Abstract
Obesity, insulin resistance, type 2 diabetes mellitus (T2DM) and metabolic syndrome have been largely correlated to a reduction in bacterial load and diversity, resulting in a condition known as intestinal dysbiosis. The recent emergence of novel antidiabetic medications has been demonstrated to exert a favourable influence on the composition of the intestinal microbiota. Incretin-based therapy exerts a multifaceted influence on the composition of the gut microbiota, leading to alterations in bacterial flora. Of particular significance is the capacity of numerous metabolites produced by the gut microbiota to modulate the activity and hormonal secretion of enteroendocrine cells. This review examines the effects of dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists and GLP-1/gastric inhibitory polypeptide (GIP) receptor dual agonists on the composition of the gut microbiota in both mice and human subjects. The nature of this interaction is complex and bidirectional. The present study demonstrates the involvement of the incretinic axis in modulating the microbial composition, with the objective of providing novel preventative strategies and potential personalised therapeutic targets for obesity and T2DM.
1. Introduction
Obesity is regarded as a global epidemic, with prevalence figures exceeding 2 billion. The prevailing definition of obesity, as outlined by the World Health Organization, is a body mass index (BMI) of 30 kg/m2 or above [1]. However, it is important to note that obesity is not solely defined by the BMI; rather, it is a complex condition influenced by individual factors, including genetic and epigenetic influences, and lifestyle factors such as unhealthy diet, overeating, and low physical activity [2]. This complex condition is associated with an elevated risk of several chronic conditions, including diabetes, hypercholesterolemia, cardiovascular disease (from hypertension to heart failure), and cancer [3]. Type 2 diabetes mellitus (T2DM) is a prevalent chronic metabolic disorder, characterised by hyperglycaemia resulting from a combination of insulin resistance and inadequate insulin secretion [4,5,6,7]. The diagnosis of T2DM is typically made on the basis of fasting plasma glucose values, 2 h plasma glucose values derived from a 75 g oral glucose tolerance test, or haemoglobin A1C (HbA1c) levels [5]. It is well established that carrying excess weight, or being obese, is a major risk factor for the development of T2DM [8,9]. The prevalence and incidence of both obesity and T2DM are steadily increasing and reflect each other. An unhealthy diet, characterised by a high consumption of carbohydrates and lipids, and a low intake of fibre, is a major contributing factor to the development of obesity and, potentially, T2DM. In individuals with T2DM and a BMI in the overweight or obese range, weight reduction has been shown to enhance glycaemic control and decrease reliance on glucose-lowering medications [9]. The management of T2DM necessitates a multifaceted, person-centred approach. The promotion of healthy lifestyle behaviours and diabetes self-management is to be emphasised alongside any pharmacotherapy [10].
Furthermore, obesity, insulin resistance and type 2 diabetes mellitus have been largely correlated to a reduction in bacterial load and diversity, resulting in a condition known as intestinal dysbiosis. The recent emergence of novel antidiabetic medications has been demonstrated to exert a favourable influence on the composition of the intestinal microbiota. In this review, the extant data on the bidirectional interactions between the gut microbiota and incretin-based therapies, including GLP-1R agonists, DPP-4 inhibitors and GLP-1/GIP receptor dual agonists, are summarised.
2. Incretins and Incretin-Based Therapies
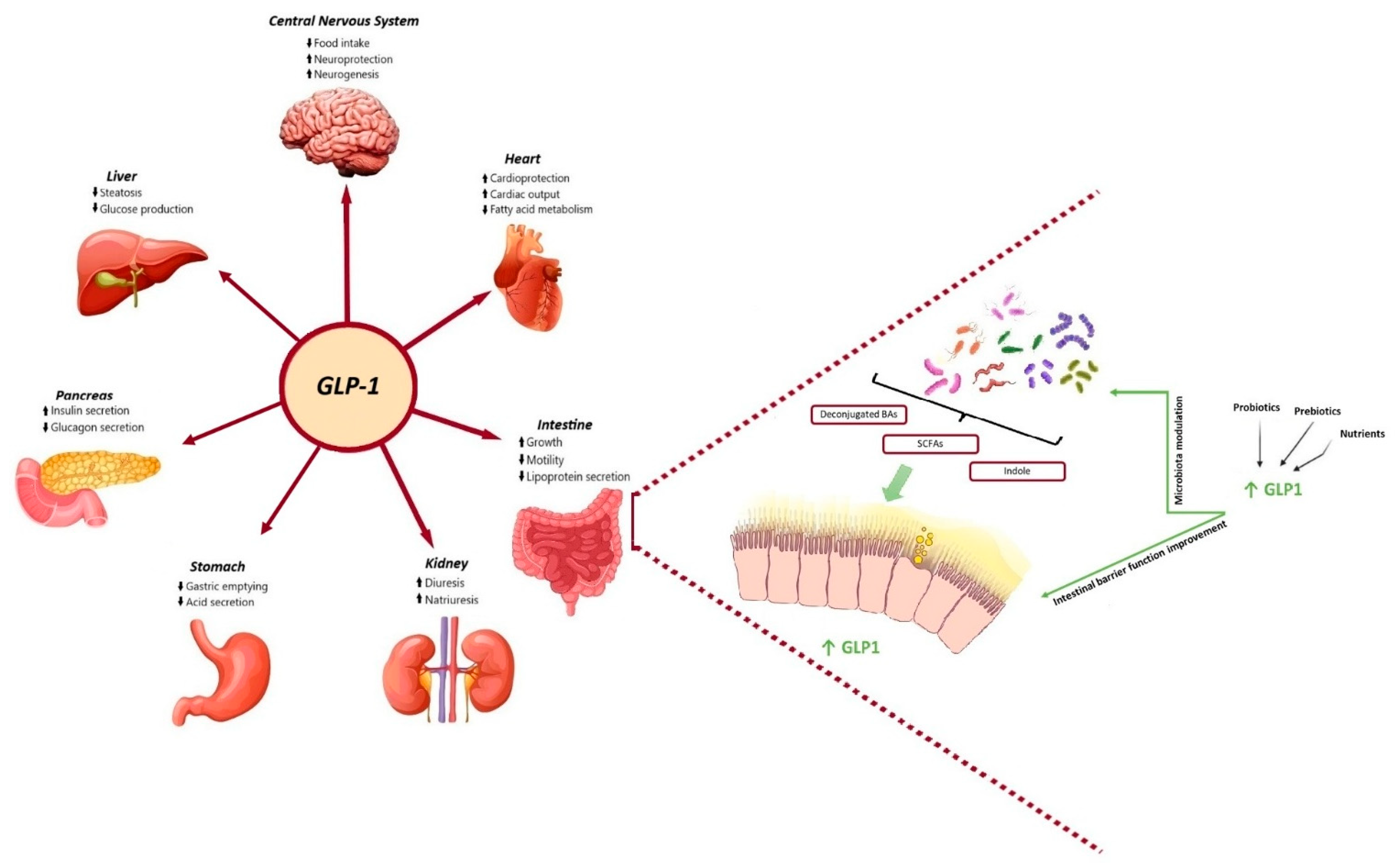
Incretin-based therapies represent a novel treatment for both T2DM and obesity, relying on the insulinotropic actions of the gut hormone glucagon-like peptide-1 (GLP-1) and, most recently, on the combined action of GLP-1 and the gastric inhibitory polypeptide (GIP) hormones. Incretins are gastrointestinal hormones that promote postprandial insulin secretion in a glucose-dependent manner. There are two types of incretins: GLP-1 and GIP. Both are secreted by intestinal cells in response to meals, with GLP-1 being secreted from L cells that are found in the large and lower bowel and GIP from K cells in the upper bowel [11]. There is a specific threshold, between 1 and 2 kcal/min, for glucose-induced GLP-1 secretion in the small intestine, while the secretion of GIP is more sensitive [12]. GLP-1 subsequently binds to GLP-1 receptors (GLP-1R), which are widely expressed in multiple organs and tissues, including the gastrointestinal tract, the endocrine pancreas, the heart, and the central nervous system, thereby exerting pleiotropic functions (Figure 1).
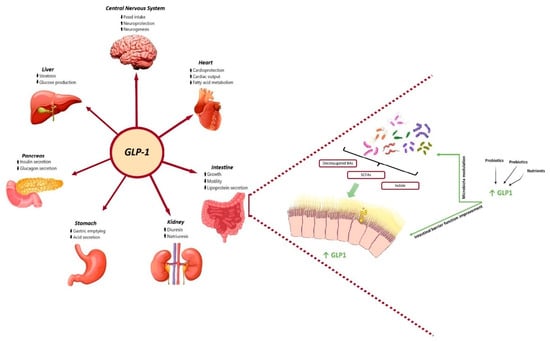
Figure 1.
Physiological benefits of GLP-1 and gut microbiota metabolites and their bidirectional interaction. On the one hand, GLP-1 promotes insulin synthesis and secretion in pancreatic beta cells and then improves glucose homeostasis, delays gastric emptying and reduces gastric acid secretion, reduces intestinal motility and lipoprotein secretion, promotes diuresis and natriuresis, increases liver glycogen storage and decreases liver sugar output, and suppresses appetite in the hypothalamus of brain. On the other hand, SCFAs, secondary bile acids and indole, have been shown to directly stimulate the release of incretins from intestinal L-cells and represent the key candidates involved in the crosstalk between microbes and host cells. Microbial-based therapeutics, including probiotics, nutrients and prebiotics, could directly target the gut microbiota or act as an adjunctive to the GLP-1 receptor agonist to restore the balance of several certain dysbiotic gut microbiota and improve gut barrier function, therefore improving glycaemic control and glucose tolerance.
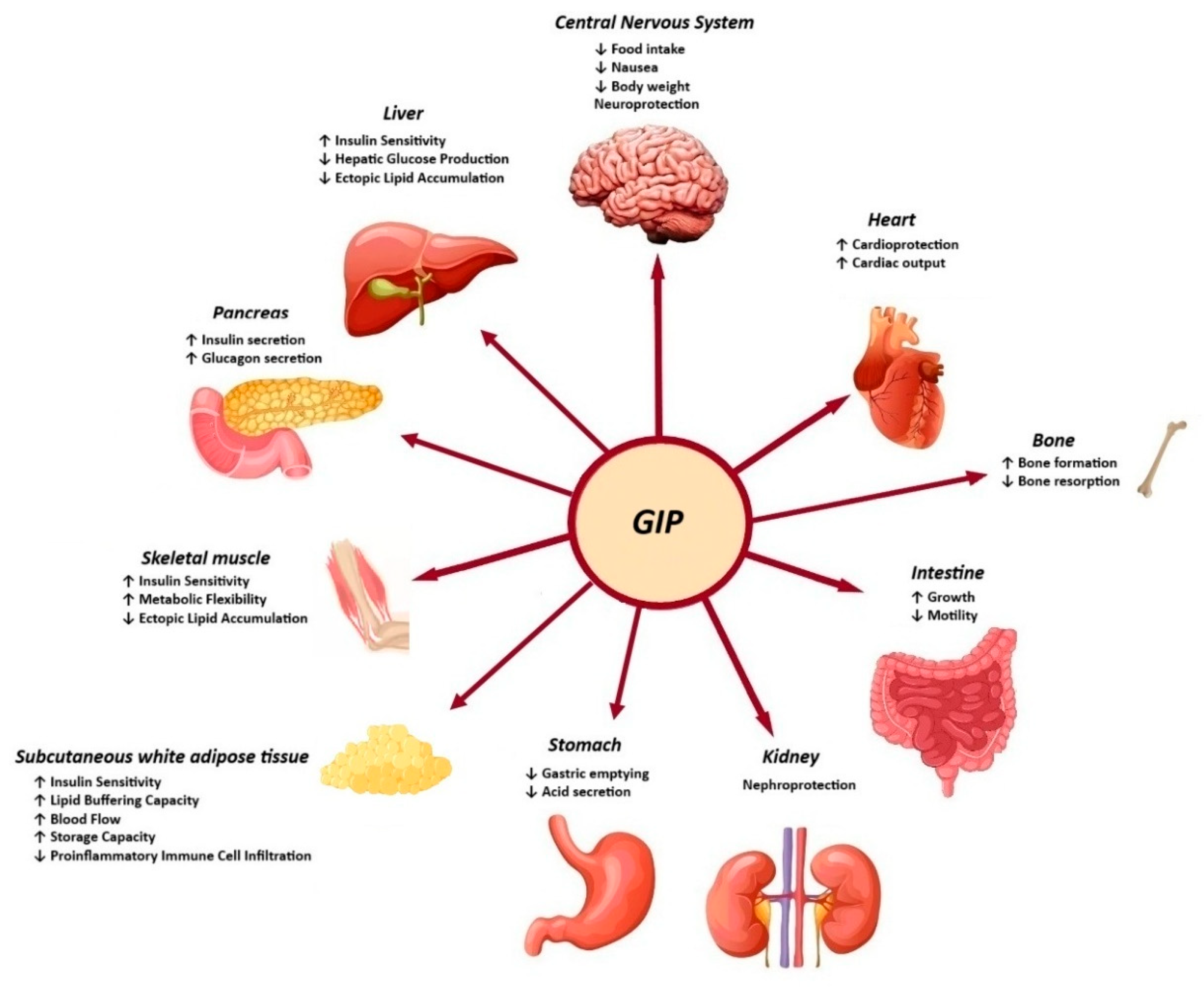
The biological action of GIP is promoted through binding to the GIP receptor (GIPR), a class B G-protein-coupled receptor that is similar to GLP-1R and belongs to the glucagon receptor family. The GIPR is expressed in the endocrine pancreas, adipocytes, myeloid cells, the endothelium of the heart and blood vessels, the inner layers of the adrenal cortex and the central nervous system. The role of GIP in regulating energy balance is achieved through cell surface receptor signalling in the brain and adipose tissue [13]. It is evident that both GLP-1 and GIP play a substantial role in the function of beta cells, including the promotion of increased beta-cell mass, enhanced insulin secretion, and the preservation of beta-cell survival [14]. Moreover, the GIP-dependent reduction in insulin clearance contributes to the enhancement of peripheral insulin levels, thereby ensuring the maintenance of normal blood glucose levels (Figure 2) [15].
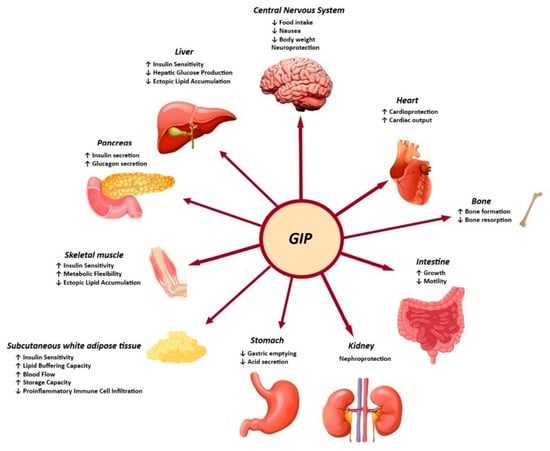
Figure 2.
Effects of GIP in peripheral tissues.
However, GLP-1 and GIP are rapidly degraded in plasma by dipeptidyl peptidase IV (DPP-4), resulting in a very short half-life of approximately 1.5 min [16,17,18].
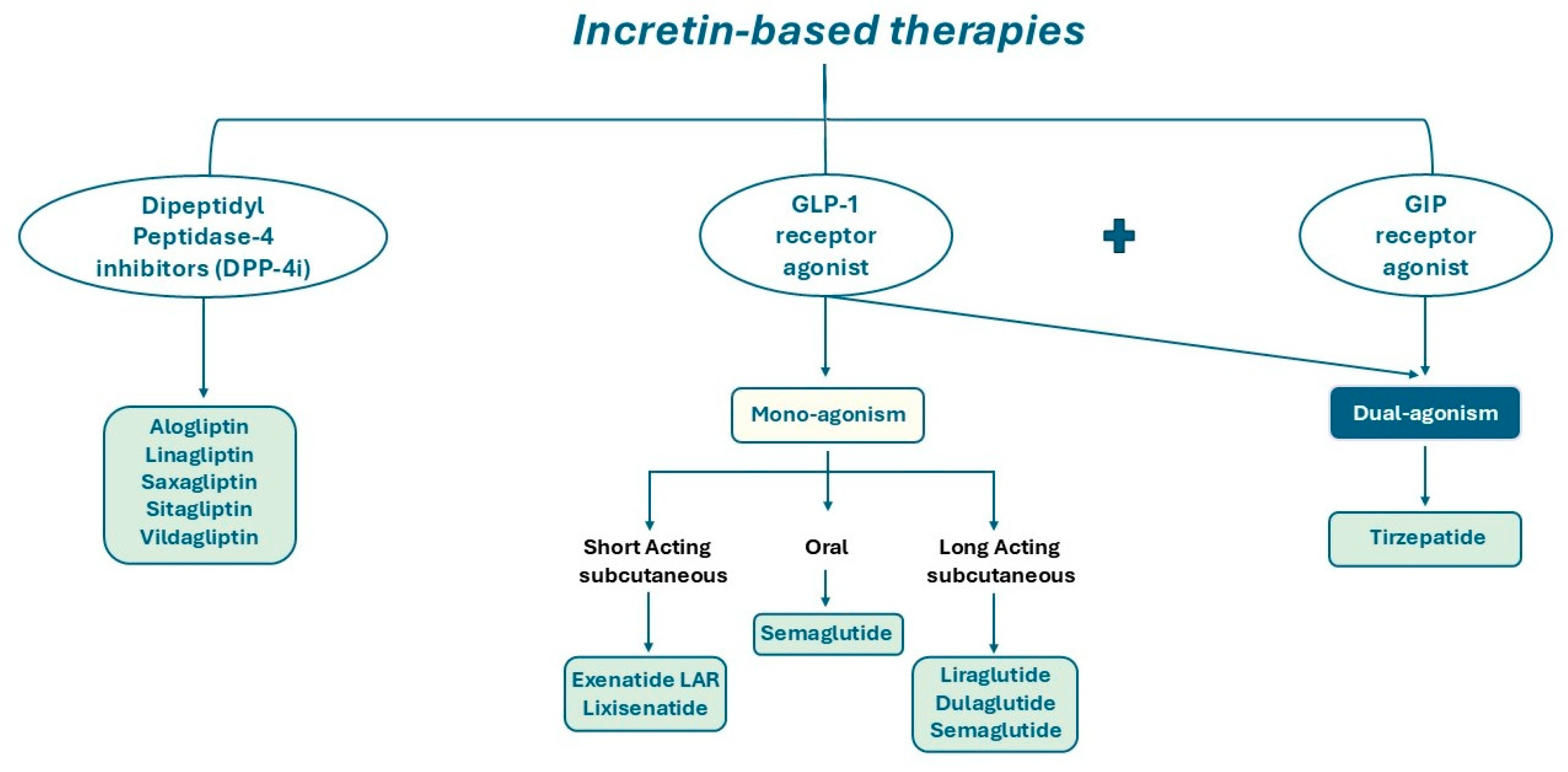
DPP-4 is a type II integral transmembrane glycoprotein with enzymatic activity; it also exists in a soluble form in the plasma, lacking the cytoplasmic and transmembrane domains [19,20]. The primary substrates for DPP-4’s catalytic activity are incretins responsible for glucose metabolism, including GIP, GLP-1 and GLP-2 [21,22,23]. Consequently, DPP-4 inhibitors may have a pivotal role in prolonging the circulating half-life of GLP-1 and GIP, thereby preventing their cleavage [24]. Indeed, DPP-4 inhibitors represent a therapeutic approach for the management of patients with T2DM (Figure 3).
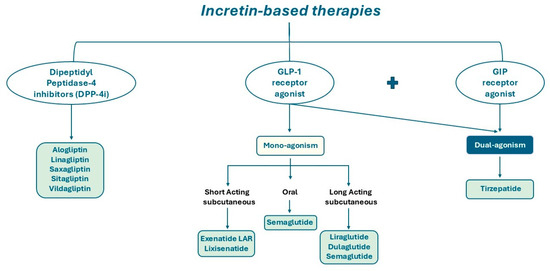
Figure 3.
Schematic illustration of DPP-4 inhibitors, mono-agonists and dual-agonists of GLP-1 and GIP receptor.
These inhibitors reduce blood glucose levels, enhance glycated haemoglobin levels, and do not induce hypoglycaemia. There is evidence that the rate of gastric emptying (GE) may influence the magnitude of effect on glucose-lowering to DPP-4 inhibition. Indeed, the ability of DPP-4 inhibitors to reduce postprandial glycaemia increases when GE is faster, partly because this leads to enhanced GLP-1 secretion, but also because higher postprandial glucose levels are necessary for GLP-1-mediated insulin secretion and glucagon suppression [25,26].
Moreover, nutritional strategies may improve the lowering of blood glucose levels induced by DPP-4 inhibitors. A promising approach to optimise DPP-4 inhibitor action involves the administration of a small quantity of a specific macronutrient at a fixed interval before a meal. The presence of nutrients in the small intestine induces the release of gut peptides, including endogenous GLP-1 and GIP; these peptides improve the glycaemic response to the subsequent meal and slow gastric emptying [27,28].
DPP-4 inhibitors have been shown to improve β-cell function and inhibit α-cell secretion, with the potential to enhance insulin sensitivity, as evidenced by increased β-cell mass in animal studies.
Furthermore, many studies demonstrated that DPP-4 gene-deficient mice exhibited enhanced postprandial glucose control and resistance to developing hyperinsulinemia and obesity. Exogenous DPP-4 inhibitors improve glucose tolerance in wild-type mice, but not in DPP-4 knockout mice [29]. Beyond its catalytic functions, DPP-4 may also regulate the immune system through the cleavage of various chemokines and cytokines, such as erythropoietin, stromal cell-derived factor-1, granulocyte colony-stimulating factor, Interleukin-3, and granulocyte-macrophage colony-stimulating factor [30]. Additionally, DPP-4 exert non-catalytic functions, including the reduction in dendritic cell/macrophage-mediated adipose tissue inflammation in obesity by affecting macrophage migration (CD11b+, CD11c+, and Ly6Chi) [31,32]. Moreover, DPP-4 enhances regulatory T cell (Treg) expansion and transforming growth factor beta levels in non-obese diabetic mice whilst concomitantly promoting T-cell activation via its interaction with adenosine deaminase [33,34].
GLP-1R agonists used in clinical settings (Figure 3) are structurally modified to exhibit relative resistance to DPP-4 cleavage, resulting in a long-circulating half-life [22,35].
The rapid cleavage of the N-terminal His–Ala residues of GLP-1 by DPP-4 expressed on surrounding tissues results in the inactivation of GLP-1 and a consequent short half-life [19]. GLP-1 analogues exert various pharmacological actions by increasing GLP-1 levels 10-fold or more. GLP-1R agonists have been shown to restore the sensitivity of β-cells to insulin, induce insulin secretion [19], and modulate body weight loss by potentially inhibiting GE [36]. In the central nervous system, GLP-1R agonists regulate glycaemic control and possess a protective effect on neuronal damage [37]. Furthermore, GLP-1 may also act on the heart and gut, directly or indirectly through the sympathetic nervous system, to regulate heart rate and gut peristalsis via the nitric oxide-mediated suppression of intestinal motility [38,39,40]. Common adverse effects include nausea, diarrhoea and constipation [41]. The relationship between intestinal norepinephrine and GLP-1 remains to be fully elucidated [11]. The acute stimulation of the β-adrenergic receptor by isoproterenol induces a significant increase in the secretion of GLP-1 and PYY. This suggests that the activation of β2-adrenergic receptors on intestinal L cells could promote the release of these hormones [42]. At the same time, it has been observed that a prolonged increase in catecholamine production may inhibit GLP-1 secretion [43].
Recently, a new pharmaceutical agent was developed, which has the capacity to simultaneously co-activate both the GIPR and the GLP-1R (Figure 3). Tirzepatide, a drug that has been hailed as a pioneering innovation in its field, was shown to improve glycaemic control by increasing insulin sensitivity and lipid metabolism and reducing body weight. The co-activation of these two receptors enhances β cell function, offering a more effective treatment for diabetes and obesity with a reduced adverse effect profile compared to selective GLP-1R agonists. Until now, clinical trials have demonstrated the remarkable effectiveness of this compound, once-weekly injected, in achieving optimal glycaemic control and substantial weight reduction [44,45]. The magnitude of the effects of tirzepatide on glycaemia and weight loss marks the commencement of a new era in diabetes therapy, with the potential to treat a significant proportion of patients according to currently established targets.
Even the SURMOUNT-3 study evaluated the efficacy and safety of tirzepatide in the treatment of obesity. This study demonstrated that the combination of tirzepatide with intensive behavioural therapy resulted in significant weight loss, with a mean reduction of 25.3% after 84 weeks of treatment, a result that is superior to the 20.9% weight loss reported in the SURMOUNT-1 study at week 72, which used tirzepatide alone without intensive behavioural therapy. The sequential therapy (intensive behavioural therapy followed by tirzepatide) may have an additive effect on weight loss, but further studies are needed to confirm this result. But the main study’s results highlight the importance of combining new obesity medications with lifestyle modification interventions to achieve optimal results [46].
3. Gut Microbiota
In the course of its development, the human gastrointestinal tract accumulates a dense, varied population of microorganisms called ‘microbiota’. The bacteria populating human intestines are in the process of evolving and coevolving with their host in a system of beneficial symbiosis [47].
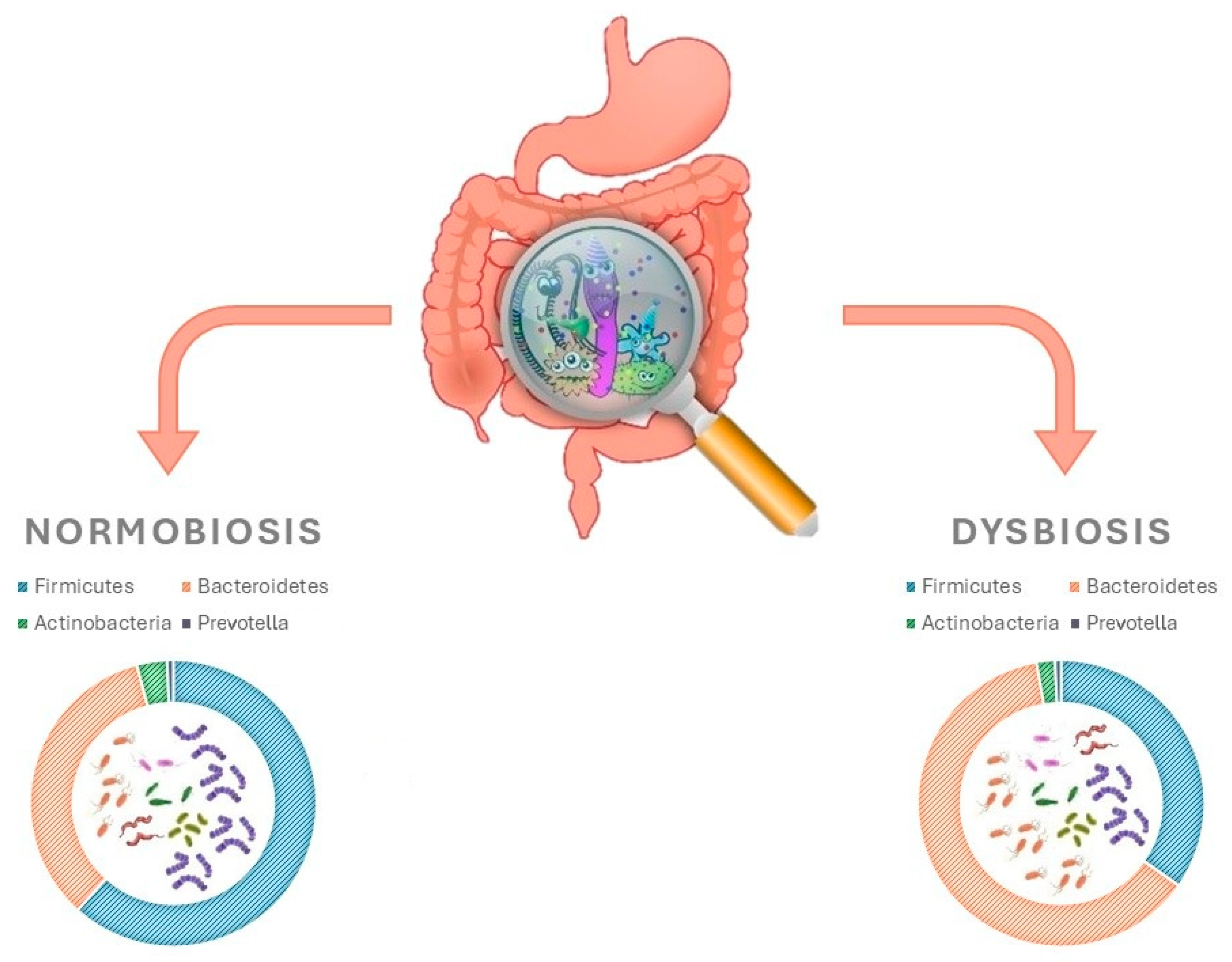
Until a decade ago, information available on the human microbiota was based upon traditional culture techniques. More recently, the use of genomic sequencing methods has enhanced the capabilities of studying the gut microbiota [47,48]. Numerous fragments from MetaHit and Human Microbiome Project databases have contributed towards building a better and more exhaustive understanding of the microorganisms present in the human gut [49,50,51]. In a typical adult, gut microbiota composition includes fungi, viruses, and parasites in addition to bacteria. The total number of species of microbes in the gut microbiota can reach up to 5000, with a combined weight of approximately 2000 g. This is equivalent to 10 times the amount of microbial cells found in the entire human body [52]. Studies that have been carried out have contributed to the identification and isolation of 2172 bacterial species, which have been subsequently categorised into 12 different phyla. The main species are, in order of abundance, Firmicutes (Ruminococcus, Clostridium and Eubacterium), Bacteroidetes, Actinobacteria and Prevotella (Figure 4) [52,53,54].
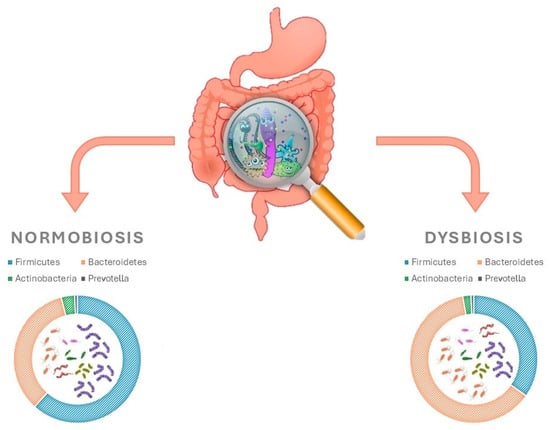
Figure 4.
Gut microbiota and how they differ in intestinal homoeostasis vs. dysbiosis.
Features of the small intestine such as high levels of acids, oxygen, and antimicrobials and a short transit time inhibit the growth of bacteria [47,51]. Thus, in this environment, only facultative anaerobes can survive, which rapidly grow and are membranous epithelium-adhering and permeable to oxygen [47,51]. On the contrary, the shallow characteristics of the large intestine support the existence of a highly diverse microbial community, predominantly anaerobes trained on complex carbohydrates and polysaccharides not utilised in the small intestine [47,51].
The microbiota present within the gastrointestinal tract are understood to exert a pivotal influence on host physiology, engaging in interactions with both the endocrine and immune systems within the gastrointestinal tissues.
The bacteria within the gut microbiome are involved in many biological processes, including the harvesting of energy from food (degrading fibres and complex polysaccharides) and the manufacturing of neurotransmitters (e.g., serotonin, vitamins and enzymes). The production of microbial metabolites can be categorised into three distinct pathways: the first involves the fermentation of food elements by intestinal microorganisms, such as 2-oleoyl glycerol (derived from dietary fats); the second pathway entails the direct production of microbial metabolites by the intestinal microbiota, including lipopolysaccharides (LPSs); and the third pathway involves the synthesis of these microbial metabolites by the host, followed by subsequent modification by bacteria. In addition, the gut microbiota protects the body from pathogens with a competitive mechanism and through the production of antimicrobial substances. In summary, the intestinal microbiota modulate the immune system and help maintain the integrity of the intestinal barrier by strengthening tight junctions between enterocytes and stimulating mucus production [52].
Nutritional intake exerts a profound influence on the equilibrium between beneficial and opportunistic microbial composition, with dietary modifications capable of affecting this ratio [52]. In addition to nutrient availability, various factors influence fluctuations and stabilities of bacterial levels, such as osmolality, pH, temperature, hormones, neurotransmitters derived from the host, cytokines, and viruses that infect bacteria (bacteriophages) [55,56,57,58]. Advances in sequencing technologies and population-scale studies have revealed that the microbiome assists in the expansion of host genomes by facilitating the host’s physiology and metabolism and associating with several diseases [59,60]. An imbalance of the microbiota called dysbiosis has been associated with numerous diseases, including obesity, diabetes, cardiovascular diseases, chronic inflammatory bowel diseases, autoimmune diseases and neurological disorders [61].
4. Incretin-Based Drugs and Gut Microbiota
Incretin-based therapies represent a novel treatment for both T2DM and obesity, relying on the insulinotropic actions of the gut hormone GLP-1 and, most recently, on the combined action of GLP-1 and the GIP hormones. The gut microbiota is a complex community consisting of more than 500 microbial species and it is involved in many biological processes, including energy metabolism, inflammatory response, immunity and gut–brain neural circuits. Despite the numerous preclinical and clinical studies conducted on the interaction between incretin-based drugs and gut microbiota, there are still several aspects of this interaction that are not yet fully understood.
4.1. Preclinical Studies
A number of studies conducted on mice have indicated that both DPP-4 inhibitors and GLP-1-R agonists have the capacity to modify the bacterial composition of the intestinal microbiome. The primary outcome of interest pertained to the variation in the balance between Firmicutes and Bacteroidetes. In a study by Yan et al. [62], it was demonstrated that a high-fat diet led to a reduction in Bacteroidetes and an increase in Firmicutes and Tenericutes. The administration of sitagliptin resulted in the reversal of these gut microbiota changes and the modification of a set of bacteria producing short-chain fatty acids (SCFAs) in rats with T2DM who were fed a high-fat diet [62].
In a similar vein, other studies have shown that the use of vildagliptin led to an increase in Bacteroidetes and a decrease in Firmicutes, a decrease in the Firmicutes/Bacteroidetes ratio, and an increase in butyrate-producing bacteria in obese and diabetic mice (Figure 4) [63,64]. Moreover, it has been reported that vildagliptin can exert positive effects through the variation in the intestinal microbiota by increasing Lactobacilli spp., promoting the production of propionate and decreasing Oscillibacter spp. [65]. In order to elucidate the mechanisms by which vildagliptin modifies the intestinal microbiota, the experiments conducted demonstrated the drug’s ability to indirectly reduce the hepatic gene expression of proinflammatory cytokines, reduce Toll-like receptor ligands in caecum contents, promote the expression of antimicrobial peptides, and restore the depth of the ileum crypts [65]. Furthermore, Liao X. et al. and Silva-Veiga et al. showed that sitagliptin and linagliptin, respectively, increase the abundance of succinate and Bacteroidetes in non-diabetic mice [66,67].
In order to evaluate the hypothesis that alterations of the gut microbiota induced by DPP-4 inhibitors treatment improve glucose homeostasis, a comparison has been made of the effects of DPP-4 inhibitors with those of α-glucosidase inhibitors. While both altered the composition of the gut microbiome, the hypoglycaemic effect was attributed to the modulation of the gut microbiome by DPP-4 inhibitors. Specifically, these drugs reversed the changes in 68.6% of intestinal bacteria genera induced by a high-fat diet. Furthermore, a study on faecal microbiota transplantation demonstrated that DPP-4 inhibitors led to a favourable alteration in the microbiome and an enhancement in glucose tolerance in colonised mice, while acarbose did not exhibit this effect [66].
However, a separate preclinical study involving 60 C57BL/6 ApoE/mice (half of which received streptozotocin) and randomised to 8 weeks of treatment with liraglutide or saxagliptin revealed that only the former substantially modified the composition of the intestinal microbiota, particularly the concentration of phylotypes relevant to weight [68].
The acute administration of liraglutide (a GLP-1R agonist) to mice increased both caecal levels of the caseinolytic protease B, a component of Escherichia coli, and norepinephrine. However, these events were blocked by chemical sympathectomy. Furthermore, in vitro studies revealed that norepinephrine was found to permeate the intestinal lumen, and the c-Fructooligosaccharides (Fos) staining of the intermediolateral nucleus was interpreted as indirect evidence of intestinal tract sympathetic nervous system activation by GLP-1R agonists. Under normal conditions, the increase in Escherichia coli did not affect the host. However, in murine models of colitis, a bacterial translocation, accompanied by the attenuation of tight junction gene expression, was observed [69].
The administration of semaglutide, a long-acting GLP-1R agonist, has been demonstrated to modify the bacterial composition of the intestinal microbiome. A preclinical study demonstrated that a high-fat diet in mice induced a significant increase in Lachnospiraceae and Bacteroides and a significant decrease in Akkermansia. The use of semaglutide has been demonstrated to mitigate microbial dysbiosis, restore lost flora and suppress excessive bacterial abundance [70,71]. In a similar manner, in a mouse model of non-alcoholic fatty liver disease (NAFLD) with a db/db genetic background, semaglutide modified the gut microbiota, influencing Alloprevotella, Alistipes, Ligilactobacillus and Lactobacillus, and improved the integrity of the gut barrier [72]. Finally, in a mouse model of T2DM, semaglutide increased Bacterioidetes while significantly decreasing the abundance of Firmicutes [73,74].
In polycystic ovary syndrome (PCOS) mice, both semaglutide and liraglutide modulates both the alpha and beta diversity of the gut microbiota. Liraglutide increases the Bacillota-to-Bacteroidota ratio through up-regulating the abundance of butyrate-producing members of Bacillota, such as Lachnospiraceae. Conversely, semaglutide increases the abundance of Helicobacter [75].
Of particular interest was the observation that the gut microbiota composition of obese mice became phylogenetically similar after treatment with liraglutide or with a dual GLP-1/GLP-2 receptor agonist, thus suggesting that GLP-2 receptor stimulation played a minimal role in the modulation of the gut microbiome [76]. The observed changes were primarily characterised by variations in the distribution of the phelotypes of Proteobacteria and Verrucomicrobia, without, however, modifying the proportion of Firmicutes [76].
Moreover, liraglutide treatment was observed to moderate glucose intolerance and insulin sensitivity in a dose-dependent manner in diabetic male rats. Furthermore, it was demonstrated that both the diabetic state and liraglutide administration profoundly changed the composition of intestinal microbiota. The pyrosequencing of the V3-V4 region of the 16S rRNA genes revealed a significant alteration in the structure of the gut microbiota in male rats treated with liraglutide in comparison to male rats with diabetes. Specifically, the study noted a selective enhancement of SCFA-producing bacteria, including Bacteroides, Lachnospiraceae, and probiotic bacteria, such as Bifidobacterium, in male rats treated with liraglutide [77]. Finally, liraglutide was shown to enhance glucose-induced insulin secretion through the increase in the Bacteroidetes-to-Firmicutes ratio, which was achieved by enhancing certain immune cells (regulatory T cells and innate lymphoid cells 1 and 3) and by reducing the Th1 cell frequency [78]. In an experiment involving a mouse model of NAFLD, liraglutide treatment resulted in a change in the gut microbiota, reducing the Helicobacter genus and Proteobacteria phylum, and weight loss, consequently improving glucose homeostasis [79].
Yuan et al. hypothesised that GLP-1R agonists could help to modify the abundance and diversity of the gut microbiota, thus restoring balance in the gut microbiota [80]. Specifically, the study noted an enhancement in several SCFA-producing bacteria, including Bacteroidetes, Lachnospiraceae, and probiotic bacteria such as Bifidobacterium, in diabetic male rats treated with liraglutide compared to those given a placebo [80]. However, in another experiment, a significant weight reduction was associated with an increase in the ratio of Firmicutes to Bacteroides, regardless of glycaemic status, in both obese and diabetic subjects [81]. Furthermore, liraglutide treatment increased the abundance of intestinal Akkermansia muciniphila in mice [79,82,83].
As previously reported, tirzepatide is a dual agonist of GIP and GLP-1R, and it has been approved by the Food and Drug Administration for the treatment of T2DM [84]. Preliminary data indicate that tirzepatide exerts a regulatory influence on the gut microbiota and bile acid (BA) metabolism in diabetic mice [85]. Notably, tirzepatide improves the abundance of beneficial genera such as Akkermansia while concomitantly reducing farnesoid X receptor (FXR) expression in intestinal tissues and elevating the ratio of FXR antagonists (glycoursodeoxycholic acid, β-muricholic acid, hyodeoxycholic acid, and ursodeoxycholic acid) to natural agonists (cholic acid, lithocholic acid, chenodeoxycholic acid, glycocholic acid and taurodeoxycholic acid) [85].
Furthermore, tirzepatide treatment restores intestinal barrier integrity, mitigates possible endotoxemia through anti-inflammatory signalling pathways, and reverses intestinal dysbiosis in an obese, diabetic ovariectomised mice model [86]. A number of studies have demonstrated that GLP-1R agonism reduces systemic inflammation and alleviates experimental gut inflammation [87,88,89]. However, the actions of GIP in reducing inflammation in different tissue compartments are not as well understood. In preclinical studies, Hammoud R. et al. demonstrated that activation of GIPR signalling attenuates 5-fluorouracil-induced gut inflammation, whereas the loss of the GIPR exacerbates the extent of gut inflammation in the murine small intestine [90]. While these findings are preliminary, they suggest the potential of GIP to play a physiological and pharmacological role in response to gut injury.
4.2. Clinical Studies
Conversely, the existing body of human research is limited and frequently yields contradictory results. For instance, Ying X et al. demonstrated that liraglutide significantly increases the diversity of the gut microbiota, particularly Bacteroidetes, Bacilli and Proteobacteria [91]. However, a subsequent study, which included 37 T2DM patients who had been administered metformin, demonstrated that those who had been given liraglutide (after metformin had been ceased) exhibited significantly different microbiota compositions to those who had been given metformin [86]. The latter group also exhibited a greater number of genus Akkermansia bacteria [92]. The precise causative agent remains uncertain as metformin is recognised for its influence on the gut microbiota [93]. On the other hand, a novel randomised controlled trial suggested that the 12-week treatment with either the GLP-1R agonist liraglutide or the DPP-4 inhibitor sitagliptin, when used as add-on therapy to metformin or sulfonylureas, induced beneficial effects on glucose metabolism, body weight, and BA production, with no change in the alpha or beta diversity on faecal microbiota [94]. Additionally, a fixed combination of degludec and liraglutide administered over a period of six months in very old subjects with T2DM showed no change in gut microbiota structure [95]. A pilot study, performed on 52 subjects with T2DM, evaluated the association between GLP-1R agonist treatment (liraglutide and dulaglutide) and intestinal flora [96]. The subjects were divided into efficacious and non-efficacious groups based on whether GLP-1R agonist administration was efficacious or not after 12 weeks of treatment. The subsequent beta-diversity analysis revealed significant disparities in the gut flora between these two groups. The authors observed a negative correlation between the abundance of Prevotella, Bacteroidales, Ruminococcaceae, Dialister succinatiphilus, Eubacterium coprostanoligenes, Mitsuokella, Alistipes obesi, Mitsuokella, and Lactobacillus mucosae and insulin resistance. Conversely, a positive link was observed between the abundance of Butyricicoccus and Lachnoclostridium and a decrease in blood glucose levels. The study indicated a close association between GLP-1R agonist treatment and intestinal flora in T2DM [96]. Liang L. et al. have recently evaluated the structural changes in the composition of the intestinal flora of T2DM subjects after 1 and 48 weeks of dulaglutide therapy. The administration of dulaglutide for a single week did not result in any significant alterations in the intestinal flora. However, after 48 weeks of dulaglutide, a significant change in the composition of the intestinal flora was observed, with a substantial reduction in the abundance of intestinal flora [97]. Furthermore, the authors demonstrated a close association between fasting glucose levels, HbA1c levels, and the BMI on the one hand and changes in intestinal flora on the other [97].
5. Gut Metabolites and Incretins
A number of studies have shown that gut microbiota metabolites, including SCFAs and/or indole, can directly stimulate the release of incretins from colonic enteroendocrine cells (EECs) (Figure 1). This influences host satiety and food intake.
SCFAs play a pivotal role in the communication between host cells and microbes, directly stimulating the release of GLP-1 from enteroendocrine cells. The induction of cell-specific signalling cascades by SCFAs is achieved through the activation of specific receptors. Two distinct receptor types, designated as free fatty acid receptor (FFAR) 3 and FFAR2 (also termed G-protein-coupled receptor (GPR) 41 and GPR43, respectively), have been identified on EECs [98,99]. The activation of these receptors enhances GLP-1 secretion through increased intracellular calcium [100].
5.1. Preclinical Studies
Preclinical studies have demonstrated that GPR43 protects mice from obesity and hyperphagia. Moreover, GPR43-deficient mice exhibit impaired glucose tolerance on a normal diet, a consequence of the role that SCFAs and GPR43 play in the release of intestinal hormones [98]. Tolhurst et al. proved that in GPR43 knockout colonic tissue from mice, SCFAs-induced GLP-1 secretion was attenuated, while in mice knockout for GPR43, SCFAs did not lead to increased GLP-1 secretion [100].
It has been hypothesised that the fermentation of non-digestible carbohydrates in the gut could promote the differentiation of L-cells in the intestine, also increasing their numbers. A study conducted on rats found that administration of nondigestible carbohydrates results in an increase in the number of enteroendocrine L cells in the proximal colon, resulting in increased GLP-1 production (mediated by SCFAs) [101]. Psichas et al. showed clearly that the intra-colonic injection of short-chain fatty acid propionate led to a significant increase in circulating plasma levels of GLP-1 and anorexigenic gut hormone peptide YY (PYY) in anaesthetised rats compared to the saline control [102].
SCFAs are produced by intestinal bacterial fermentation from dietary prebiotic fibres, such as FOS and inulin. Several studies revealed that the oral administration of FOS and inulin in rats is associated with improved glucose tolerance and hepatic insulin sensitivity through increased GLP-1 secretion, mediated by increased SCFAs [103].
5.2. Clinical Studies
Human-based studies appear to substantiate the correlation between the gut microbiota fermentation of non-digestible carbohydrates and incretin secretion. Piche T. et al. reported that the administration of a low-residue diet (i.e., 10 g fibre/day) in nine patients within reflux disease was associated with a significant increase in plasma GLP-1 after a meal [104]. The relevance of fermentation arising from the large intestine to the release of GLP-1 and lowering of postprandial glycaemia were also well supported in a clinical trial on T2DM patients, where GLP-1 release was indirectly quantified by the resultant hydrogen production in breath samples [27]. Conversely, the ingestion of lactulose (20 g/day) or the intracolonic administration of SCFAs in healthy volunteers has been associated with an increased production of incretins [105].
Furthermore, Cani P.D. et al. conducted a study in which 10 healthy adults were enrolled and divided into two groups. Each group was administered either prebiotic fibre or maltose dextrin for a period of two weeks [106]. The results of this study indicated that prebiotic administration was associated with increased blood GLP-1 and PYY and improved plasma responses to postprandial hyperglycaemia [106]. Although several studies suggest that the gut microbiota may play an important role in GLP-1 secretion through the production of SCFAs, additional studies are required to determine whether alterations of the gut microbiota are responsible for these effects.
In addition to SCFAs, the secretory activity of EECs can be modulated by several gut bacteria metabolites, including secondary BAs and indole derivatives.
Secondary BAs regulate GLP-1 secretion through the action of Takeda G protein-coupled receptor 5 on intestinal L cells [107,108]. These acids inhibit GLP-1 secretion by a mechanism that involves the activation of the farnesoid X receptor (FXR) [109,110]. In a study conducted by Rune E. et al., the intraluminal administration of BAs in an anaesthetised rat resulted in the stimulation of GIP, GLP-1, insulin, and C-peptide secretion [111].
A similar outcome was observed in a human study that was single-blinded; ten patients with obesity and T2DM received an intrarectal infusion of taurocholate, and GLP-1, insulin and PYY were increased in a dose-dependent manner [112]. The same result was also obtained in healthy subjects; ten men received taurocholic acid enemas, which induced a dose-dependent increase in GLP-1 and PYY; the participants also reported an increase in satiety [113].
In another study, Tongzhi et al. divided 10 healthy subjects into two groups in a double-blind order [114]. In each group, a jejunal catheter was placed. In addition, a balloon was inflated to 30 cm beyond the pylorus with the aim of aspirating bile. The administration of taurocholic acid, a secondary bile acid, or a saline control, both with and without glucose, was conducted through the catheter. The results demonstrated that the administration of TCA in the small intestine led to an increase in GLP-1 and a significant reduction in small intestine glucose levels, as well as an increase in the peptide C/glucose ratio [114].
Another product of the gut microbiota is indole, which is produced from tryptophan. This molecule has been observed to exert a dual effect on GLP-1 release. Short exposures result in increased GLP-1 secretion in immortalised and primary mouse colonic L cells, whereas secretion is reduced following longer exposures. Consequently, indole could be considered a molecule through which the microbiota exert their influence on host metabolism [115].
6. The Relationship Between the Gut Microbiota and Incretin-Based Therapies: A Complex Bidirectional Interaction
The intestinal microbiota plays an important role in many biological processes, including gut barrier function, digestion, the modulation of the immune system and/or glucose metabolism. Obesity, insulin resistance, T2DM and metabolic syndrome have been largely correlated to a reduction in bacterial load and diversity, configuring a picture termed intestinal dysbiosis [116,117]. The ratio between the bacterial species Firmicutes and Bacteroidetes is widely regarded as playing a significant role in disease and health [60,61,118].
A multitude of metabolites produced by the gut microbiota modulate the activity of enteroendocrine cells and their hormonal secretion. Such metabolites include 5-hydroxytryptamine [119], indole [111], LPS [120], secondary BAs [99] and SCFAs [94]. These increase GLP-1 secretion, either directly or indirectly, through a variety of mechanisms [88,96,121,122,123,124]. Of particular interest is the role of SCFAs, which activate various receptors, including GPR41 (FFA3) and GPR43 (FFA2), expressed by enteroendocrine L cells. The activation of these receptors have been shown to enhance GLP-1 secretion through increased intracellular calcium [94,96,123]. Furthermore, the gut microbiota exerts a regulatory effect on GLP-1 secretion through Takeda G protein-coupled receptor 5 on intestinal L cells via secondary BAs metabolism [96,97]. Conversely, some metabolic diseases, including T2DM, are partly related to a decreased production of SCFAs [118,125], which are predominantly formed in the colon.
Moreover, a plethora of evidence suggests that the intestinal microbiota can not only influence incretin secretion, but in turn can also be influenced by incretin-based therapies and modulate the individual response to such drugs [96]. This interaction is complex and bidirectional. Numerous studies have indicated that the administration of DPP-4 inhibitors and the GLP-1R agonist could potentially modify the intestinal microbiota (Table 1).

Table 1.
Effects of DPP-4 inhibitors and GLP-1R agonist on gut microbiota composition.
The mechanisms by which DDP-IV inhibitors and GLP-1R agonists modify the composition of the gut microbiota are still not clear. As suggested by Zeng Y. et al. [126] and as reported in our manuscript (see Section 4.1 and Section 4.2, Preclinical Studies and Clinical Studies, respectively), DDP-IV inhibitors impact the composition and function of the gut microbiota and they correct the dysbiosis of the microbiota. It is hypothesised that DPP-4 inhibitors can normalise the faecal microbiota composition and promote a functional shift in the gut microbiome. Numerous studies have demonstrated that the administration of DPP-4 inhibitors exerts a substantial influence on the composition of the gut microbiota, as evidenced by an augmentation in the abundance of Bacteroidetes, a concomitant escalation in the production of succinate, and an amelioration of microbiota dysbiosis in obese and T2DM mice [66,67]. These changes may contribute to the observed beneficial effects. As previously reported, DPP-4 inhibitors improve glucose metabolism and increase plasma GLP-1 concentrations. However, the magnitude of GLP-1 secretion is dependent on the rate and load of nutrient entry to the small intestine, so the individual basal rate of gastric emptying may well explain the different response to DPP-4 inhibitors [27].
It is plausible that the shifts in gut microbiota could contribute to the modulation of glucose homeostasis by increasing GLP-1 levels.
Furthermore, liraglutide treatment increases the Firmicutes to Bacteroides ratio in diabetic mice with normal weight, thereby indicating that liraglutide has the capacity to modulate the gut microbiota composition in a positive manner. Collectively, the findings from animal studies imply that GLP-1R agonists and DPP-4 inhibitors may have a significant impact on the composition of the gut microbiota. Treatment with GLP-1R agonists increases the richness and diversity of the gut microbiota and changes the overall structure of the gut microbiota, especially some bacteria related to intestinal inflammation and glucolipid inflammation [76,77,91,127]. In addition, preclinical models suggest that changes in the gut microbiota induced by DPP-4 inhibitors and GLP-1R agonists could be linked to metabolic improvements, such as the attenuation of endotoxaemia and the increased production of short-chain fatty acids.
However, the paucity of human studies has yielded equivocal results to date. Additional studies and research are necessary due to the heterogenicity in human studies. Some human studies have suggested that incretin-based therapies do not result in any change to the structure of the gut microbiota using incretin-based therapies [94,95]. One potential explanation for this observation is that these medications are often administered in conjunction with other therapies, which may obscure the impact on the gut microbiota. Indeed, both DPP-4 inhibitors and GLP-1R agonists are invariably used in conjunction with metformin, and it has been observed that metformin dosage affects gut microbiota diversity [128]. It can be hypothesised that patients undergoing metformin treatment already possess a more favourable microbiota profile, thereby resulting in a diminished impact of DPP-4 inhibitors and GLP-1-R agonist therapies. Furthermore, these studies demonstrated that diet is the most significant factor in modulating the composition of the intestinal microbiota. The composition of the intestinal microbiota is influenced by the type of food consumed, which in turn affects the absorption of nutrients.
Especially, the Mediterranean diet increases Bifidobacterium, Faecalibacterium prausnitzii, Roseburia, and Lachnospiraceae (polyphenols and unsaturated fats appear to be the main drivers of the observed benefits); vegetarian diets increase microbial diversity and promote SCFA-producing bacteria like Akkermansia and Eubacterium; Ketogenic diets, in mouse models, improve insulin sensitivity and reduce inflammation, but their effects in humans are less clear; and Western diets reduce microbial diversity, increase gut permeability, and promote proinflammatory metabolites like TMAO (Trimethylamin N-Oxid) and LPS [129].
The microbiome plays a pivotal role in nutrient extraction, energy production, and low-grade inflammation, all of which have the potential to contribute to the development of obesity and T2DM [130,131].
As established, GLP-1R agonists induce a decrease in caloric intake. Consequently, there is a possibility that alterations to the gut microbiome may be observed if the diet is not standardised. Currently, few studies have directly compared different dietary profiles during incretin therapies. Most research focuses on the effect of incretin on the gut microbiota, without considering the interaction with specific dietary regimens. This lack of data makes it difficult to interpret discrepancies between clinical and preclinical trials, where dietary conditions can vary significantly.
In the context of animal experiments, the provision of a uniform diet to all subjects during the intervention period serves to mitigate the potential for confounding variables. This may provide a rationale for the observed discrepancies between animal studies and human trials. To fully understand the interaction between diet, gut microbiota and incretin-based therapies, studies are needed to compare directly different dietary profiles (e.g., the Mediterranean diet, ketogenic diet, vegetarian diet, Western diet, etc.) during treatment with incretins, evaluate changes in microbiota composition and functionality in response to specific combinations of diet and therapy, and analyse the metabolic and clinical effects resulting from these interactions.
Conversely, the increasingly frequent use of non-caloric artificial sweeteners (NASs) in diabetic and obese populations has led to their independent role in modifying the gut microbiota. Indeed, most of the NASs cross the gastrointestinal tract without being absorbed and therefore come into direct contact with the microbiota.
Suez et al. demonstrated that the consumption of NASs (firstly saccharin), both in mice and in humans, increases the risk of glucose intolerance, and changes in the composition of the microbiota in bacterial species are associated with the onset of type 2 diabetes in humans, first of all manifesting as the overexpression of Bacteroides [132].
However, recent studies have demonstrated a close association between GLP-1R agonist treatment and intestinal flora in T2DM subjects [98].
The changes in intestinal flora appear to be related to the modifications in metabolic parameters (e.g., fasting glucose levels, glycated haemoglobin levels, and BMI) induced by GLP-1R agonist therapies. Furthermore, Liang L. et al. reported that alterations in intestinal flora were time-related. Indeed, no significant changes in intestinal flora were observed after one week of GLP-1R agonist therapy in T2DM subjects; however, during long-term use, there was a significant change in the structure and overall composition of the flora and a significant decrease in the abundance of intestinal microorganisms compared to those before drug administration [97]. It is important to note that there is another relevant point of interaction between GLP-1 receptor agonists or tirzepatide and microbiota. This interaction is particularly significant in terms of their profound effects on both gastric emptying [133,134] and small intestinal transit [135]. Indeed, the inverse relationships in duodenal microbiota between the relative abundances of Streptococcus and Prevotella and the relative abundance of Veillonella spp with gastric emptying time is demonstrated. Consequently, this variation in gastric emptying and the exposure of nutrients to the small intestine warrants further investigations to delineate the underlying mechanisms and clarify its relevance to the risk of alterations in the gut microbiota [136].
Finally, several results show that alterations in the gut microbiota associated with metabolic diseases are different in men and women, and these characteristics may influence sex differences in the development and prevalence of metabolic diseases. Sex steroids, mainly oestrogen and testosterone, play a prominent role in the sexual dimorphism of gut microbiota [137]. Furthermore, there is recent evidence that the secretion of the GLP-1 may also be influenced by sex difference, probably through oestrogen signalling [138]. Therefore, in the complex interaction between the gut microbiota and incretin drugs, the interaction with sex hormones plays an important role, so the therapies may have sex-specific effects.
Despite numerous preclinical and clinical studies, the interaction between incretin drugs and the gut microbiota still shows several dark sides. Human data remain largely correlative, and correlations between improvements in metabolic parameters (e.g., reduced blood glucose levels, enhanced glycated haemoglobin levels, weight loss, improved hepatic steatosis) and specific microbial taxa have not established a causal link. Therefore, claims that DPP-4 inhibitors and GLP-1R agonists can significantly modulate the microbiota must be interpreted with caution, recognising that the observed changes may be secondary effects of metabolic improvement rather than primary therapeutic mechanisms.
To better understand the relationship between microbiota and drugs, multiomics techniques, such as metagenomics, metatranscriptomics, and metabolomics, should be applicated. These techniques enable the analysis of the composition and activity of the microbiota in a detailed manner, identifying specific patterns of gene expression and metabolic activity associated with drug response [139]. However, studies that have highlighted, through multiomics and strain-specific metabolite techniques, bidirectional interactions between the gut microbiota and incretin treatments are currently limited.
7. Conclusions
In conclusion, as demonstrated by several studies, incretin-based therapies have the capacity to influence the composition of the gut microbiota, thereby resulting in alterations to the bacterial flora. In addition, a lot of evidence suggests that the intestinal microbiota can not only influence incretin secretion, but in turn can also be influenced by incretin-based therapies and modulate the individual response to such drugs. The potential mechanisms by which the microbiota modulates drug metabolism and efficacy may involve the microbial-mediated metabolism of xenobiotics (oral drugs) and microbial metabolites (short-chain fatty acids and BAs). However, despite numerous preclinical and clinical studies, the interaction between incretin drugs and the gut microbiota still shows several dark sides. Taxonomic data are important, but gut microbiota–incretin crosstalk should be studied not only through metagenomic description (what the bacteria are) but also in the metabolomic direction (how they interact). An important limitation is the heterogeneity of clinical trials due to interindividual complexity (age, gender, and ethnicity), which includes dietary variability and polypharmacy.
Further research is necessary to determine whether targeting the gut microbiota could enhance the endogenous production of incretins.
Author Contributions
Conceptualisation, V.T. and A.D.; methodology, V.T., R.S. and F.A.; software, F.A.; validation, V.T., R.S. and F.A.; formal analysis, F.A.; data curation, V.T., M.R.N., A.D. and F.A.; writing—original draft preparation, V.T., A.D., M.R.N., F.G., C.V., M.M., F.C., S.S. and F.I.; writing—review and editing, V.T., A.D., M.R.N. and F.A.; visualisation, R.S. and F.A.; supervision, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| BAs | Bile acids |
| DPP-4 | Dipeptidyl peptidase IV |
| EECs | Colonic enteroendocrine cells |
| FFAR | Free fatty acid receptor |
| FOS | Fructooligosaccharides |
| FXR | Farnesoid X receptor |
| GIP | Gastric inhibitory polypeptide |
| GIPR | GIP receptor |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1R | GLP-1 receptors |
| GPR | G-protein-coupled receptor |
| LPS | Lipopolysaccharide |
| NAFLD | Non-alcoholic fatty liver disease |
| PCOS | Polycystic ovary syndrome |
| PYY | Peptide YY |
| SCFAs | Short-chain fatty acids |
| T2DM | Type 2 diabetes mellitus |
| TMAO | Trimethylamine N-oxide |
| Treg | Regulatory T cell |
| NAS | Non-caloric artificial sweetener |
References
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’I, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Andujar-Plata, P.; Pi-Sunyer, X.; Laferrere, B. Metformin effects revisited. Diabetes Res. Clin. Pract. 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef]
- Rojas, L.B.A.; Gomes, M.B. Metformin: An old but still the best treatment for type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 6. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40, Erratum in Diabetes Care 2023, 46, 1106. https://doi.org/10.2337/dc23-er05. Erratum in Diabetes Care 2023, 46, 1715. https://doi.org/10.2337/dc23-ad08. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S128–S139. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S140–S157. [Google Scholar] [CrossRef]
- Seino, Y.; Yabe, D. Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: Incretin actions beyond the pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef] [PubMed]
- Schirra, J.; Katschinski, M.; Weidmann, C.; Schäfer, T.; Wank, U.; Arnold, R.; Göke, B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J. Clin. Investig. 1996, 97, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Akindehin, S.E.; Orsso, C.E.; Waldner, R.C.; DiMarchi, R.D.; Müller, T.D.; Haqq, A.M. Recent Advances in Incretin-Based Pharmacotherapies for the Treatment of Obesity and Diabetes. Front. Endocrinol. 2022, 13, 838410. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L.; Drucker, D.J. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology 2004, 145, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Rudovich, N.N.; Rochlitz, H.J.; Pfeiffer, A.F. Reduced hepatic insulin extraction in response to gastric inhibitory polypeptide compensates for reduced insulin secretion in normal-weight and normal glucose tolerant first-degree relatives of type 2 dia-betic patients. Diabetes 2004, 53, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R.; Gallwitz, B.; Schmidt, W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993, 214, 829–835. [Google Scholar] [CrossRef]
- Brubaker, P.L.; Crivici, A.; Izzo, A.; Ehrlich, P.; Tsai, C.H.; Drucker, D.J. Circulating and tissue forms of the intestinal growth factor, glucagon-like peptide-2. Endocrinology 1997, 138, 4837–4843. [Google Scholar] [CrossRef]
- Hartmann, B.; Harr, M.B.; Jeppesen, P.B.; Wojdemann, M.; Deacon, C.F.; Mortensen, P.B.; Holst, J.J. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2884–2888. [Google Scholar] [CrossRef]
- Zhong, J.; Maiseyeu, A.; Davis, S.N.; Rajagopalan, S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015, 116, 1491–1504. [Google Scholar] [CrossRef]
- Zhong, J.; Rao, X.; Deiuliis, J.; Braunstein, Z.; Narula, V.; Hazey, J.; Mikami, D.; Needleman, B.; Satoskar, A.R.; Rajagopalan, S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 2013, 62, 149–157. [Google Scholar] [CrossRef]
- Drucker, D.J. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology 2002, 122, 531–544. [Google Scholar] [CrossRef]
- Gupta, A.; Jelinek, H.F.; Al-Aubaidy, H. Glucagon like peptide-1 and its receptor agonists: Their roles in management of Type 2 diabetes mellitus. Diabetes Metab. Syndr. 2017, 11, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Rao, X.; Rajagopalan, S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implica-tions in cardiovascular disease. Atherosclerosis 2013, 226, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B. GLP-1 agonists and dipeptidyl-peptidase IV inhibitors. Handb. Exp. Pharmacol. 2011, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.E.; Buttfield, M.; Wu, T.; Hatzinikolas, S.; Pham, H.; Lange, K.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Effects of sitagliptin on gastric emptying of, and the glycaemic and blood pressure responses to, a carbohydrate meal in type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, X.; Trahair, L.G.; Bound, M.J.; Little, T.J.; Deacon, C.F.; Horowitz, M.; Jones, K.L.; Rayner, C.K. Small Intestinal Glucose Delivery Affects the Lowering of Blood Glucose by Acute Vildagliptin in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 4769–4778. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bound, M.J.; Zhao, B.R.; Standfield, S.D.; Bellon, M.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of a D-xylose preload with or without sitagliptin on gastric emptying, glucagon-like peptide-1, and postprandial glycemia in type 2 diabetes. Diabetes Care 2013, 36, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Little, T.J.; Bound, M.J.; Borg, M.; Zhang, X.; Deacon, C.F.; Horowitz, M.; Jones, K.L.; Rayner, C.K. A Protein Preload Enhances the Glucose-Lowering Efficacy of Vildagliptin in Type 2 Diabetes. Diabetes Care 2016, 39, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Marguet, D.; Baggio, L.; Kobayashi, T.; Bernard, A.M.; Pierres, M.; Nielsen, P.F.; Ribel, U.; Watanabe, T.; Drucker, D.J.; Wagtmann, N. Enhanced insulin secretion and improved glu-cose tolerance in mice lacking CD26. Proc. Natl. Acad. Sci. USA 2000, 97, 6874–6879. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Hoggatt, J.; O’Leary, H.A.; Mantel, C.; Chitteti, B.R.; Cooper, S.; Messina-Graham, S.; Hangoc, G.; Farag, S.; Rohrabaugh, S.L.; et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat. Med. 2012, 18, 1786–1796. [Google Scholar] [CrossRef]
- Shah, Z.; Kampfrath, T.; Deiuliis, J.A.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-term dipeptidyl-peptidase 4 inhibition reduces ath-erosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011, 124, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Rajagopalan, S. Dipeptidyl peptidase-4 regulation of SDF-1/CXCR4 axis: Implications for cardiovascular disease. Front. Immunol. 2015, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, J.; Tanaka, T.; Nojima, Y.; Schlossman, S.F.; Morimoto, C. Direct association of adenosine deaminase with a T cell acti-vation antigen, CD26. Science 1993, 261, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.; Klemann, C.; Stephan, M.; von Hörsten, S. Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins. Clin. Exp. Immunol. 2016, 184, 265–283. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Burcelin, R.; Gourdy, P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes. Rev. 2017, 18, 86–98. [Google Scholar] [CrossRef]
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like peptide-1 and the central/peripheral nervous system: Crosstalk in diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103. [Google Scholar] [CrossRef]
- Baggio, L.L.; Ussher, J.R.; McLean, B.A.; Cao, X.; Kabir, M.G.; Mulvihill, E.E.; Mighiu, A.S.; Zhang, H.; Ludwig, A.; Seeley, R.J.; et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metab. 2017, 6, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression Within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Cinci, L.; Rotondo, A.; Serio, R.; Faussone-Pellegrini, M.S.; Vannucchi, M.G.; Mulè, F. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol. Motil. 2010, 22, 664-e203. [Google Scholar] [CrossRef] [PubMed]
- Gorgojo-Martínez, J.J.; Mezquita-Raya, P.; Carretero-Gómez, J.; Castro, A.; Cebrián-Cuenca, A.; de Torres-Sánchez, A.; García-de-Lucas, M.D.; Núñez, J.; Obaya, J.C.; Soler, M.J.; et al. Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. J. Clin. Med. 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Claustre, J.; Brechet, S.; Plaisancie, P.; Chayvialle, J.A.; Cuber, J.C. Stimulatory effect of beta-adrenergic agonists on ileal L cell secretion and modulation by alpha-adrenergic activation. J. Endocrinol. 1999, 162, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chen, Y.; Lai, F.; Chen, L.; Zeng, R.; Pei, L.; Wu, L.; Wang, C.; Li, Y.; Xiao, H.; et al. Circulating GLP-1 Levels in Patients with Pheochromocytoma/Paraganglioma. Int. J. Endocrinol. 2022, 2022, 4203018. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. New Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Chao, A.M.; Machineni, S.; Kushner, R.; Ard, J.; Srivastava, G.; Halpern, B.; Zhang, S.; Chen, J.; Bunck, M.C.; et al. Author Correction: Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: The SURMOUNT-3 phase 3 trial. Nat. Med. 2024, 30, 1784, Erratum in Nat. Med. 2023, 29, 2909–2918. https://doi.org/10.1038/s41591-023-02597-w. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Poretsky, R.; Rodriguez-R, L.M.; Luo, C.; Tsementzi, D.; Konstantinidis, K.T.; Rodriguez-Valera, F. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS ONE 2014, 9, e93827. [Google Scholar] [CrossRef]
- Hugon, P.; Dufour, J.C.; Colson, P.; Fournier, P.E.; Sallah, K.; Raoult, D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect. Dis. 2015, 15, 1211–1219. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, E.P.; Dunbar, S.B.; Dunlop, A.L.; Corwin, E.J. 20 things you didn’t know about the human gut microbiome. J. Cardiovasc. Nurs. 2014, 29, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.D.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Entero-types of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Espin-Garcia, O.; Xu, W.; Silverberg, M.S.; Kevans, D.; Smith, M.I.; Guttman, D.S.; Griffiths, A.; Panaccione, R.; Otley, A.; et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat. Genet 2016, 48, 1413–1417. [Google Scholar] [CrossRef]
- Burton, C.L.; Chhabra, S.R.; Swift, S.; Baldwin, T.J.; Withers, H.; Hill, S.J.; Williams, P. The growth response of Escherichia coli to neu-rotransmitters and related catecholamine drugs requires a functional enterobactin biosynthesis and uptake system. Infect. Immun. 2002, 70, 5913–5923. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Human virome and disease: High-throughput sequencing for virus discovery, identifcation of phage-bacteria dysbiosis and development of therapeutic approaches with emphasis on the human gut. Viruses 2019, 11, 656. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Bacteriophages as New Human Viral Pathogens. Microorganisms 2018, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, M.; Leclerc, M.; Tinsley, C.R.; Petit, M.A. Bacteriophages: An underestimated role in human and animal health? Front. Cell. Infect. Microbiol. 2014, 4, 39. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, X.; Feng, B.; Li, P.; Tang, Z.; Wang, L. Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: An animal study. J. Diabetes Res. 2016, 2016, 2093171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Li, M.; Yu, M.; Ping, F.; Zheng, J.; Wang, T.; Wang, X. Vildagliptin Increases Butyrate-Producing Bacteria in the Gut of Diabetic Rats. PLoS ONE 2017, 12, e0184735. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Patterson, E.; Carafa, I.; Mandal, R.; Wishart, D.S.; Dinan, T.G.; Cryan, J.F.; Tuohy, K.M.; Stanton, C.; Ross, R.P. Metformin and Dipeptidyl Peptidase-4 Inhibitor Differentially Modulate the Intestinal Microbiota and Plasma Metabolome of Metabolically Dysfunctional Mice. Can. J. Diabetes 2020, 44, 146–155.e2. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Neyrinck, A.M.; Pötgens, S.A.; Beaumont, M.; Salazar, N.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. The DPP-4 inhibitor vildagliptin impacts the gut microbiota and prevents disruption of intestinal homeostasis induced by a Western diet in mice. Diabetologia 2018, 61, 1838–1848. [Google Scholar] [CrossRef]
- Liao, X.; Song, L.; Zeng, B.; Liu, B.; Qiu, Y.; Qu, H.; Zheng, Y.; Long, M.; Zhou, H.; Wang, Y.; et al. Alteration of gut microbiota induced by DPP-4i treatment improves glucose homeostasis. EBioMedicine 2019, 44, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Silva-Veiga, F.M.; Miranda, C.S.; Vasques-Monteiro, I.M.L.; Souza-Tavares, H.; Martins, F.F.; Daleprane, J.B.; Souza-Mello, V. Peroxisome proliferator-activated receptor-alpha activation and dipeptidyl peptidase-4 inhibition target dysbiosis to treat fatty liver in obese mice. World J. Gastroenterol. 2022, 28, 1814–1829. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, P.; Tang, Z.; Yan, X.; Feng, B. Structural modulation of the gut microbiota and the relationship with body weight: Compared evaluation of liraglutide and saxagliptin treatment. Sci. Rep. 2016, 6, 33251. [Google Scholar] [CrossRef]
- Kato, S.; Sato, T.; Fujita, H.; Kawatani, M.; Yamada, Y. Effects of GLP-1 receptor agonist on changes in the gut bacterium and the underlying mechanisms. Sci. Rep. 2021, 11, 9167. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, L.; Liao, Y.; Lin, Z.; Guo, C.; Luo, S.; Wang, F.; Zou, Z.; Zeng, Z.; Chen, C.; et al. Semaglutide alleviates gut microbiota dysbiosis induced by a high-fat diet. Eur. J. Pharmacol. 2024, 969, 176440. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Teng, Z.; Yang, Y.; Liu, J.; Chen, S. Effects of semaglutide on gut microbiota, cognitive function and inflammation in obese mice. PeerJ 2024, 12, e17891. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Zhang, C.; Yang, S.; Bi, Y.; Li, M.; Yu, J. Semaglutide alters gut microbiota and improves NAFLD in db/db mice. Biochem. Biophys. Res. Commun. 2024, 710, 149882. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, I.H.R.; da Silva, R.S.; Mendonça, I.P.; de Souza, J.R.B.; Peixoto, C.A. Semaglutide Attenuates Anxious and Depressive-Like Behaviors and Reverses the Cognitive Impairment in a Type 2 Diabetes Mellitus Mouse Model Via the Microbiota-Gut-Brain Axis. J. Neuroimmune Pharmacol. 2024, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, J.E.; Zeng, H.; Zhang, Y.; Yang, S.; Liu, J. Semaglutide alleviates the pancreatic β cell function via the METTL14 signaling and modulating gut microbiota in type 2 diabetes mellitus mice. Life Sci. 2025, 361, 123328. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Wu, J.; Ma, Y.; Li, N.; Wang, X.; Li, Y.; Ding, X. Effects of Glucagon-Like Peptide-1 Receptor Agonists on Gut Microbiota in Dehydroepiandrosterone-Induced Polycystic Ovary Syndrome Mice: Compared Evaluation of Liraglutide and Semaglutide Intervention. Diabetes Metab. Syndr. Obes. 2024, 17, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.S.A.; Holm, J.B.; Pallejà, A.; Wismann, P.; Fabricius, K.; Rigbolt, K.; Mikkelsen, M.; Sommer, M.; Jelsing, J.; Nielsen, H.B.; et al. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci. Rep. 2019, 9, 15582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. Featured article: Structure moderation of gut microbiota in liraglutide-treated diabetic male rats. Exp. Biol. Med. 2018, 243, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, J.; Briand, F.; Lelouvier, B.; Servant, F.; Azalbert, V.; Puel, A.; Christensen, J.E.; Waget, A.; Branchereau, M.; Garret, C.; et al. Liraglutide Targets the Gut Microbiota and the In-testinal Immune System to Regulate Insulin Secretion. Acta Diabetol. 2021, 58, 881–897. [Google Scholar] [CrossRef]
- Moreira, G.; Azevedo, F.; Ribeiro, L.; Santos, A.; Guadagnini, D.; Gama, P.; Liberti, E.; Saad, M.; Carvalho, C. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J. Nutr. Biochem. 2018, 62, 143–154. [Google Scholar] [CrossRef]
- Yuan, X.; Ni, H.; Chen, X.; Feng, X.; Wu, Q.; Chen, J. Identification of therapeutic effect of glucagon-like peptide 1 in the treatment of STZ-induced diabetes mellitus in rats by restoring the balance of intestinal flora. J. Cell. Biochem. 2018, 119, 10067–10074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, Y.; Xia, F.; Abudukerimu, B.; Zhang, W.; Guo, Y.; Wang, N.; Lu, Y. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front. Endocrinol. 2018, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cai, B.Y.; Zhu, L.X.; Xin, X.; Wang, X.; An, Z.M.; Li, S.; Hu, Y.Y.; Feng, Q. Liraglutide modulates gut microbiome and attenuates nonalcoholic fatty liver in db/db mice. Life Sci. 2020, 261, 118457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Li, Y.; Luo, S.; Ye, J.; Lu, Z.; Li, X.; Lu, H. The HIF-2α/PPARα pathway is essential for liraglutide-alleviated, lipid-induced hepatic steatosis. Biomed. Pharmacother. 2021, 140, 111778. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315, Erratum in Molecules 2025, 30, 1190. https://doi.org/10.3390/molecules30061190. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gong, W.; Yang, F.; Cheng, R.; Zhang, G.; Gan, L.; Zhu, Y.; Qin, W.; Gao, Y.; Li, X.; et al. Dual GIP and GLP-1 receptor agonist tirzepatide alleviates hepatic steatosis and modulates gut microbiota and bile acid metabolism in diabetic mice. Int. Immunopharmacol. 2025, 147, 113937. [Google Scholar] [CrossRef]
- Silva-Veiga, F.M.; Marinho, T.S.; de Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), positively impacts the altered microbiota of obese, diabetic, ovariectomized mice. Life Sci. 2024, 361, 123310. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; El Essawy, B.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef] [PubMed]
- Yusta, B.; Baggio, L.L.; Koehler, J.; Holland, D.; Cao, X.; Pinnell, L.J.; Johnson-Henry, K.C.; Yeung, W.; Surette, M.G.; Bang, K.A.; et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes 2015, 64, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Yusta, B.; Koehler, J.A.; Baggio, L.L.; McLean, B.A.; Matthews, D.; Seeley, R.J.; Drucker, D.J. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 2022, 34, 1514–1531.e7. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, R.; Kaur, K.D.; Koehler, J.A.; Baggio, L.L.; Wong, C.K.; Advani, K.E.; Yusta, B.; Efimova, I.; Gribble, F.M.; Reimann, F.; et al. Glucose-dependent insulinotropic polypeptide receptor signaling alleviates gut inflammation in mice. J. Clin. Investig. 2024, 10, e174825. [Google Scholar] [CrossRef]
- Ying, X.; Rongjiong, Z.; Kahaer, M.; Chunhui, J.; Wulasihan, M. Therapeutic efficacy of liraglutide versus metformin in modulating the gut microbiota for treating type 2 diabetes mellitus complicated with nonalcoholic fatty liver disease. Front. Microbiol. 2023, 14, 1088187. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Saha, S.; Van Horn, S.; Thomas, E.; Traini, C.; Sathe, G.; Rajpal, D.K.; Brown, J.R. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol. Diabetes Metab. Case Rep. 2018, 1, e00009. [Google Scholar] [CrossRef]
- Smits, M.M.; Fluitman, K.S.; Herrema, H.; Davids, M.; Kramer, M.H.; Groen, A.K.; Belzer, C.; de Vos, W.M.; Cahen, D.L.; Nieuwdorp, M.; et al. Liraglutide and sitagliptin have no effect on intestinal microbiota composition: A 12-week randomized placebo-controlled trial in adults with type 2 diabetes. Diabetes Metab. 2021, 47, 101223. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Pietrucci, D.; Longo, S.; Menghini, R.; Teofani, A.; Piciucchi, G.; Montagna, M.; Federici, M. Impact of insulin De-gludec/Liraglutide fixedcombination on the gut microbiomes of elderly patients with type 2 diabetes: Results from A Suba-nalysis of A small non-randomised single arm study. Aging Dis. 2023, 14, 319–324. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Lu, H.C.; Chou, Y.H.; Liu, P.Y.; Chen, H.Y.; Huang, M.C.; Lin, C.H.; Tsai, C.N. Gut Microbial Signatures for Glycemic Responses of GLP-1 Receptor Agonists in Type 2 Diabetic Patients: A Pilot Study. Front. Endocrinol. 2021, 12, 814770. [Google Scholar] [CrossRef]
- Liang, L.; Su, X.; Guan, Y.; Wu, B.; Zhang, X.; Nian, X. Correlation between intestinal flora and GLP-1 receptor agonist dulaglutide in type 2 diabetes mellitus treatment—A preliminary longitudinal study. iScience 2024, 27, 109784. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Freeland, K.R.; Wolever, T.M.S. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-α. Br. J. Nutr. 2010, 103, 460–466. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate gluca-gon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Cani, P.D.; Hoste, S.; Guiot, Y.; Delzenne, N.M. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br. J. Nutr. 2007, 98, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Dewever, C.; Delzenne, N.M. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br. J. Nutr. 2004, 92, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Piche, T.; des Varannes, S.B.; Sacher-Huvelin, S.; Holst, J.J.; Cuber, J.C.; Galmiche, J.P. Colonic fermentation influences lower esopha-geal sphincter function in reflux disease. Gastroenterology 2003, 124, 894–902. [Google Scholar] [CrossRef]
- Ropert, A.; Cherbut, C.; Roze, C.; Le Quellec, A.; Holst, J.; Fu-Cheng, X.; Bruley des Varannes, S.; Galmiche, J.P. Colonic fermentation and proximal gastric tone in humans. Gastroenterology 1996, 111, 289–296. [Google Scholar] [CrossRef]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Pols, T.W.H.; Nomura, M.; Maida, A.; Watanabe, M.; Auwerx, J.; Schoonjans, K. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci. Rep. 2012, 2, 430. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Trabelsi, M.-S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef]
- Ducastel, S.; Touche, V.; Trabelsi, M.-S.; Boulinguiez, A.; Butruille, L.; Nawrot, M.; Peschard, S.; Chávez-Talavera, O.; Dorchies, E.; Vallez, E.; et al. The nuclear receptor FXR inhibits glucagon-like peptide-1 secretion in response to microbiota-derived short-chain fatty acids. Sci. Rep. 2020, 10, 174. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Albrechtsen, N.J.W.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.E.; Gariballa, S.; Parekh, K.A.; Thomas, S.A.; Saadi, H.; Al Kaabi, J.; Nagelkerke, N.; Gedulin, B.; Young, A.A. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 2012, 55, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Gedulin, B.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes. Metab. 2013, 15, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J. Clin. Endocrinol. Metab. 2013, 98, E718–E722. [Google Scholar] [CrossRef] [PubMed]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secre-tion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Hartstra, A.V.; Bouter, K.E.C.; Bäckhed, F.; Nieuwdorp, M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin cells are gut Chemosensors that couple to sensory neural pathways. Cell 2017, 170, 185–198. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Lenaerts, K.; Kiers, D.; Pais de Barros, J.-P.; Le Guern, N.; Plesnik, J.; Thomas, C.; Bourgeois, T.; Dejong, C.H.C.; Kox, M.; et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 2017, 21, 1160–1168. [Google Scholar] [CrossRef]
- Chaudhari, S.N.; Luo, J.N.; Harris, D.A.; Aliakbarian, H.; Yao, L.; Paik, D.; Subramaniam, R.; Adhikari, A.A.; Vernon, A.H.; Kiliç, A.; et al. A microbial metabolite Remodels the gut-liver axis following Bariatric surgery. Cell Host Microbe 2021, 29, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Johansson-Boll, E.V.; Björck, I.M.E. Increased gut hormones and insulin sensitivity index following a 3-d interven-tion with a barley kernel-based product: A randomised cross-over study in healthy middle-aged subjects. Br. J. Nutr. 2015, 114, 899–907. [Google Scholar] [CrossRef]
- Koopen, A.; Witjes, J.; Wortelboer, K.; Majait, S.; Prodan, A.; Levin, E.; Herrema, H.; Winkelmeijer, M.; Aalvink, S.; Bergman, J.; et al. Duodenal Anaerobutyricum soehngenii infusion stimulates GLP-1 production, ameliorates glycaemic control and beneficially shapes the duodenal transcriptome in metabolic syndrome subjects: A randomised double-blind placebo-controlled cross-over study. Gut 2022, 71, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Rosenkilde, M.M.; Knop, F.K.; Wellner, N.; Diep, T.A.; Rehfeld, J.F.; Andersen, U.B.; Holst, J.J.; Hansen, H.S. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J. Clin. Endocrinol. Metab. 2011, 96, E1409–E1417. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between glucagon-like peptide 1 and gut microbiota in metabolic diseases. mBio 2024, 15, e0203223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gofron, K.K.; Wasilewski, A.; Małgorzewicz, S. Effects of GLP-1 Analogues and Agonists on the Gut Microbiota: A Systematic Review. Nutrients 2025, 17, 1303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Metformin and gut microbiota: Their interactions and their impact on diabetes. Hormones 2019, 18, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The role of the gut microbiota in the Relationship between diet and human health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Type 2 Diabetes. 2019. Available online: https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val=https://www.cdc.gov/diabetes/basics/type2.html (accessed on 12 February 2021).
- Baothman, O.A.; Zamzami, M.A.; Taher, I.; Abubaker, J.; Abu-Farha, M. The role of Gut Microbiota in the development of obesity and Diabetes. Lipids Health Dis. 2016, 15, 108. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Rayner, C.K.; Watson, L.E.; Phillips, L.K.; Lange, K.; Bound, M.J.; Grivell, J.; Wu, T.; Jones, K.L.; Horowitz, M.; Ferrannini, E.; et al. Effects of sustained treatment with lixisenatide on gastric emptying and postprandial glucose metabolism in type 2 diabetes: A randomized controlled trial. Diabetes Care 2020, 43, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Huynh, L.Q.; Hatzinikolas, S.; Rigda, R.S.; Phillips, L.K.; Pham, H.T.; Marathe, C.S.; Wu, T.; Malbert, C.H.; Stevens, J.E.; et al. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentrations. Diabetes Obes. Metab. 2020, 22, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Marathe, C.S.; Wu, T.; Chang, J.; Khoo, J.; Kuo, P.; Checklin, H.L.; Bound, M.J.; Rigda, R.S.; Crouch, B.; et al. The glucagon-like peptide 1 receptor agonist exenatide inhibits small intestinal motility, flow, transit, and absorption of glucose in healthy subjects and patients with type 2 diabetes: A randomized controlled trial. Diabetes 2016, 65, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, E.R.; Kang, S.; Staudacher, H.; Shah, A.; Do, A.; Burns, G.; Chachay, V.S.; A Koloski, N.; Keely, S.; Walker, M.M.; et al. Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in functional dyspepsia. Gut 2023, 72, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Santos-Marcos, J.A.; Mora-Ortiz, M.; Tena-Sempere, M.; Lopez-Miranda, J.; Camargo, A. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol. Sex Differ. 2023, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Huang, W.; Sun, Y.; Xiang, C.; Trahair, L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Disparities in the Glycemic and Incretin Responses to Intraduodenal Glucose Infusion Between Healthy Young Men and Women. J. Clin. Endocrinol. Metab. 2023, 108, e712–e719. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, C.; Liu, H.; Wu, J.; Tan, L.; Liu, S.; Lv, H.; Wang, C.; Wang, F.; Liu, J. Integrated Multi-Omics Analysis Reveals Mountain-Cultivated Ginseng Ameliorates Cold-Stimulated Steroid-Resistant Asthma by Regulating Interactions among Microbiota, Genes, and Metabolites. Int. J. Mol. Sci. 2024, 25, 9110, Erratum in Int. J. Mol. Sci. 2024, 25, 10021. https://doi.org/10.3390/ijms251810021. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).