Is It Possible to Access the Uterus of Sheep by Endoscopy: Studies of Vaginoscopy and Hysteroscopy with Transcervical Uterine Access in Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
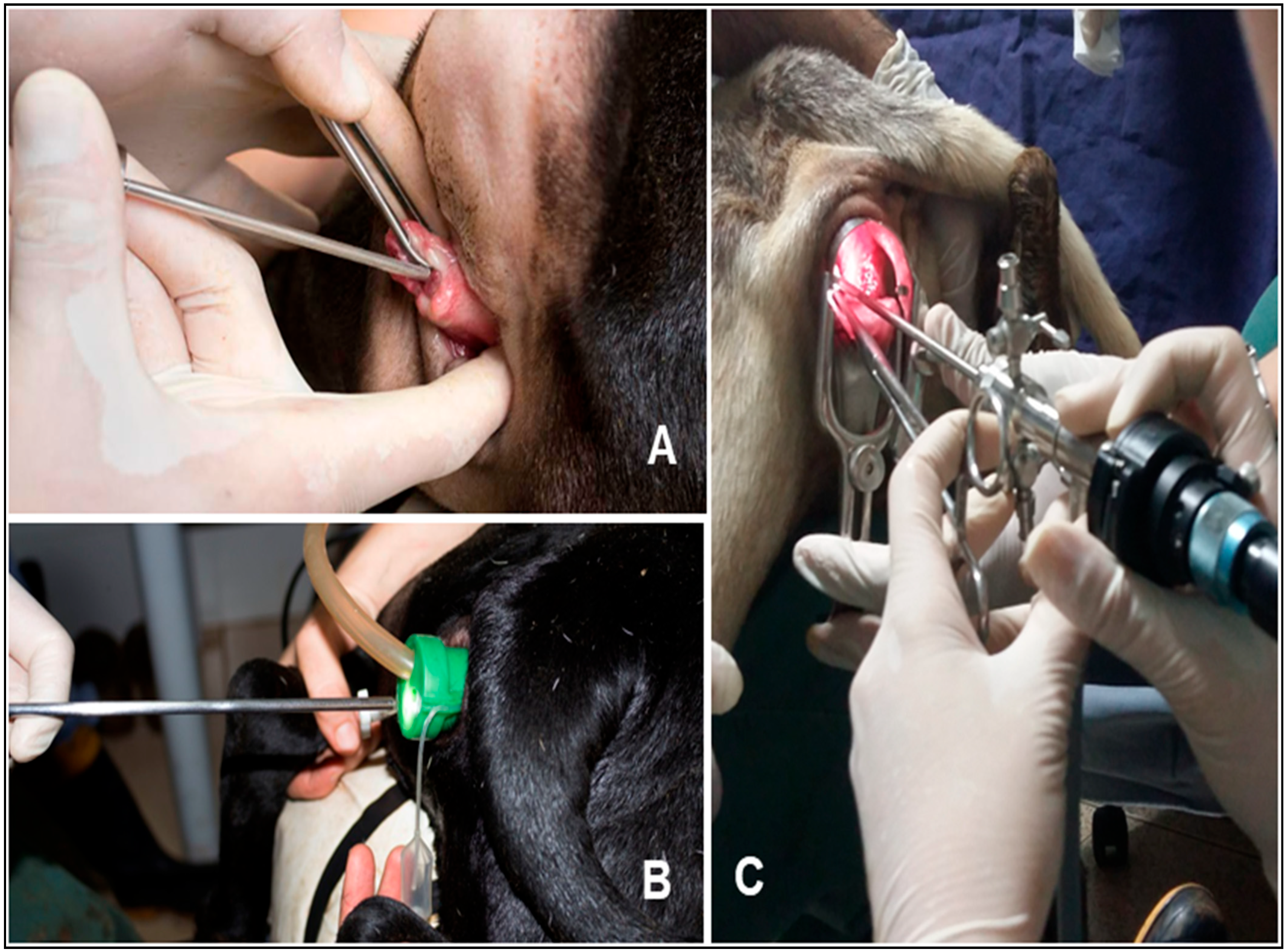
2.2. Pilot Study of Access to the Uterus
2.3. Second Pilot Study of Uterine Access with Fixed-Time Artificial Insemination (FTAI) Protocol
2.4. Study After Adaptations of the Previous Phase
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Insemination |
| FTAI | Fixed-time Artificial Insemination |
| CP | Crude Protein |
| TDN | Total Digestible Nutrients |
| CG | Control Group with cervical traction |
| EPG | Endoscopic Pilot Group without protocol |
| EPGp | Endoscopic Pilot Group with protocol |
| EVG | Endoscopic Vaginoscopy Group |
| eCG | equine Chorionic Gonadotropin |
References
- Casali, R.; Pinczak, A.; Cuadro, F.; Guillen-Munoz, J.M.; Mazzalira, A.; Menchaca, A. Semen deposition by cervical, transcervical and intrauterine route for fixed-time artificial insemination (FTAI) in the ewe. Theriogenology 2017, 103, 30–35. [Google Scholar] [CrossRef] [PubMed]
- El Amiri, B.; Rahim, A. Exploring Endogenous and Exogenous Factors for Successful Artificial Insemination in Sheep: A Global Overview. Vet. Sci. 2024, 11, 86. [Google Scholar] [CrossRef]
- Padilha, L.C.; Teixeira, P.P.M.; Pires-Buttler, E.A.; Apparicio, M.; Motheo, T.F.; Savi, P.A.P.; Vicente, W.R.R. In vitro maturation of oocytes from Santa Ines ewes subjected to consecutive sessions of follicular aspiration by laparoscopy. Reprod. Domest. Anim. 2014, 49, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Easley, J.; Shasa, D.; Hackett, E. Vaginoscopy in Ewes Utilizing a Laparoscopic Surgical Port Device. J. Vet. Med. 2017, 30, 290. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Riemma, G.; Alonso Pacheco, L.; Carugno, J.; Haimovich, S.; Tesarik, J.; De Franciscis, P. Hysteroscopic endometrial biopsy: From indications to instrumentation and techniques. A call to action. Minim. Invasive Ther. Allied Technol. 2021, 30, 251–262. [Google Scholar] [CrossRef]
- Di Spiezio Sardo, A.; Saccone, G.; Carugno, J.; Pacheco, L.A.; Zizolfi, B.; Haimovich, S.; Clark, T.J. Endometrial biopsy under direct hysteroscopic visualisation versus blind endometrial sampling for the diagnosis of endometrial hyperplasia and cancer: Systematic review and meta-analysis. Facts Views Vis. Obgyn. 2022, 14, 103–110. [Google Scholar] [CrossRef]
- Vitale, S.G.; Buzzaccarini, G.; Riemma, G.; Pacheco, L.A.; Di Spiezio Sardo, A.; Carugno, J.; Chiantera, V.; Török, P.; Noventa, M.; Haimovich, S.; et al. Endometrial biopsy: Indications, techniques and recommendations. An evidence-based guideline for clinical practice. J. Gynecol. Obstet. Hum. Reprod. 2023, 52, 102588. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Della Corte, L.; Ciebiera, M.; Carugno, J.; Riemma, G.; Lasmar, R.B.; Lasmar, B.P.; Kahramanoglu, I.; Urman, B.; Mikuš, M.; et al. Hysteroscopic Endometrial Ablation: From Indications to Instrumentation and Techniques-A Call to Action. Diagnostics 2023, 13, 339. [Google Scholar] [CrossRef]
- Rodrigues, G.J.; Monteiro, B.M.; Viana, R.B.; da Silva, A.O.A.; Monteiro, F.D.O.; Teixeira, P.P.M. New method of video-assisted vaginoscopy in Nellore heifers. Vet. Med. Sci. 2023, 9, 2781–2785. [Google Scholar] [CrossRef]
- Vall, E.; Blanchard, M.; Sib, O.; Cormary, B.; González-García, E. Standardized body condition scoring system for tropical farm animals (large ruminants, small ruminants, and equines). Trop. Anim. Health Prod. 2025, 57, 106. [Google Scholar] [CrossRef]
- Kershaw, M.C.; Khalid, M.; McGowan, M.R.; Ingram, K.; Leethongdee, S.; Wax, G.; Scaramuzzi, R.J. The anatomy of the sheep cervix and its influence on the transcervical passage of an inseminating pipette into the uterine lumen. Theriogenology 2005, 64, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Taqueda, G.S.; Azevedo, H.C.; Santos, E.M.; Matos, J.E.; Bittencourt, R.F.; Bicudo, S.D. Influence of anatomical and technical aspects on fertility rate based on sheep transcervical artificial insemination performance. ARS Vet. 2011, 27, 127–133. [Google Scholar] [CrossRef]
- Millar, H.R.; Simpson, J.G.; Stalker, A.L. An evaluation of the heat precipitation method for plasma fibrinogen estimation. J. Clin. Pathol. 1971, 24, 827–830. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Pearson, K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philos. Mag. 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Colagross-Schouten, A.; Allison, D.; Brent, L.; Lissner, E. Successful Use of Endoscopy for Transcervical Cannulation Procedures in the Goat. Reprod. Domest. Anim. 2014, 49, 909–912. [Google Scholar] [CrossRef]
- McCool, K.E.; Marks, S.L.; Hawkins, E.C. Endoscopy Training in Small Animal Internal Medicine: A Survey of Residency Training Programs in North America. J. Vet. Med. Educ. 2022, 49, 515–523. [Google Scholar] [CrossRef]
- Önder, N.T.; Batı, Y.U.; Gökdemir, T.; Kılıç, M.C.; Şahin, O.; Öğün, M.; Öztürkler, Y. Oxidative response of sheep to transcervical applications. Reprod. Domest. Anim. 2023, 58, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fabjan, J.M.G.; Oliveira, M.E.F.; Guimarães, M.P.P.; Brandão, F.Z.; Bartlewski, P.M.; Fonseca, J.F. Review: Non-surgical artificial insemination and embryo recovery as safe tools for genetic preservation in small ruminants. Animal 2023, 17, 100787. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.C. Essentials of Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1993; p. 417. [Google Scholar]
- Sabes, A.F.; Girardi, A.M.; Fagliari, J.J.; de Oliveira, J.A.; Marques, L.C. Serum proteinogram in sheep with acute ruminal lactic acidosis. Int. J. Vet. Sci. Med. 2017, 5, 35–40. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Haas, C.S.; Ferreira, C.E.R.; Goularte, K.L.; Pegoraro, L.M.C.; Gasperin, B.G.; Vieira, A.D. Inflammatory markers in ewes submitted to surgical or transcervical embryo collection. Small Rumin. Res. 2018, 158, 15–18. [Google Scholar] [CrossRef]
- Önder, N.T.; Gökdemir, T.; Kilic, M.C.; Şahin, O.; Demir, M.C.; Yildiz, S.; Öztürkler, Y. A novel “Onder Speculum” to visualize and retract the cervix during transcervical procedures in small ruminants. Vet. Fak. Derg. 2024, 30, 101–106. [Google Scholar] [CrossRef]
- Duarte, G.S.; Galindo, D.J.; Baldini, M.H.M.; da Fonseca, J.F.; Duarte, J.M.B.; Oliveira, M.E.F. Transcervical artificial insemination in the brown brocket deer (Subulo gouazoubira): A promising method for assisted reproduction in deer. Sci. Rep. 2023, 13, 17369. [Google Scholar] [CrossRef]
- Masoudi, R.; Shahneh, A.Z.; Towhidi, A.; Kohram, H.; Akbarisharif, A.; Sharafi, M. Fertility response of artificial insemination methods in sheep with fresh and frozen-thawed semen. Cryobiology 2017, 74, 77–80. [Google Scholar] [CrossRef]
- Silva, M.A.M.; Teixeira, P.P.M.; Coutinho, L.N.; Gutierrez, R.R.; Macente, B.I.; Vicente, W.R.R.; Brun, M.V. Resection of vaginal neoplasms by video-vaginoscopy in bitches. Acta Sci. Vet. 2014, 42, 1–5. [Google Scholar]
- Franco, M.C.; dos Santos, J.F.; Maciel, T.A.; Duarte Neto, P.J.; Oliveira, D. Morphology of the cervix of Santa Ines adult sheep in luteal and folicular phases. Ciênc. Anim. Bras. 2014, 15, 495–501. [Google Scholar] [CrossRef]
- Moura, D.S.; Lourenço, T.T.; Moscardini, M.M.; Scott, C.; Fonseca, P.O.; Souza, F.F. Aspectos morfológicos da cérvice de ovelhas. Pesq. Vet. Bras. 2011, 31, 33–38. [Google Scholar] [CrossRef]
- Rekha, A.; Zohara, B.F.; Bari, F.; Alam, M.G.S. Comparison of commercial Triladyl extender with a tris-fructose-egg-yolk extender on the quality of frozen semen and pregnancy rate after transcervical AI in Bangladeshi indigenous sheep (Ovis aries). Small Rumin. Res. 2016, 134, 39–43. [Google Scholar] [CrossRef]
- Lima, A.C.N.; Pereira, E.T.N.; Almeida, I.C.; Xavier, E.D.; Oliveira, D.C.F.; Almeida, A.C. Reproductive disorders and reconception of beef cows subjected to timed artificial insemination. Ciênc. Anim. Bras. 2022, 23, e70384. [Google Scholar] [CrossRef]
- Russi, L.S.; Costa-e-Silva, E.V.; Zúccari, C.E.S.N.; Recalde, C.S.; Cardoso, N.G. Impact of the quality of life of inseminators on the results of artificial insemination programs in beef cattle. Rev. Bras. Zootec. 2010, 39, 1457–1463. [Google Scholar] [CrossRef]
- Purdy, P.H.; Spiller, S.F.; McGuire, E.; McGuire, K.; Koepke, K.; Lake, S.; Blackburn, H.D. Critical factors for non-surgical artificial insemination in sheep. Small Rumin. Res. 2020, 191, 106179. [Google Scholar] [CrossRef]
- Teixeira, T.A.; da Fonseca, J.F.; de Souza-Fabjan, J.M.G.; de Rezende Carvalheira, L.; de Moura Fernandes, D.A.; Brandão, F.Z. Efficiency of different hormonal treatments for estrus synchronization in tropical Santa Inês sheep. Trop. Anim. Health Prod. 2016, 48, 545–551. [Google Scholar] [CrossRef]
- Spanner, E.A.; de Graaf, S.P.; Rickard, J.P. Factors affecting the success of laparoscopic artificial insemination in sheep. An. Reprod. Sci. 2024, 264, 107453. [Google Scholar] [CrossRef]
- Myers, A.; Ramanathan, K. Laparoscopic artificial insemination in sheep: Review and cost benefit analysis. Clin. Theriog. 2025, 17, 1–16. [Google Scholar] [CrossRef]
- Faigl, V.; Vass, N.; Jávor, A.; Kulcsár, M.; Solti, L.; Amiridis, G.; Cseh, S. Artificial insemination of small ruminants—A review. Acta Vet. Hung. 2012, 60, 115–129. [Google Scholar] [CrossRef] [PubMed]
| Parameter | EPGp | EPG | CG | EVG | p-Value |
|---|---|---|---|---|---|
| Cervical passage rate (%) | 10 (1/10) | 100 5/5 | – | – | <0.001 |
| Intrauterine deposition (%) | 10 (1/10) | 10 (1/10) | 20 (2/10) | 20 (2/10) | 0.0078 |
| Pregnancy rate (%) | 10 (1/10) | – | 20 (2/10) | 20 (2/10) | 1.0 |
| Insemination time (s) | 249 ± 101 | – | 158 ± 50 | 129 ± 29 | 0.0017 |
| Group | Cervical Rings (Mean ± SD) | Intrauterine (%) | Deep Cervical (%) | Superficial Cervical (%) |
|---|---|---|---|---|
| EPG | - | 100 (5/5) | - | - |
| EPGp | 10 (1/10) | - | 90 (9/10) | |
| CG | 3.5 ± 3.3 | 20 (2/10) | 80 (8/10) | – |
| EVG | 1.6 ± 1.2 | 20 (2/10) | – | 80 (8/10) |
| Time Interval | EVG Trend | CG Trend | p-Values |
|---|---|---|---|
| 20 min to 24 h | Increase | – | 0.0017 |
| 24 h to 72 h and beyond | Decrease | Decrease (lowest at 72 h) | 0.003/0.006 |
| Cervical os Type | Frequency (n) | Prevalence (%) |
|---|---|---|
| Papilla | 10 | 33 (10/30) |
| Smooth | 7 | 23 (7/30) |
| Rosette | 6 | 20 (6/10) |
| Flap | 7 | 17 (7/10) |
| Duckbill | 7 | 7 (2/30) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, A.R.; Cardoso, T.d.S.; Amanajas, R.L.; Landers, R.S.G.M.; da Silva, P.D.Á.; Santos, V.J.C.; Rodrigues, N.N.; Vrisman, D.P.; Barros, F.F.P.d.C.; Monteiro, F.D.d.O.; et al. Is It Possible to Access the Uterus of Sheep by Endoscopy: Studies of Vaginoscopy and Hysteroscopy with Transcervical Uterine Access in Sheep. Life 2025, 15, 846. https://doi.org/10.3390/life15060846
Taira AR, Cardoso TdS, Amanajas RL, Landers RSGM, da Silva PDÁ, Santos VJC, Rodrigues NN, Vrisman DP, Barros FFPdC, Monteiro FDdO, et al. Is It Possible to Access the Uterus of Sheep by Endoscopy: Studies of Vaginoscopy and Hysteroscopy with Transcervical Uterine Access in Sheep. Life. 2025; 15(6):846. https://doi.org/10.3390/life15060846
Chicago/Turabian StyleTaira, Augusto Ryonosuke, Thiago da Silva Cardoso, Renata Levy Amanajas, Renata Sitta Gomes Mariano Landers, Priscila Del Águila da Silva, Victor Jóse Correia Santos, Naiara Nantes Rodrigues, Dayane Priscila Vrisman, Felipe Faria Pereira da Câmara Barros, Francisco Décio de Oliveira Monteiro, and et al. 2025. "Is It Possible to Access the Uterus of Sheep by Endoscopy: Studies of Vaginoscopy and Hysteroscopy with Transcervical Uterine Access in Sheep" Life 15, no. 6: 846. https://doi.org/10.3390/life15060846
APA StyleTaira, A. R., Cardoso, T. d. S., Amanajas, R. L., Landers, R. S. G. M., da Silva, P. D. Á., Santos, V. J. C., Rodrigues, N. N., Vrisman, D. P., Barros, F. F. P. d. C., Monteiro, F. D. d. O., Albuquerque, R. d. S., Salvarani, F. M., Vicente, W. R. R., & Teixeira, P. P. M. (2025). Is It Possible to Access the Uterus of Sheep by Endoscopy: Studies of Vaginoscopy and Hysteroscopy with Transcervical Uterine Access in Sheep. Life, 15(6), 846. https://doi.org/10.3390/life15060846







