Radiation-Induced Cardiotoxicity in Hypertensive Salt-Sensitive Rats: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
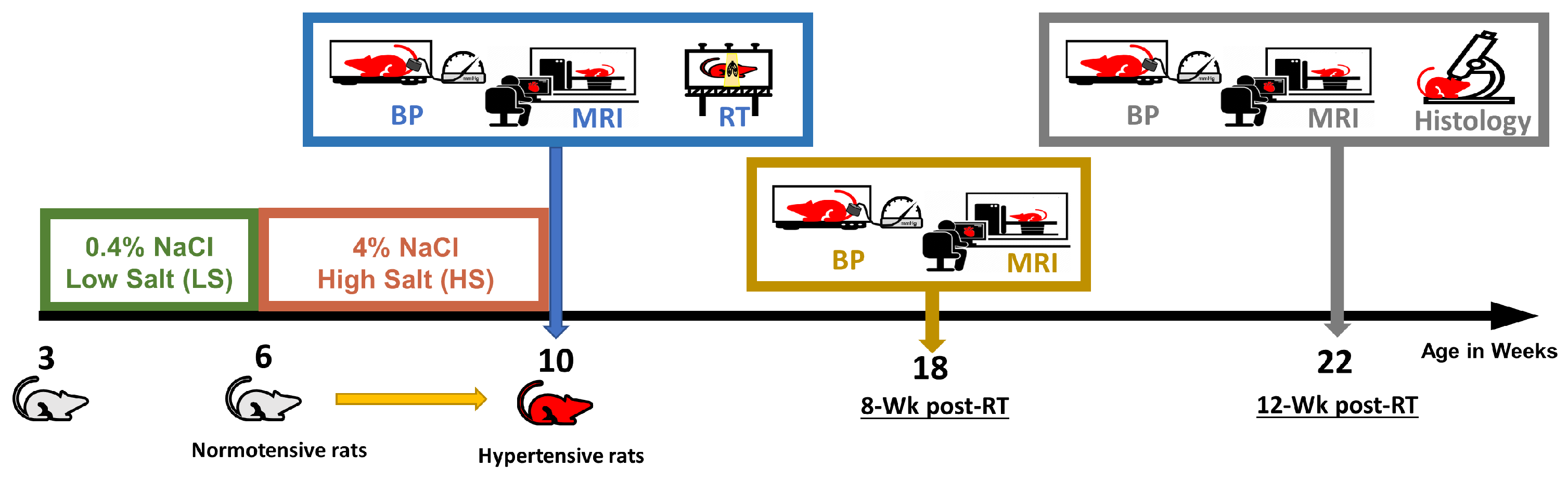
2.1. Animal Model and Irradiation Procedure
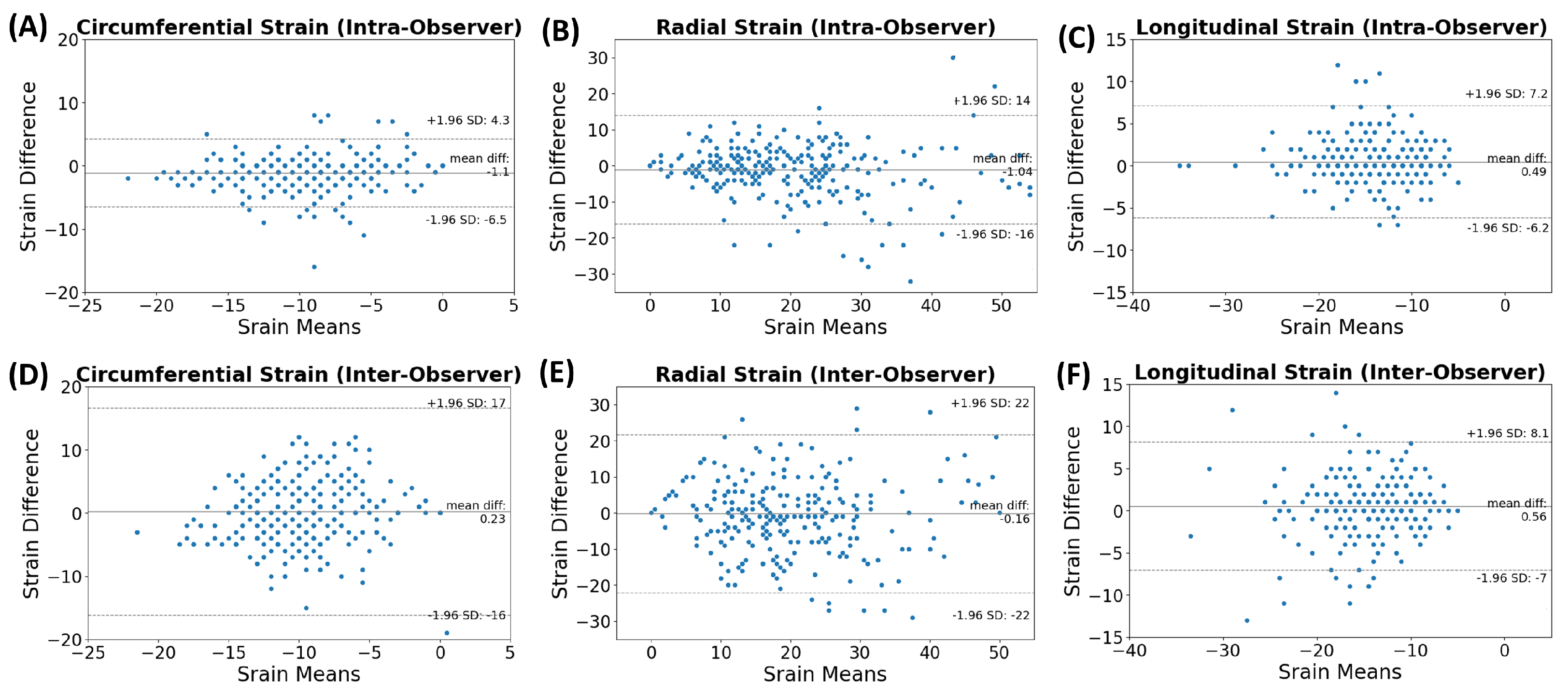
2.2. MRI Scans and Image Analysis
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
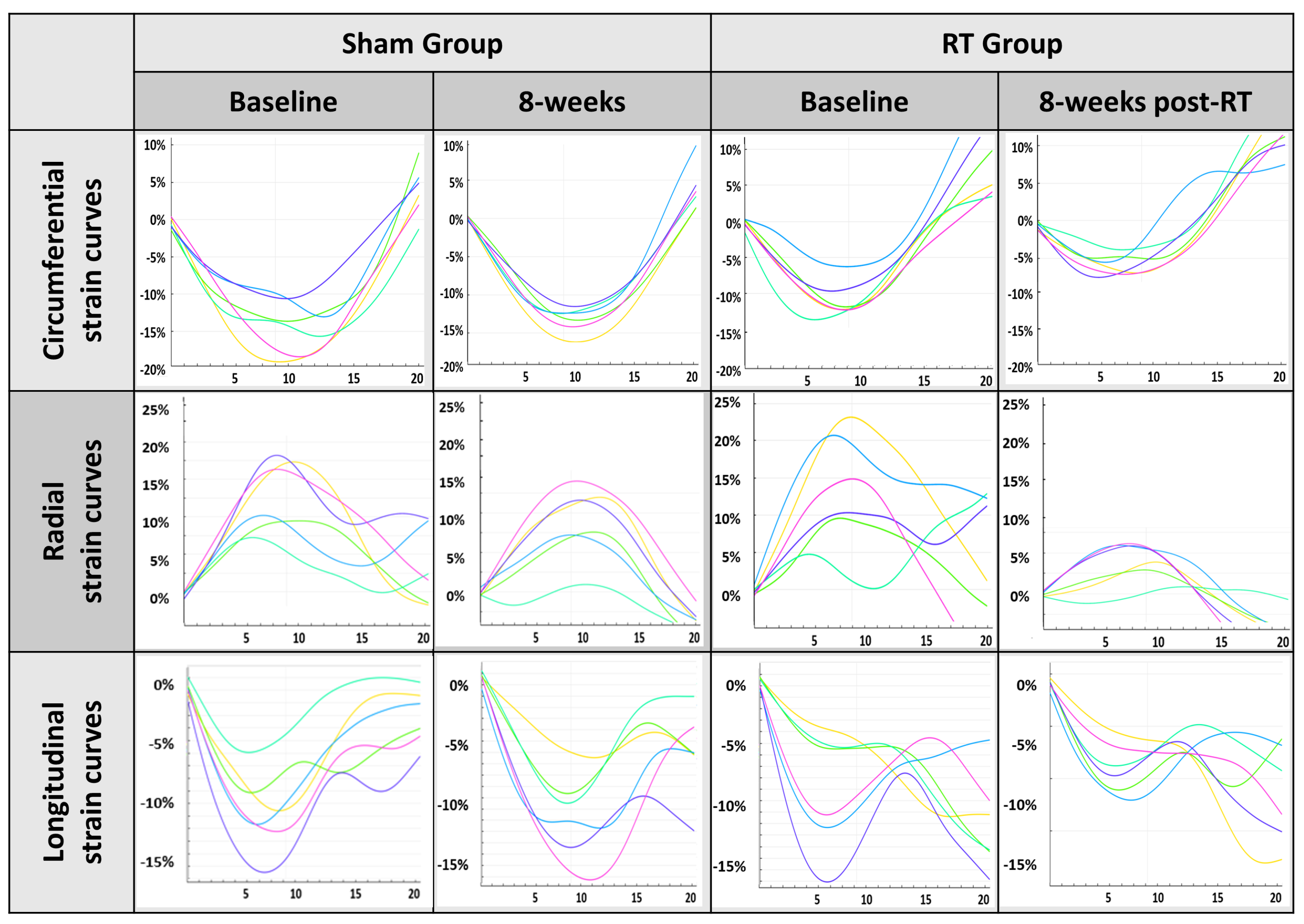
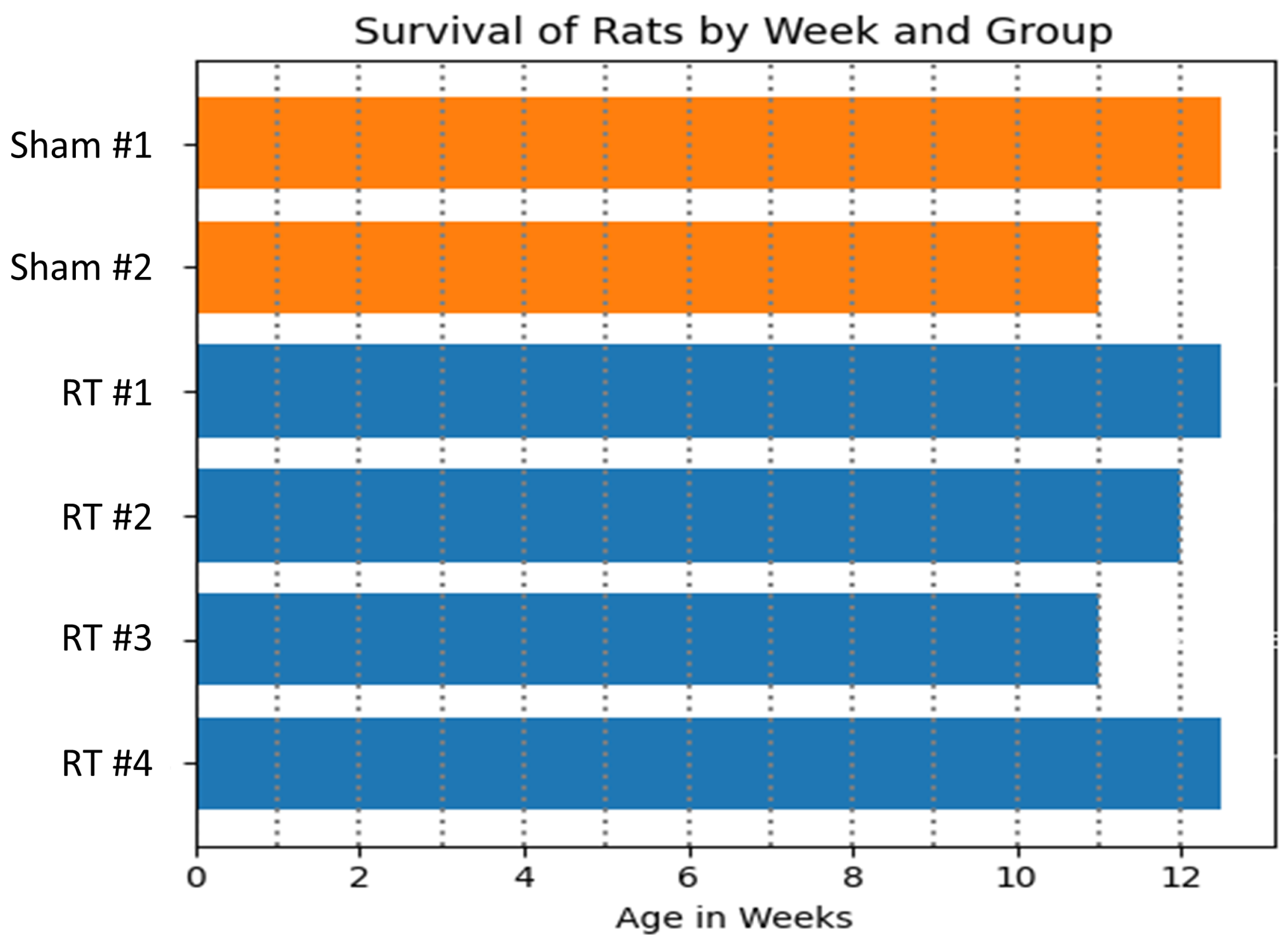
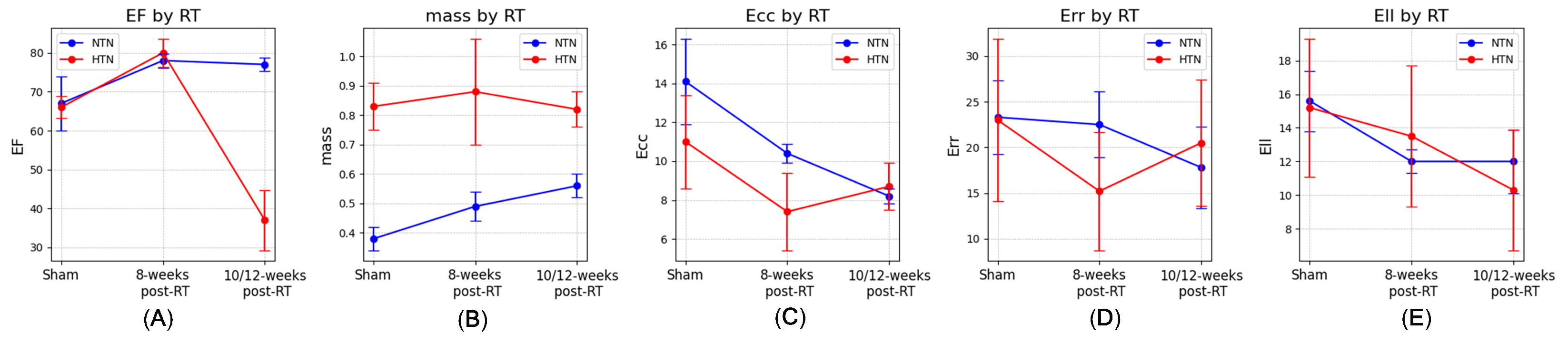
3.1. Physiological Results and MRI-Derived Cardiac Function
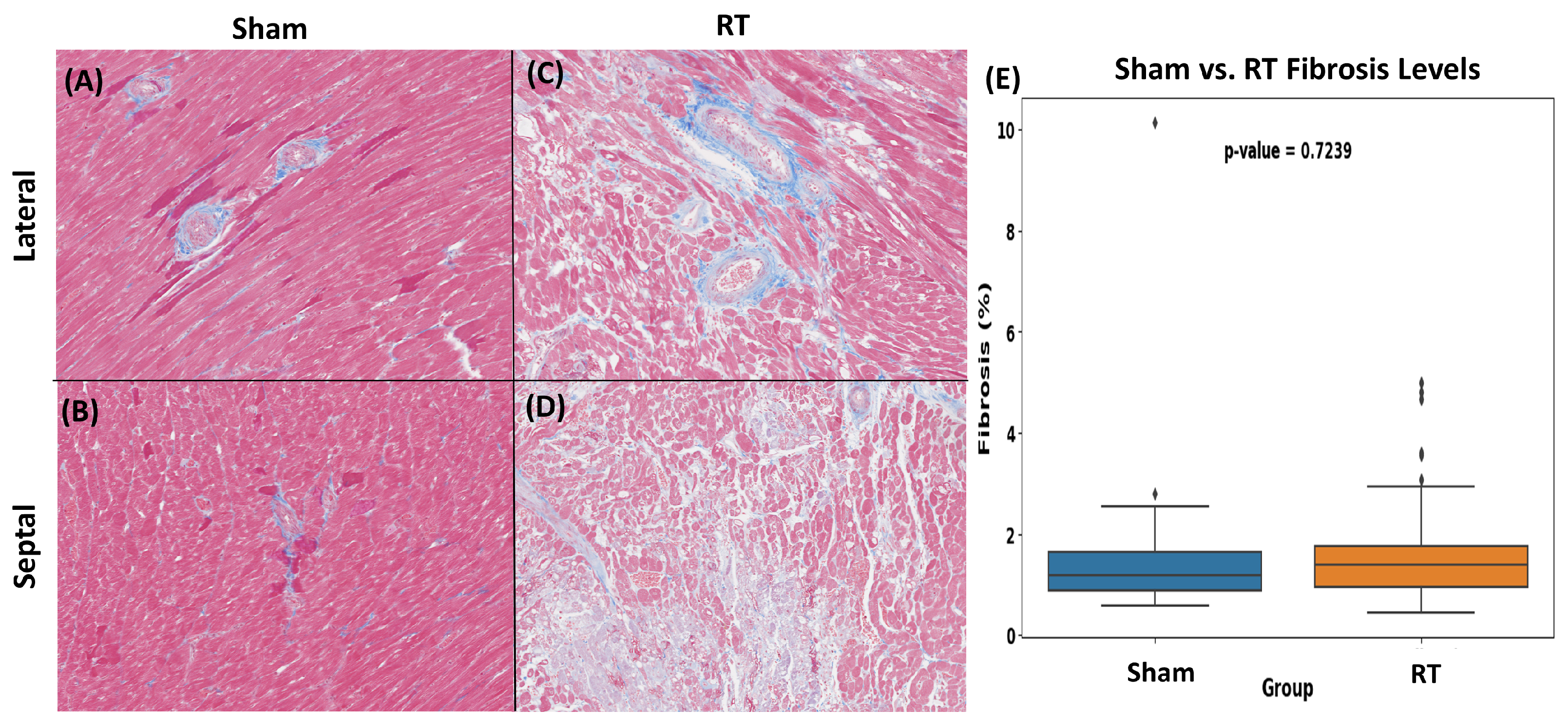
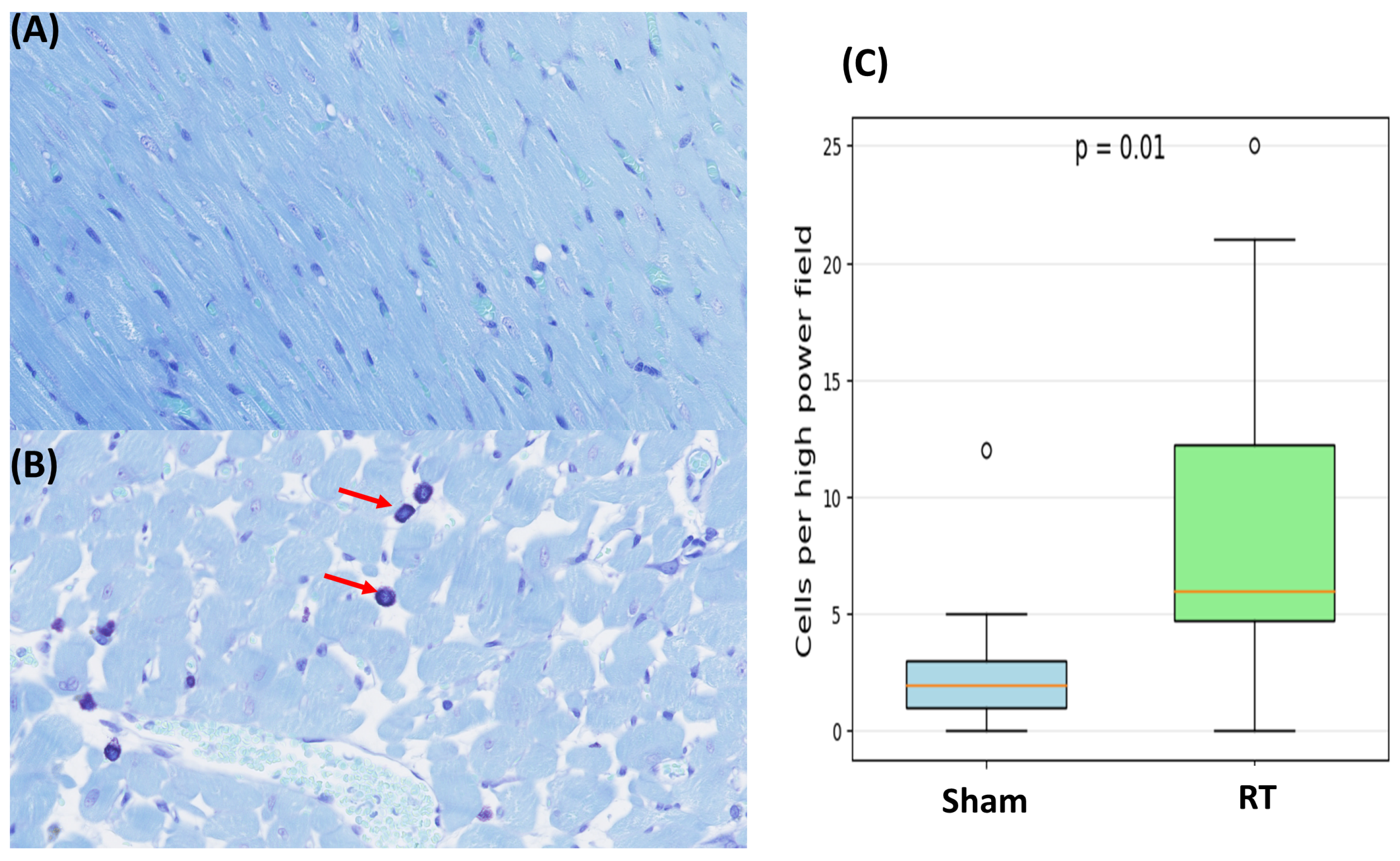
3.2. Histopathologic Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RT | Radiation therapy |
| SS | Salt-sensitive |
| EF | Ejection fraction |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| Ecc | Circumferential strain |
| Err | Radial strain |
| Ell | Longitudinal strain |
| SAX | Short axis |
| LAX | Long axis |
| LV | Left ventricle |
| TR | Repetition time |
| TE | Echo time |
| EDV | End-diastolic volume |
| ESV | End-systolic volume |
| SV | Stroke volume |
| H&E | Hematoxylin and eosin |
| SD | Standard deviation |
References
- Baskar, R.; Lee, K.A.; Yeo, R. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Shore, R.E. Radiation-related heart disease: Current knowledge and future prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Henson, K.E.; McGale, P.; Taylor, C.; Darby, S.C. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br. J. Cancer 2013, 108, 179–182. [Google Scholar] [CrossRef]
- Yaromina, A.; Krause, M.; Baumann, M. Individualization of cancer treatment from radiotherapy perspective. Mol. Oncol. 2012, 6, 211–221. [Google Scholar] [CrossRef]
- Badiyan, S.N.; Puckett, L.L.; Vlacich, G.; Schiffer, W.; Pedersen, L.N.; Mitchell, J.D.; Bergom, C. Radiation-induced cardiovascular toxicities. Curr. Treat. Options Oncol. 2022, 23, 1388–1404. [Google Scholar] [CrossRef]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-induced cardiovascular disease: Review of an underrecognized pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef] [PubMed]
- Erven, K.; Florian, A.; Slagmolen, P.; Sweldens, C.; Jurcut, R.; Wildiers, H.; Voigt, J.U.; Weltens, C. Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1172–1178. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Pedersen, L.N.; Schiffer, W.; Mitchell, J.D.; Bergom, C. Radiation-induced cardiac dysfunction: Practical implications. Kardiol. Pol. 2022, 80, 256–265. [Google Scholar] [CrossRef]
- Zhu, D.; Li, T.; Zhuang, H.; Cui, M. Early detection of cardiac damage by two-dimensional speckle tracking echocardiography after thoracic radiation therapy: Study protocol for a prospective cohort study. Front. Cardiovasc. Med. 2022, 26, 735265. [Google Scholar] [CrossRef]
- Imazio, M.; Colopi, M.; De Ferrari, G.M. Pericardial diseases in patients with cancer: Contemporary prevalence, management and outcomes. Heart 2020, 106, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Szpakowski, N.; Desai, M.Y. Radiation-associated pericardial disease. Curr. Cardiol. Rep. 2019, 21, 97. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128. [Google Scholar] [CrossRef]
- Carlson, L.E.; Watt, G.P.; Tonorezos, E.S.; Chow, E.J.; Yu, A.F.; Woods, M.; Lynch, C.F.; John, E.M.; Mellemkjær, L.; Brooks, J.D.; et al. Coronary artery disease in young women after radiation therapy for breast cancer: The WECARE Study. Cardio Oncol. 2021, 3, 381–392. [Google Scholar] [CrossRef]
- da Silva, R.M.F.L. Effects of radiotherapy in coronary artery disease. Curr. Atheroscler. Rep. 2019, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Kirresh, A.; White, L.; Mitchell, A.; Ahmad, S.; Obika, B.; Davis, S.; Ahmad, M.; Candilio, L. Radiation-induced coronary artery disease: A difficult clinical conundrum. Clin. Med. 2022, 22, 251–256. [Google Scholar] [CrossRef]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular heart disease epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Cutter, D.J.; Darby, S.C.; Yusuf, S.W. Risks of heart disease after radiotherapy. Tex. Heart Inst. J. 2011, 38, 257. [Google Scholar]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Donnellan, E.; Phelan, D.; McCarthy, C.P.; Collier, P.; Desai, M.; Griffin, B. Radiation-induced heart disease: A practical guide to diagnosis and management. Clevel. Clin. J. Med. 2016, 83, 914–922. [Google Scholar] [CrossRef]
- Bergom, C.; Bradley, J.A.; Ng, A.K.; Samson, P.; Robinson, C.; Lopez-Mattei, J.; Mitchell, J.D. Past, present, and future of radiation-induced cardiotoxicity: Refinements in targeting, surveillance, and risk stratification. Cardio Oncol. 2021, 3, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G.; Constine, L.S.; Moiseenko, V.; Correa, C.; Pierce, L.J.; Allen, A.M.; Marks, L.B. Radiation dose–volume effects in the heart. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S77–S85. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.; Schachat, A.P.; He, Q.; Leske, M.C. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch. Ophthalmol. 2000, 118, 351–358. [Google Scholar] [CrossRef]
- Janeway, T.C. A clinical study of hypertensive cardiovascular disease. Arch. Intern. Med. 1913, 12, 755–798. [Google Scholar] [CrossRef]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. J. Hypertens. 2001, 37, 1053–1059. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Klein, B.E.; Tielsch, J.M.; Hubbard, L.; Nieto, F.J. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv. Ophthalmol. 2001, 46, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3673–3780. [Google Scholar] [CrossRef]
- Kirova, Y.; Tallet, A.; Aznar, M.C.; Loap, P.; Bouali, A.; Bourgier, C. Radio-induced cardiotoxicity: From physiopathology and risk factors to adaptation of radiotherapy treatment planning and recommended cardiac follow-up. Cancer Rad. Ther. 2020, 24, 576–585. [Google Scholar] [CrossRef]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular disease and breast cancer: Where these entities intersect: A scientific statement from the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Finn, C.F. Cardiac toxicity of thoracic radiotherapy: Existing evidence and future directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef]
- Ibrahim, E.S.; Sosa, A.; Brown, S.A.; An, D.; Klawikowski, S.; Baker, J.; Bergom, C. Myocardial contractility pattern characterization in radiation-induced cardiotoxicity using magnetic resonance imaging: A pilot study with ContractiX. Tomography 2022, 9, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Abais-Battad, J.M.; Saravia, F.L.; Lund, H.; Dasinger, J.H.; Fehrenbach, D.J.; Alsheikh, A.J.; Zemaj, J.; Kirby, J.R.; Mattson, D.L. Dietary influences on the Dahl SS rat gut microbiota and its effects on salt-sensitive hypertension and renal damage. Acta Physiol. 2021, 232, e13662. [Google Scholar] [CrossRef]
- Mattson, D.L.; Dwinell, M.R.; Greene, A.S.; Kwitek, A.E.; Roman, R.J.; Jacob, H.J.; Cowley, A.W., Jr. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am. J. Physiol.-Ren. Physiol. 2008, 295, F837–F842. [Google Scholar] [CrossRef]
- Qu, P.; Hamada, M.; Ikeda, S.; Hiasa, G.; Shigematsu, Y.; Hiwada, K. Time-course changes in left ventricular geometry and function during the development of hypertension in Dahl salt-sensitive rats. Hypertens. Res. 2000, 23, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Rapp, J. Dahl salt-susceptible and salt-resistant rats. A review. J. Hypertens. 1982, 4, 753–763. [Google Scholar] [CrossRef]
- Schlaak, R.A.; SenthilKumar, G.; Boerma, M.; Bergom, C. Advances in preclinical research models of radiation-induced cardiac toxicity. J. Cancer 2020, 12, 415. [Google Scholar] [CrossRef]
- Geurts, A.M.; Mattson, D.L.; Liu, P.; Cabacungan, E.; Skelton, M.M.; Kurth, T.M.; Yang, C.; Endres, B.T.; Klotz, J.; Liang, M.; et al. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. J. Hypertens. 2015, 65, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, A.J.; Greene, A.S. Substitution of Brown Norway chromosome 16 preserves cardiac function with aging in a salt-sensitive Dahl consomic rat. Physiol. Genom. 2008, 36, 35–42. [Google Scholar] [CrossRef]
- Schlaak, R.A.; Frei, A.; Schottstaedt, A.M.; Tsaih, S.W.; Fish, B.L.; Harmann, L.; Liu, Q.; Gasperetti, T.; Medhora, M.; North, P.E.; et al. Mapping genetic modifiers of radiation-induced cardiotoxicity to rat chromosome 3. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1267–H1280. [Google Scholar] [CrossRef]
- Ibrahim, E.-S.H.; Baruah, D.; Budde, M.; Rubenstein, J.; Frei, A.; Schlaak, R.; Gore, E.; Bergom, C. Optimized cardiac functional MRI of small-animal models of cancer radiation therapy. Magn. Reson. Imaging 2020, 73, 130–137. [Google Scholar] [CrossRef]
- Ibrahim, E.S.-H.; Baruah, D.; Croisille, P.; Stojanovska, J.; Rubenstein, J.C.; Frei, A.; Schlaak, R.A.; Lin, C.Y.; Pipke, J.L.; Lemke, A.; et al. Cardiac magnetic resonance for early detection of radiation therapy-induced cardiotoxicity in a small animal model. Cardio Oncol. 2021, 3, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.S.; Stojanovska, J.; Hassanein, A.; Duvernoy, C.; Croisille, P.; Pop-Busui, R.; Swanson, S.D. Regional cardiac function analysis from tagged MRI images. Comparison of techniques: Harmonic-Phase (HARP) versus Sinusoidal-Modeling (SinMod) analysis. Magn. Reson. Imaging 2018, 54, 271–282. [Google Scholar] [CrossRef]
- Arts, T.; Prinzen, F.W.; Delhaas, T.; Milles, J.R.; Rossi, A.C.; Clarysse, P. Mapping displacement and deformation of the heart with local sine-wave modeling. IEEE Trans. Med. Imaging 2010, 29, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Lenarczyk, M.; Kronenberg, A.; Mäder, M.; North, P.E.; Komorowski, R.; Cheng, Q.; Little, M.P.; Chiang, I.H.; LaTessa, C.; Jardine, J.; et al. Age at exposure to radiation determines severity of renal and cardiac disease in rats. Radiat. Res. 2019, 192, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Zietara, A.; Spires, D.R.; Juffre, A.; Costello, H.M.; Crislip, G.R.; Douma, L.G.; Levchenko, V.; Dissanayake, L.V.; Klemens, C.A.; Nikolaienko, O.; et al. Knockout of the circadian clock protein PER1 (Period1) exacerbates hypertension and increases kidney injury in dahl salt-sensitive rats. J. Hypertens. 2022, 79, 2519–2529. [Google Scholar] [CrossRef]
- Mayet, J.; Hughes, A. Cardiac and vascular pathophysiology in hypertension. Heart 2003, 89, 1104–1109. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Pfeffer, J.A.; Mirsky, I.S.; Iwai, J.U. Cardiac hypertrophy and performance of Dahl hypertensive rats on graded salt diets. J. Hypertens. 1984, 6, 475–481. [Google Scholar] [CrossRef]
- Klotz, S.; Hay, I.; Zhang, G.; Maurer, M.; Wang, J.; Burkhoff, D. Development of heart failure in chronic hypertensive Dahl rats: Focus on heart failure with preserved ejection fraction. J. Hypertens. 2006, 47, 901–911. [Google Scholar] [CrossRef]


| Baseline | 8 Weeks Post-RT | 12 Weeks Post-RT | ||||
|---|---|---|---|---|---|---|
| Sham | RT | Sham | RT | Sham | RT | |
| SBP (mmHg) | 232.5 ± 12.0 | 223 ± 37.4 | 280 ± 8.5 | 223.3 ± 38.5 | 306 | 181 ± 54.6 |
| DBP (mmHg) | 174 ± 35.4 | 176.3 ± 51.9 | 217 ± 29.7 | 165.3 ± 60.8 | 246 | 93.3 ± 50.1 |
| Pulse (bpm) | 433 ± 34 | 410 ± 33 | 460 ± 59 | 426 ± 39 | 414 | 399 ± 50 |
| EDV (mL) | 0.38 ± 0.01 | 0.39 ± 0.04 | 0.44 ± 0 | 0.47 ± 0.05 | 0.44 | 0.47 ± 0.07 |
| ESV (mL) | 0.14 ± 0.02 | 0.17 ± 0.06 | 0.15 ± 0.01 | 0.1 ± 0.02 | 0.2 | 0.31 ± 0.08 |
| SV (mL) | 0.24 ± 0.03 | 0.25 ± 0.04 | 0.29 ± 0.01 | 0.38 ± 0.05 | 0.24 | 0.17 ± 0.01 |
| EF (%) | 63 ± 7.1 | 64.5 ± 11.7 | 66 ± 2.8 | 80 ± 3.6 | 56 | 36.5 ± 7.8 |
| Mass (g) | 0.59 ± 0.01 | 0.61 ± 0.05 | 0.83 ± 0.08 | 0.88 ± 0.18 | 0.83 | 0.82 ± 0.06 |
| Ecc (%) | −10.7 ± 2.7 | −9.4 ± 2.3 | −11 ± 2.4 | −7.4 ± 2.0 | −13.7 ± 3.8 | −8.7 ± 1.2 |
| Err (%) | 20.3 ± 8.9 | 22.8 ± 7.8 | 23 ± 8.9 | 15.2 ± 6.5 | 28.7 ± 8.1 | 20.5 ± 6.9 |
| Ell (%) | −15.9 ± 5.0 | −14.5 ± 6.3 | −15.2 ± 4.1 | −13.5 ± 4.2 | −15.8 ± 5.4 | −10.3 ± 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, D.; Kriegel, A.; Kumar, S.; Himburg, H.; Fish, B.; Klawikowski, S.; Rowe, D.; Lenarczyk, M.; Baker, J.; Ibrahim, E.-S. Radiation-Induced Cardiotoxicity in Hypertensive Salt-Sensitive Rats: A Feasibility Study. Life 2025, 15, 862. https://doi.org/10.3390/life15060862
An D, Kriegel A, Kumar S, Himburg H, Fish B, Klawikowski S, Rowe D, Lenarczyk M, Baker J, Ibrahim E-S. Radiation-Induced Cardiotoxicity in Hypertensive Salt-Sensitive Rats: A Feasibility Study. Life. 2025; 15(6):862. https://doi.org/10.3390/life15060862
Chicago/Turabian StyleAn, Dayeong, Alison Kriegel, Suresh Kumar, Heather Himburg, Brian Fish, Slade Klawikowski, Daniel Rowe, Marek Lenarczyk, John Baker, and El-Sayed Ibrahim. 2025. "Radiation-Induced Cardiotoxicity in Hypertensive Salt-Sensitive Rats: A Feasibility Study" Life 15, no. 6: 862. https://doi.org/10.3390/life15060862
APA StyleAn, D., Kriegel, A., Kumar, S., Himburg, H., Fish, B., Klawikowski, S., Rowe, D., Lenarczyk, M., Baker, J., & Ibrahim, E.-S. (2025). Radiation-Induced Cardiotoxicity in Hypertensive Salt-Sensitive Rats: A Feasibility Study. Life, 15(6), 862. https://doi.org/10.3390/life15060862






