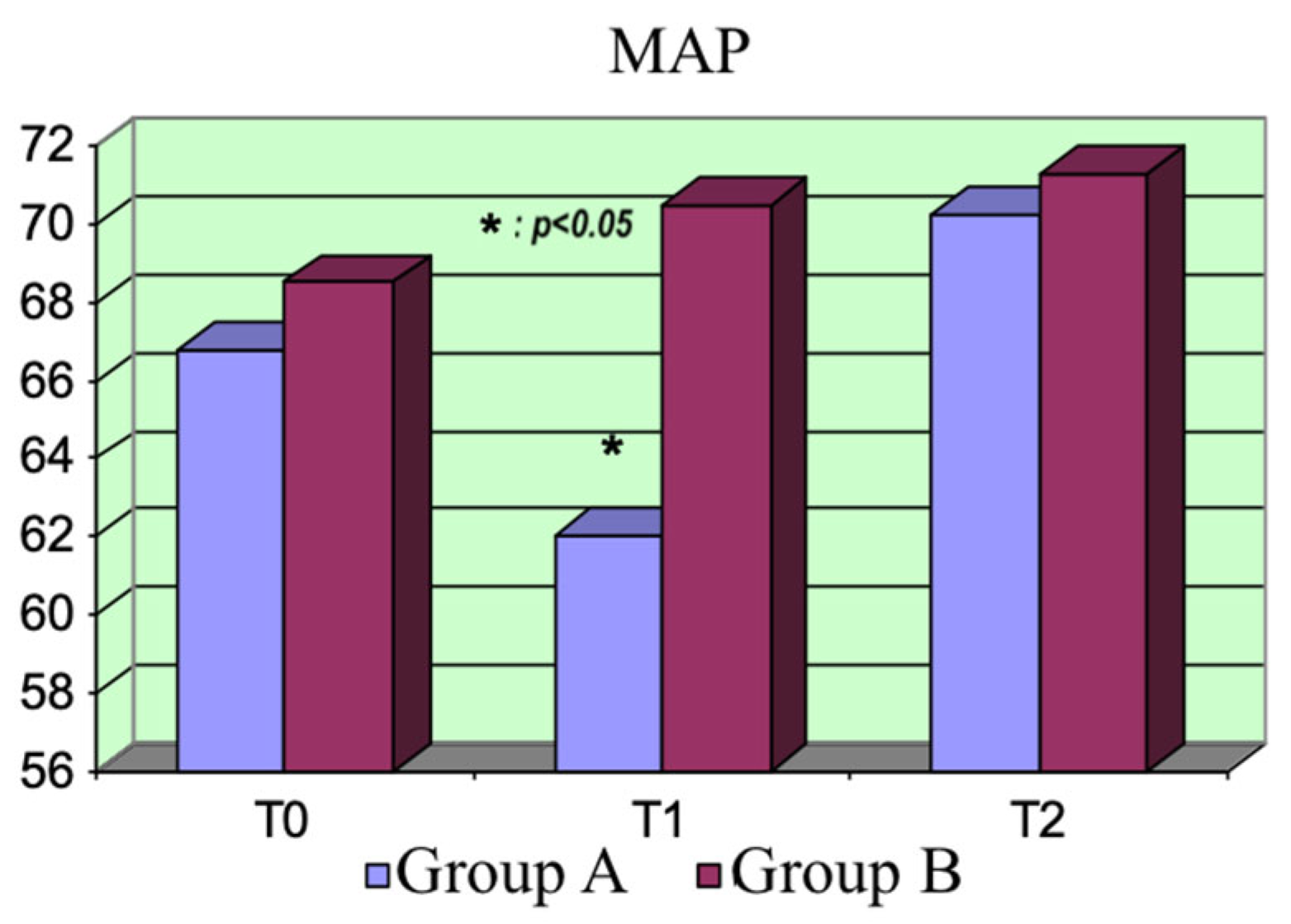
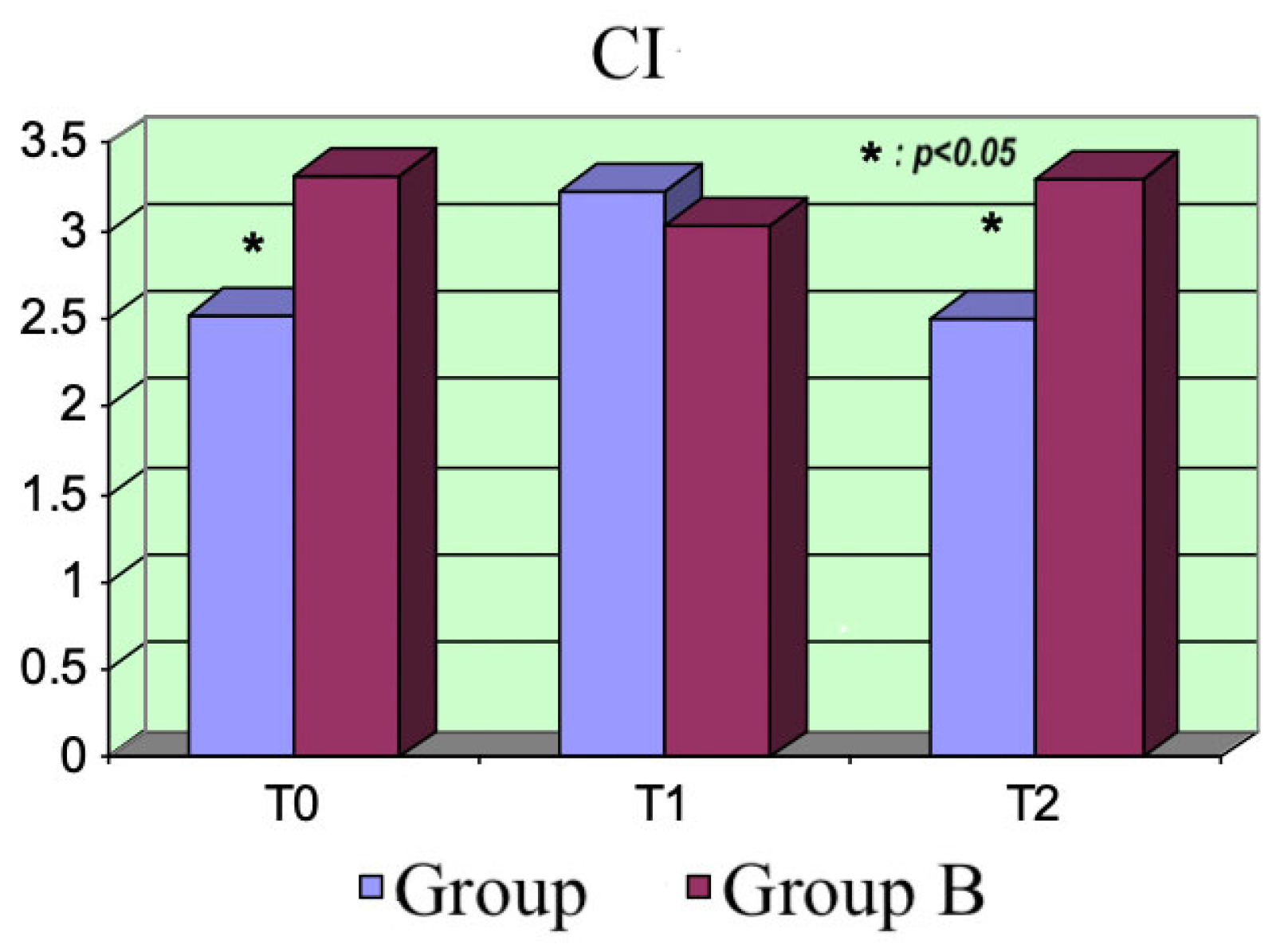
The two drugs did not exhibit significant differences regarding the hemodynamic parameters. As expected, the CI increased and the peripheral resistance dropped at the beginning in Group A, consequently reducing the MAP. Moreover, the increase in peripheral blood flow was associated with reduced peripheral resistance.
The hematic lactate concentrations were significantly reduced in the levosimendan group, probably because of the enhanced aerobic metabolism due to both the action on mitochondrial KATP channels and the better oxygen delivery to cells. Contrarily, lacticemia did not change after administering dobutamine in Group B.
These results, showing a significant reduction in hematic lactate concentrations, are significant because this parameter is related to the outcomes of septic patients.
Given that the development of mitochondrial dysfunction is one of the main causes of MOF, reversing it could represent an effective therapeutic goal in sepsis treatment.
ROS production could inactivate the respiratory chain and ATP production.
Levosimendan, binding to the K
ATP channels in mitochondria, leads to a K
+ flux via the mitochondrial membrane, maintaining cellular homeostasis and protecting against oxidative injury [
16]. It also activates the preconditioning signaling pathways protecting against ischemia–reperfusion injury [
26].
This study had limitations. It was a monocentric study including a limited number of patients. Considering an interval of confidence of 95%, a standard error of 5%, and our mean and standard deviation results, the ideal sample size could be 45 patients. The two groups differed because we included patients with a previously documented reduction in the ejection fraction of the left ventricle in the levosimendan group; conversely, we preserved the previous left ventricle ejection fraction of patients in the dobutamine group. However, this study’s primary endpoint was the assessment of the two drugs on peripheral perfusion using the PU, not their inotropic effect, and we compared their effects concerning only the dilator effect on peripheral perfusion.